Cellular Senescence and Inflammaging in the Bone: Pathways, Genetics, Anti-Aging Strategies and Interventions
Abstract
:1. Introduction
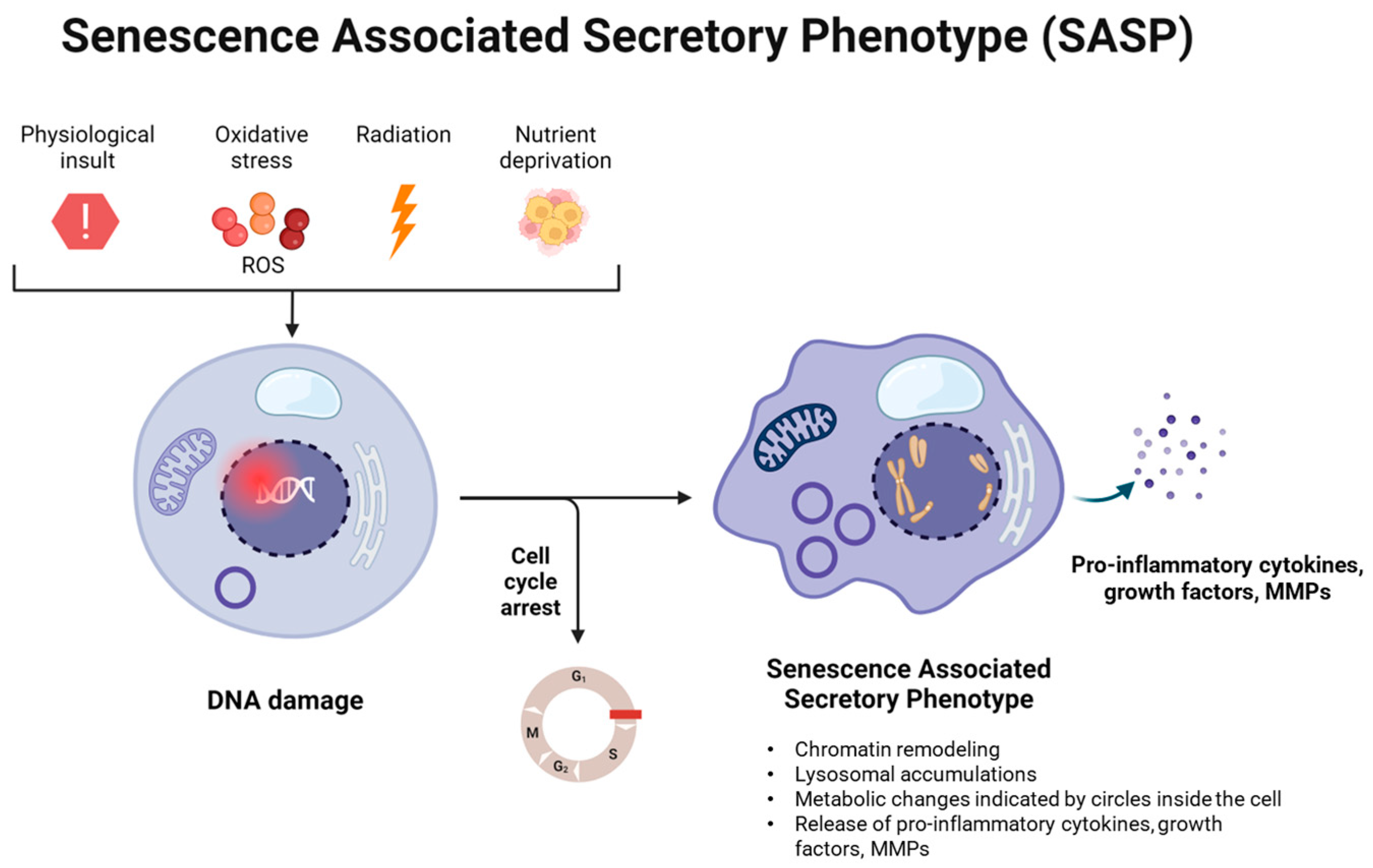
2. Cellular Senescence and Inflammaging in the Bone
3. Pathways Involved in Senescence and Inflammaging in the Bone
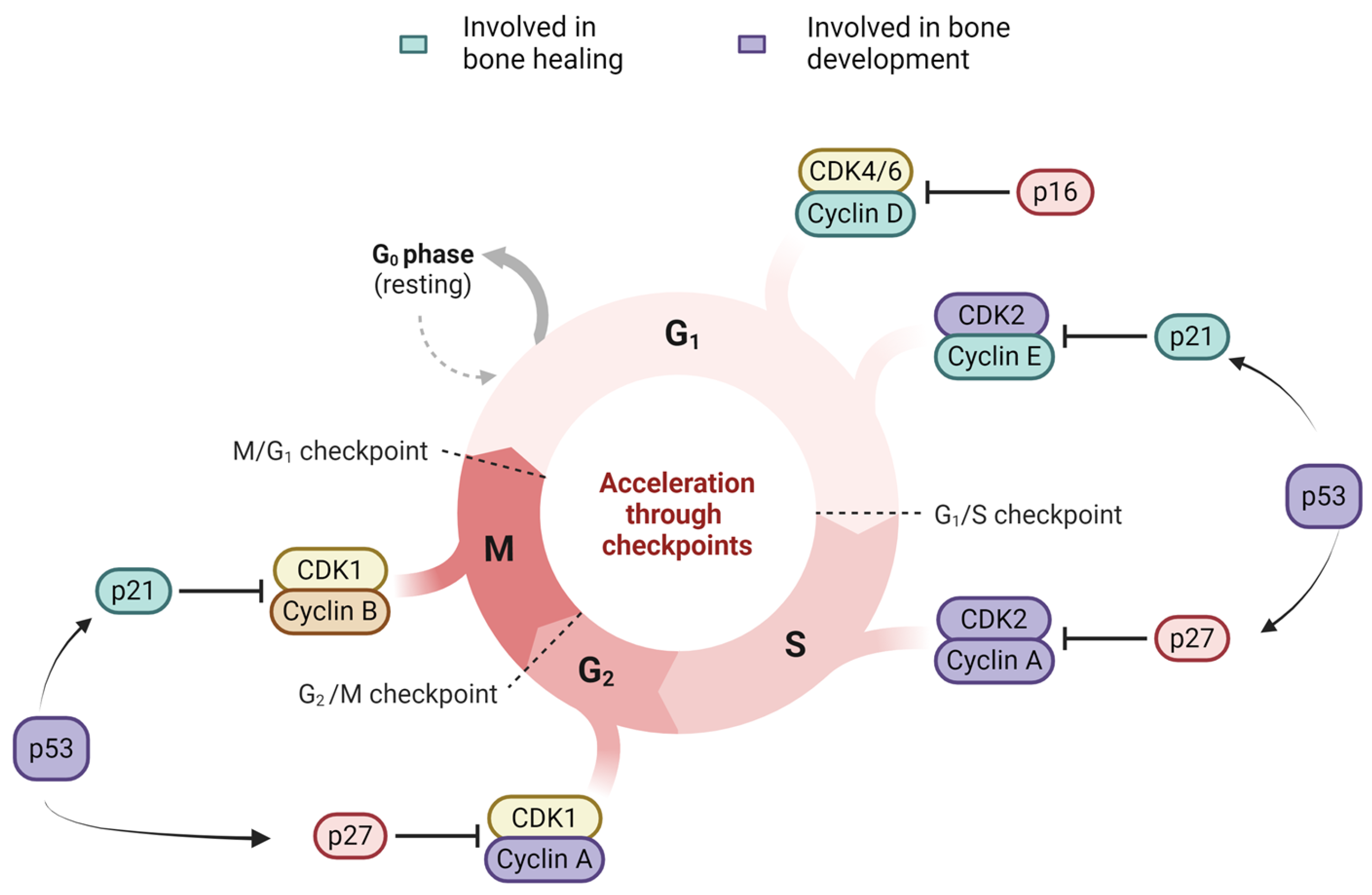
3.1. Cell Cycle Arrest: p16, p21, p53
3.1.1. p16-Rb Pathway
3.1.2. p53-p21 Pathway
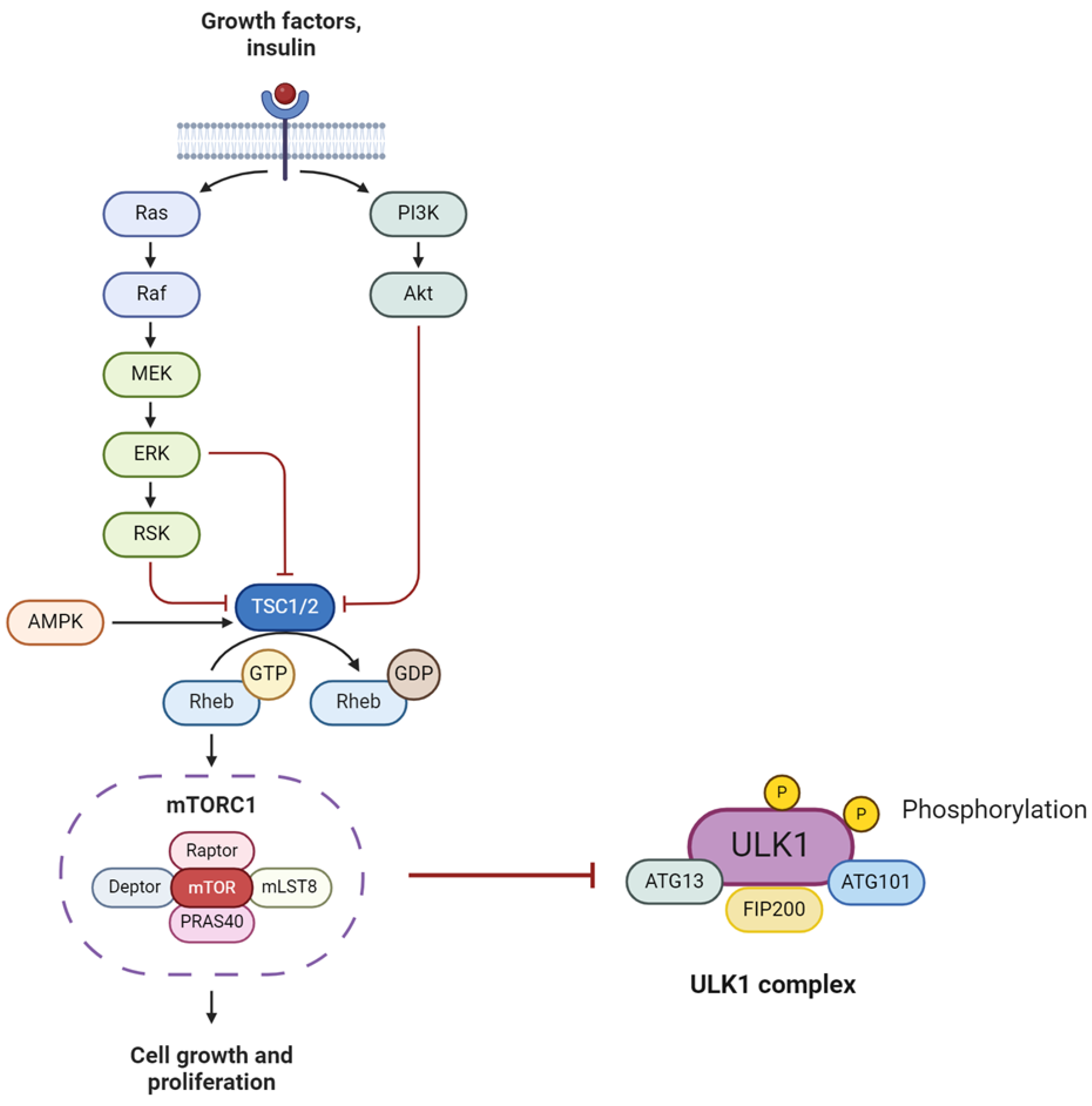
3.2. Growth Promotion Pathways—mTOR and, SIRT-1
3.2.1. mTOR
3.2.2. SIRTs
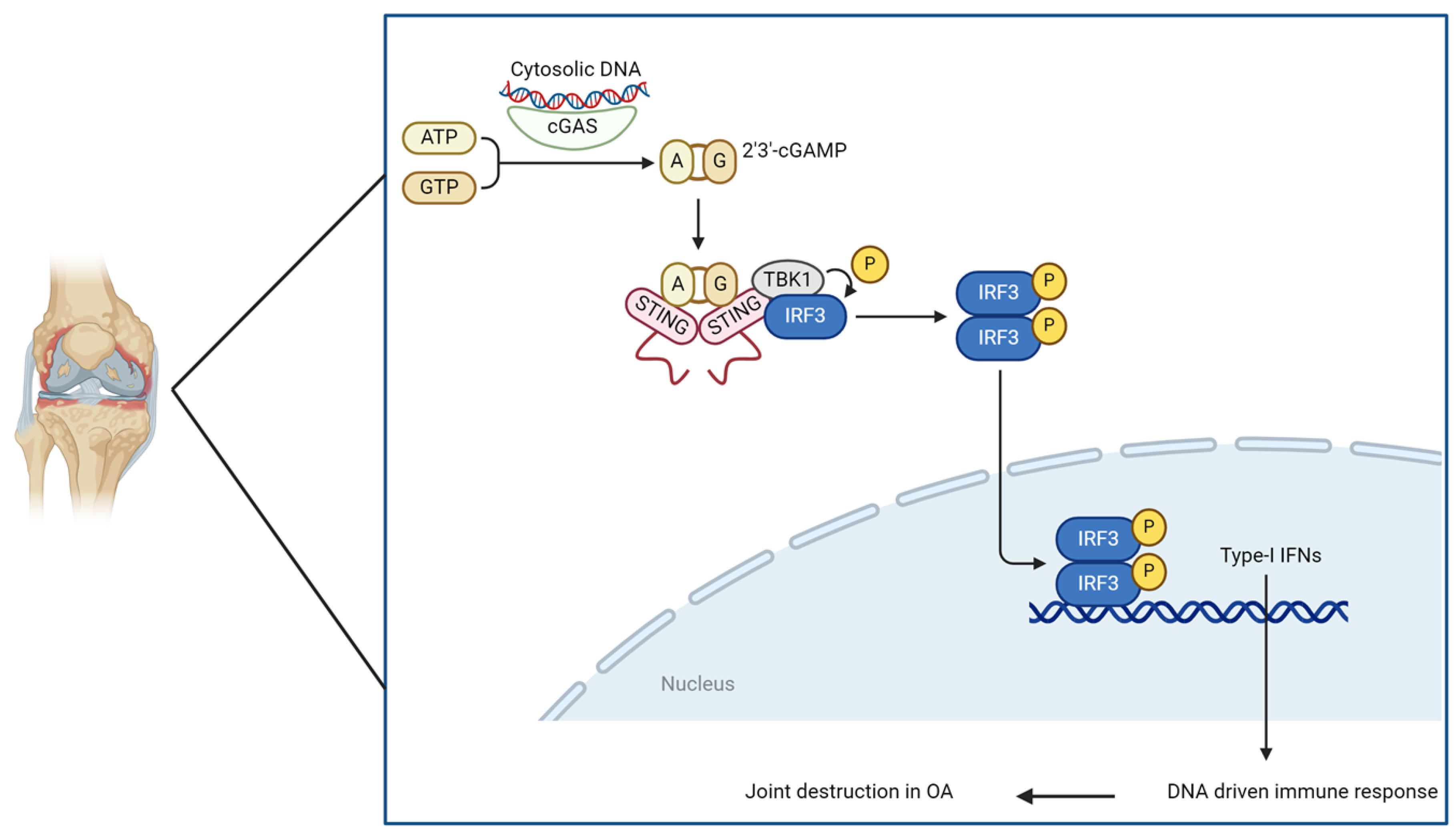
3.3. Reactive Oxygen Species (ROS)-Induced DNA Damage (Type 1 IFN and the STING Pathway)
4. Genetics of Cellular Senescence in the Bone
4.1. Key Genes Associated with SASP
4.2. NGS Studies on Bone Aging
4.3. Potential Pathways to Target in Bone Aging
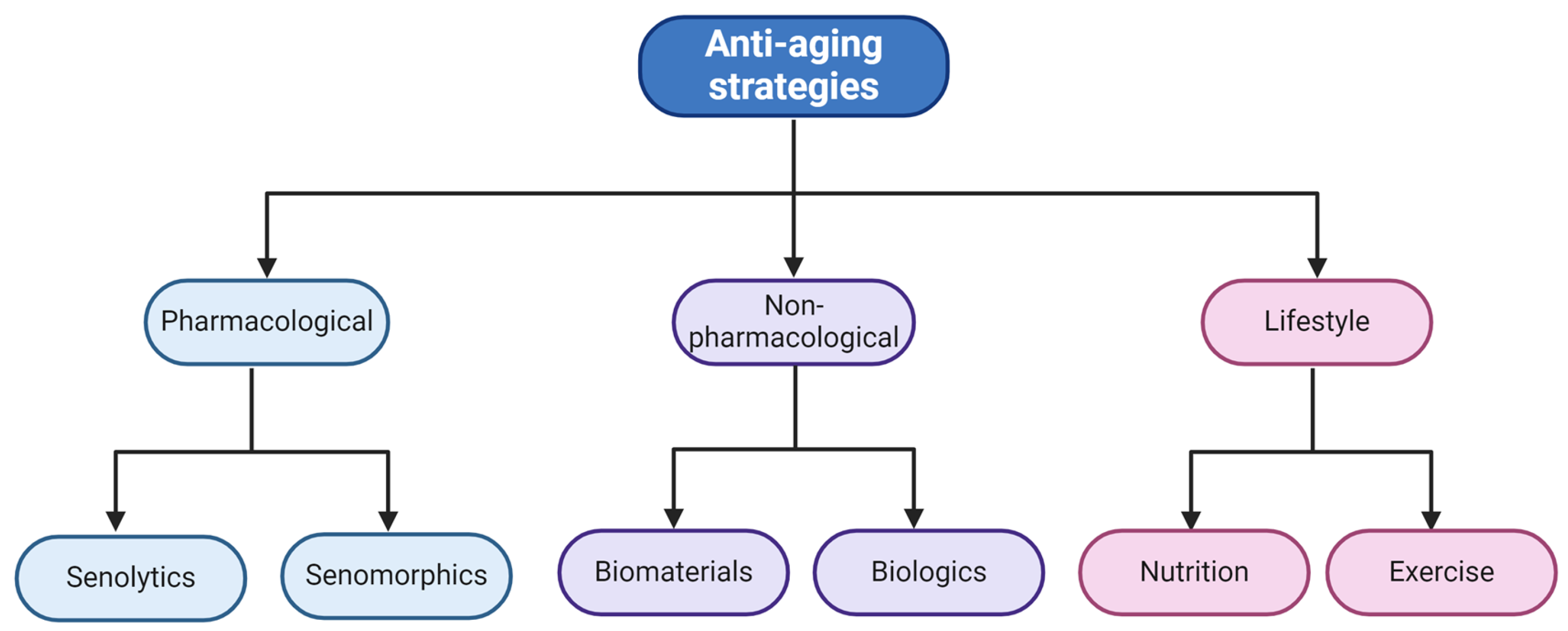
5. Combatting Senescence in the Bone
5.1. Pharmacological Approaches
5.1.1. Senolytics
5.1.2. Senomorphics
5.1.3. Seno-Inflammation
5.2. Non-Pharmacological Approaches
5.2.1. Materials for Bone Regeneration via Senescence Reduction
5.2.2. Anti-Aging Strategies Using Biologics
5.3. Lifestyle Approaches as Regeneration and Anti-Aging Strategies
| Approach | Strategy | Model | Target | Reference |
|---|---|---|---|---|
| Pharmacological, senolytic | Dasatinib (D) and/or quercetin (Q) | in vitro and in vivo | D- senescent fat progenitors, Q- human endothelial cells and mouse BM MSCs, D + Q—mouse embryonic fibroblasts | [123] |
| Pharmacological, senolytic | Dasatinib + Quercetin | in vitro and in vivo | Trabecular and cortical bone in mice | [73] |
| Pharmacological, senolytic | ABT263 | In vivo | Senescent bone marrow hematopoietic stem cells in mice | [126] |
| Pharmacological, senolytic/senomorphic | Zeldronic acid | In vitro and in vivo | Bone | [185] |
| Pharmacological, senomorphic | Ruxolitinib | In vivo | metabolism | [186] |
| Non-pharmacological, biomaterial | Shell nacre | In vitro | BM MSCs | [117] |
| Non-pharmacological, biomaterial | PEGylated hydrogel with Rapamycin nanomicelles | In vitro and in vivo | BM MSCs | [153] |
| Non-pharmacological, biologics | HA v/s PRP in RCT | In vivo | Knee OA | [173] |
| Non-pharmacological, biologics | Collagen | In vitro | BM MSCs | [157] |
| Non-pharmacological, biologics | PRP | In vitro and in vivo | Injured tendons | [174] |
| Lifestyle | Intermittent fasting | In vivo, n = 25 young males | Senescence markers linked with diet and lifestyle | [181] |
| Lifestyle | Physical functioning | In vivo, n = 1377 older adults | Senescence biomarkers linked with physical functioning in elderly | [120] |
| Lifestyle | Diet + exercise | In vivo, n = 12 young males | Senescent cells in skeletal muscle linked with diet and exercise | [187] |
| Lifestyle | Diet + exercise + community | Mixed methods, n = 57 older adults | Nutritional habits and active lifestyle linked with longevity | [182] |
| Lifestyle | Sleep deprivation | In vivo, n = 29 older adults | Sleep deprivation linked with senescent markers | [183] |
6. Conclusions, Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization: Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 March 2024).
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N. Biology of Aging and Cancer. Cancer Control 2007, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Heavener, K.S.; Bradshaw, E.M. The aging immune system in Alzheimer’s and Parkinson’s diseases. Semin. Immunopathol. 2022, 44, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Levy, G. The Relationship of Parkinson Disease with Aging. Arch. Neurol. 2007, 64, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2020, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Black, R.J.; Cross, M.; Haile, L.M.; Culbreth, G.T.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; Ong, K.L.; et al. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Marie, P.J. Bone Cell Senescence: Mechanisms and Perspectives. J. Bone Miner. Res. 2014, 29, 1311–1321. [Google Scholar] [CrossRef]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Corrillon, F.; Stamatopoulos, B.; Dubois, N.; Ruer, A.; Meuleman, N.; Bron, D.; Lagneaux, L. Age-related changes in human bone marrow mesenchymal stromal cells: Morphology, gene expression profile, immunomodulatory activity and miRNA expression. Front. Immunol. 2023, 14, 1267550. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kovtonyuk, L.V.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Toghill, B.; Pathak, S. Aging, Bone Marrow and Next-Generation Sequencing (NGS): Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12225. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.-D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef] [PubMed]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Health 2013, 2, 8. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. Senolytic therapies for healthy longevity. Science 2019, 364, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2022, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Myrianthopoulos, V. The emerging field of senotherapeutic drugs. Futur. Med. Chem. 2018, 10, 2369–2372. [Google Scholar] [CrossRef]
- Vun, J.; Iqbal, N.; Jones, E.; Ganguly, P. Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering 2023, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lei, T. Effects of autologous platelet-rich plasma injections on facial skin rejuvenation. Exp. Ther. Med. 2020, 19, 3024–3030. [Google Scholar] [CrossRef]
- Karin, O.; Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. GeroScience 2020, 43, 329–341. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Vanyushin, B.F. The Effects of Parabiosis on Aging and Age-Related Diseases. Rev. New Drug Targets Age-Relat. Disorders 2020, 1260, 107–122. [Google Scholar] [CrossRef]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds A Compend. Clin. Res. Pract. 2017, 29, 168–174. [Google Scholar]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B.; et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45lowCD271+ Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef]
- Kuranda, K.; Vargaftig, J.; de la Rochere, P.; Dosquet, C.; Charron, D.; Bardin, F.; Tonnelle, C.; Bonnet, D.; Goodhardt, M. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell 2011, 10, 542–546. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Wang, J.; Zhai, J.; Ren, L.; Zhu, G. Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling. Int. J. Mol. Sci. 2021, 22, 9323. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.H.; Lee, G.; Kim, S.-J.; Song, W.-H.; Kwon, S.-H.; Koh, J.-T.; Huh, Y.H.; Ryu, J.-H. Hypoxia-inducible factor-2α mediates senescence-associated intrinsic mechanisms of age-related bone loss. Exp. Mol. Med. 2021, 53, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; David, N.; Campisi, J.; Elisseeff, J.H. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018, 128, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Föger-Samwald, U.; Kerschan-Schindl, K.; Butylina, M.; Pietschmann, P. Age Related Osteoporosis: Targeting Cellular Senescence. Int. J. Mol. Sci. 2022, 23, 2701. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, M.J.; Valín, Á.; Usategui, A.; Ergueta, S.; Martín, E.; Municio, C.; Cañete, J.D.; Blanco, F.J.; Criado, G.; Pablos, J.L. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun. Ageing 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Chalan, P.; Berg, A.v.D.; Kroesen, B.-J.; Brouwer, L.; Boots, A. Rheumatoid Arthritis, Immunosenescence and the Hallmarks of Aging. Curr. Aging Sci. 2015, 8, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. STEM CELLS Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Farr, J.N.; Kaur, J.; Doolittle, M.L.; Khosla, S. Osteocyte Cellular Senescence. Curr. Osteoporos. Rep. 2020, 18, 559–567. [Google Scholar] [CrossRef]
- Kulaberoglu, Y.; Hergovich, A.; Gómez, V. The Role of p53/p21/p16 in DNA-Damage Signaling and DNA Repair. In Genome Stability; Academic Press: Cambridge, MA, USA, 2021; Volume 26, pp. 257–274. [Google Scholar]
- Khosla, S.; Farr, J.N.; Monroe, D.G. Cellular senescence and the skeleton: Pathophysiology and therapeutic implications. J. Clin. Investig. 2022, 132, e154888. [Google Scholar] [CrossRef] [PubMed]
- Beauséjour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lagnado, A.B.; Farr, J.N.; Doolittle, M.; Tchkonia, T.; Kirkland, J.L.; LeBrasseur, N.K.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell 2022, 21, e13602. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Zhao, Y.; Guo, J.; Gu, S.; Li, X.; Chang, C. Senescence of bone marrow mesenchymal stromal cells is accompanied by activation of p53/p21 pathway in myelodysplastic syndromes. Eur. J. Haematol. 2014, 93, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Jiang, J.; Tan, W.; Xia, Y.; Cao, H.; Meng, Y.; Da, Z.; Liu, H.; Cheng, C. p53/p21 Pathway Involved in Mediating Cellular Senescence of Bone Marrow-Derived Mesenchymal Stem Cells from Systemic Lupus Erythematosus Patients. J. Immunol. Res. 2013, 2013, 134243. [Google Scholar] [CrossRef]
- Shaikh, A.; Wesner, A.A.; Abuhattab, M.; Kutty, R.G.; Premnath, P. Cell cycle regulators and bone: Development and regeneration. Cell Biosci. 2023, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Buj, R.; Aird, K.M. p16: Cycling off the beaten path. Mol. Cell. Oncol. 2019, 6, e1677140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; He, S.-H.; Liang, X.; Li, W.; Li, T.-F.; Li, D.-F. Aging, Cell Senescence, the Pathogenesis and Targeted Therapies of Osteoarthritis. Front. Pharmacol. 2021, 12, 728100. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Lu, L.; Liu, L.; Yu, X.; Pei, F. Cellular senescence in knee osteoarthritis: Molecular mechanisms and therapeutic implications. Ageing Res. Rev. 2021, 70, 101413. [Google Scholar] [CrossRef]
- Philipot, D.; Guérit, D.; Platano, D.; Chuchana, P.; Olivotto, E.; Espinoza, F.; Dorandeu, A.; Pers, Y.-M.; Piette, J.; Borzi, R.M.; et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 2014, 16, R58. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079, Erratum in Nat. Med. 2017, 23, 1384. [Google Scholar] [CrossRef] [PubMed]
- Borrero, L.J.H.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Fang, C.-L.; Liu, B.; Wan, M. “Bone-SASP” in Skeletal Aging. Calcif. Tissue Int. 2023, 113, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A. UC2288 Improves Osteogenic Capacity of Murine Mesenchymal Stem Cells. Master’s Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2017. Available online: https://dc.uwm.edu/etd/2946/ (accessed on 4 March 2024).
- Englund, D.A.; Jolliffe, A.; Aversa, Z.; Zhang, X.; Sturmlechner, I.; Sakamoto, A.E.; Zeidler, J.D.; Warner, G.M.; McNinch, C.; White, T.A.; et al. p21 induces a senescence program and skeletal muscle dysfunction. Mol. Metab. 2022, 67, 101652. [Google Scholar] [CrossRef] [PubMed]
- Velletri, T.; Huang, Y.; Wang, Y.; Li, Q.; Hu, M.; Xie, N.; Yang, Q.; Chen, X.; Chen, Q.; Shou, P.; et al. Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin. Cell Death Differ. 2020, 28, 156–169. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Vasheghani, F.; Li, Y.-H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.-P.; et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2014, 74, 1432–1440. [Google Scholar] [CrossRef]
- Cheng, N.-T.; Meng, H.; Ma, L.-F.; Zhang, L.; Yu, H.-M.; Wang, Z.-Z.; Guo, A. Role of autophagy in the progression of osteoarthritis: The autophagy inhibitor, 3-methyladenine, aggravates the severity of experimental osteoarthritis. Int. J. Mol. Med. 2017, 39, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Liu, R.-X.; Huan, S.-W.; Tang, W.; Zeng, Y.-K.; Zhang, J.-C.; Yang, J.; Li, Z.-Y.; Zhou, Y.; Zha, Z.-G.; et al. Senescent skeletal cells cross-talk with synovial cells plays a key role in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2022, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Shan, H.; Bai, J.; Gao, T.; Chen, B.; Shen, Z.; Zhou, H.; Lu, H.; Sheng, L.; Zhou, X. Four-octyl itaconate improves osteoarthritis by enhancing autophagy in chondrocytes via PI3K/AKT/mTOR signalling pathway inhibition. Commun. Biol. 2022, 5, 641. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Liu, C.; Zhang, H. Sirtuins in osteoarthritis: Current understanding. Front. Immunol. 2023, 14, 1140653. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Deng, Z.; Liu, Y.; Zheng, Y.; Yang, S.; Lu, W.; Xiao, D.; Zhu, W. Protective Effect of SIRT1 Activator on the Knee with Osteoarthritis. Front. Physiol. 2021, 12, 661852. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef]
- Batshon, G.; Elayyan, J.; Qiq, O.; Reich, E.; Ben-Aderet, L.; Kandel, L.; Haze, A.; Steinmeyer, J.; Lefebvre, V.; Zhang, H.; et al. Serum NT/CT SIRT1 ratio reflects early osteoarthritis and chondrosenescence. Ann. Rheum. Dis. 2020, 79, 1370–1380. [Google Scholar] [CrossRef]
- Zhou, D.; Ran, Y.; Yu, R.; Liu, G.; Ran, D.; Liu, Z. SIRT1 regulates osteoblast senescence through SOD2 acetylation and mitochondrial dysfunction in the progression of Osteoporosis caused by Cadmium exposure. Chem. Biol. Interact. 2023, 382, 110632. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Yu, Q.; Katlinskaya, Y.V.; Carbone, C.J.; Zhao, B.; Katlinski, K.V.; Zheng, H.; Guha, M.; Li, N.; Chen, Q.; Yang, T.; et al. DNA-Damage-Induced Type I Interferon Promotes Senescence and Inhibits Stem Cell Function. Cell Rep. 2015, 11, 785–797. [Google Scholar] [CrossRef]
- Ganguly, P.; Burska, A.N.; Davis, C.L.; El-Jawhari, J.J.; Giannoudis, P.V.; Jones, E.A. Intrinsic Type 1 Interferon (IFN1) Profile of Uncultured Human Bone Marrow CD45lowCD271+ Multipotential Stromal Cells (BM-MSCs): The Impact of Donor Age, Culture Expansion and IFNα and IFNβ Stimulation. Biomedicines 2020, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Z.J. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, X.; Chen, J.; Zheng, G.; Xie, C.; Wu, H.; Miao, Z.; Lin, Y.; Wang, X.; Gao, W.; et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis. 2021, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Pang, Y. cGAS-STING pathway in pathogenesis and treatment of osteoarthritis and rheumatoid arthritis. Front. Immunol. 2024, 15, 1384372. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- He, X.; Hu, W.; Zhang, Y.; Chen, M.; Ding, Y.; Yang, H.; He, F.; Gu, Q.; Shi, Q. Cellular senescence in skeletal disease: Mechanisms and treatment. Cell. Mol. Biol. Lett. 2023, 28, 88. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, M.-K.; Zhang, H.; Peng, Y.-Q.; Wu, X.-P.; Wu, X.-Y.; Liao, E.-Y. Relationships between age-related biochemical markers of bone turnover and OPG, TGF-β1 and TGF-β2 in native Chinese women. Endocr. Res. 2013, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hatori, K.; Sasano, Y.; Takahashi, I.; Kamakura, S.; Kagayama, M.; Sasaki, K. Osteoblasts and osteocytes express MMP2 and -8 and TIMP1, -2, and -3 along with extracellular matrix molecules during appositional bone formation. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 277A, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Control of p53 and NF-κB signaling by WIP1 and MIF: Role in cellular senescence and organismal aging. Cell. Signal. 2011, 23, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, S.; Zhang, S.; Liu, M.; Du, H.; Sun, R.; Jing, B.; Sun, Y. Ageing characteristics of bone indicated by transcriptomic and exosomal proteomic analysis of cortical bone cells. J. Orthop. Surg. Res. 2019, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Munk, R.; Kim, K.M.; Piao, Y.; De, S.; Abdelmohsen, K.; Gorospe, M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019, 47, 11476. [Google Scholar] [CrossRef]
- Purcell, M.; Kruger, A.; Tainsky, M.A. Gene expression profiling of replicative and induced senescence. Cell Cycle 2014, 13, 3927–3937. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; DeMaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660.e4. [Google Scholar] [CrossRef]
- Jochems, F.; Thijssen, B.; De Conti, G.; Jansen, R.; Pogacar, Z.; Groot, K.; Wang, L.; Schepers, A.; Wang, C.; Jin, H.; et al. The Cancer SENESCopedia: A delineation of cancer cell senescence. Cell Rep. 2021, 36, 109441. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.E.; McDaniel, D.K.; Ringel-Scaia, V.M.; Allen, I.C. Modulating inflammation through the negative regulation of NF-κB signaling. J. Leukoc. Biol. 2018, 103, 1131–1150. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Wilson, B.J.; Owston, H.E.; Iqbal, N.; Giannoudis, P.V.; McGonagle, D.; Pandit, H.; Pampadykandathil, L.P.; Jones, E.; Ganguly, P. In Vitro Osteogenesis Study of Shell Nacre Cement with Older and Young Donor Bone Marrow Mesenchymal Stem/Stromal Cells. Bioengineering 2024, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review—The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Young, J.D. Lifestyle interventions to delay senescence. Biomed. J. 2024, 47, 100676. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. GeroScience 2022, 44, 2757–2770. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, M.L.; Monroe, D.G.; Farr, J.N.; Khosla, S. The role of senolytics in osteoporosis and other skeletal pathologies. Mech. Ageing Dev. 2021, 199, 111565. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Chen, P.; Tang, H.; Meng, H.; Cheng, X.; Wang, Y.; Zhang, Y.; Peng, J. Cellular senescence in osteoarthritis and anti-aging strategies. Mech. Ageing Dev. 2018, 175, 83–87. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Kim, E.-C.; Kim, J.-R. Senotherapeutics: Emerging strategy for healthy aging and age-related disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.-L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; DeMaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Roberts, R.L.; Benson, R.D., Jr.; Pierce, J.L.; Yu, K.; Hamrick, M.W.; McGee-Lawrence, M.E. The Senolytic Drug Navitoclax (ABT-263) Causes Trabecular Bone Loss and Impaired Osteoprogenitor Function in Aged Mice. Front. Cell Dev. Biol. 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Tang, Q.; Zou, J.; Huang, H.; Yang, J.; Gao, X.; Xu, X.; Ma, S.; Li, M.; Liang, C.; et al. Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. Acta Biomater. 2023, 157, 352–366. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Investig. 2013, 123, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell senescence, rapamycin and hyperfunction theory of aging. Cell Cycle 2022, 21, 1456–1467. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Minor, R.K.; Mitchell, S.J.; Palacios, H.H.; Licata, J.J.; Ward, T.M.; Abulwerdi, G.; Elliott, P.; Westphal, C.; Ellis, J.L.; et al. Combining a High Dose of Metformin With the SIRT1 Activator, SRT1720, Reduces Life Span in Aged Mice Fed a High-Fat Diet. J. Gerontol. Ser. A 2020, 75, 2037–2041. [Google Scholar] [CrossRef]
- Chen, M.; Tan, J.; Jin, Z.; Jiang, T.; Wu, J.; Yu, X. Research progress on Sirtuins (SIRTs) family modulators. Biomed. Pharmacother. 2024, 174, 116481. [Google Scholar] [CrossRef] [PubMed]
- Scisciola, L.; Sarno, F.; Carafa, V.; Cosconati, S.; Di Maro, S.; Ciuffreda, L.; De Angelis, A.; Stiuso, P.; Feoli, A.; Sbardella, G.; et al. Two novel SIRT1 activators, SCIC2 and SCIC2.1, enhance SIRT1-mediated effects in stress response and senescence. Epigenetics 2020, 15, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Pawge, G.; Khatik, G.L. p53 regulated senescence mechanism and role of its modulators in age-related disorders. Biochem. Pharmacol. 2021, 190, 114651. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Recalde, U.; Lorenzo-Gómez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Domínguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Alimbetov, D.; Davis, T.; Brook, A.J.C.; Cox, L.S.; Faragher, R.G.A.; Nurgozhin, T.; Zhumadilov, Z.; Kipling, D. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 2015, 17, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Mensah, K.A.; Stricker, J.; Adams, M.; Parton, A.; Cedzik, D.; Connarn, J.; Thomas, M.; Horan, G.; Schafer, P.; et al. CC-99677, a novel, oral, selective covalent MK2 inhibitor, sustainably reduces pro-inflammatory cytokine production. Arthritis Res. Ther. 2022, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Macleod, T.; Wong, C.; Harland, M.; McGonagle, D. Revisiting p38 Mitogen-Activated Protein Kinases (MAPK) in Inflammatory Arthritis: A Narrative of the Emergence of MAPK-Activated Protein Kinase Inhibitors (MK2i). Pharmaceuticals 2023, 16, 1286. [Google Scholar] [CrossRef] [PubMed]
- Bray, K. Black Box Warning for JAKis. Available online: http://www.medicalrepublic.com.au/black-box-warning-for-jakis/90848 (accessed on 10 August 2023).
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Leonard, C.; Regmi, S.C.; De Rantere, D.; Tailor, P.; Ren, G.; Ishida, H.; Hsu, C.; Abubacker, S.; Pang, D.S.; et al. Lubricin/Proteoglycan 4 binds to and regulates the activity of Toll-Like Receptors In Vitro. Sci. Rep. 2016, 6, 18910. [Google Scholar] [CrossRef]
- Alquraini, A.; Garguilo, S.; D’souza, G.; Zhang, L.X.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: An anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res. Ther. 2015, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; del Caz, M.D.P.; Gomar, F.; Castejón, M.A.; Mirabet, V.; Alcaraz, M.J. Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging 2016, 8, 1703–1717. [Google Scholar] [CrossRef]
- Chesnokova, V.; Zonis, S.; Apaydin, T.; Barrett, R.; Melmed, S. Non-pituitary growth hormone enables colon cell senescence evasion. Aging Cell 2024, e14193. [Google Scholar] [CrossRef]
- Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.U.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Manoochehri, H.; Makvandi, P.; Afshar, S.; Salahinejad, E.; Khosraviyan, P.; Saidijam, M.; Asl, S.S.; Sharifi, E. Chitosan/alginate bionanocomposites adorned with mesoporous silica nanoparticles for bone tissue engineering. J. Nanostructure Chem. 2022, 13, 389–403. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hsu, G.C.-Y.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications-design, physicochemical and biological characterization. Colloids Surfaces B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, C.; Ma, Y.; Chen, X.; Wang, Y.; Chen, K.; Xie, F.; Zhang, Y.; Yuan, Y.; Liu, C. Rejuvenating Aged Bone Repair through Multihierarchy Reactive Oxygen Species-Regulated Hydrogel. Adv. Mater. 2023, 36, e2306552. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Huang, H.; Gao, X.; Yang, J.; Tang, Q.; Xu, X.; Wu, Y.; Li, M.; Liang, C.; Tan, L.; et al. Local Elimination of Senescent Cells Promotes Bone Defect Repair during Aging. ACS Appl. Mater. Interfaces 2022, 14, 3885–3899. [Google Scholar] [CrossRef]
- Borges, R.; Genova, L.A.; Marchi, J. Microspheres for Bone Regeneration. Technol. Appl. Role Drug Deliv. Syst. 2015, 1–20. [Google Scholar]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J. Potential Application of Hydrolyzed Fish Collagen for Inducing the Multidirectional Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Biomacromolecules 2014, 15, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nagaoka, H.; Terajima, M.; Tsuda, N.; Hayashi, Y.; Yamauchi, M. Effects of fish collagen peptides on collagen post-translational modifications and mineralization in an osteoblastic cell culture system. Dent. Mater. J. 2013, 32, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A Mussel-Inspired Persistent ROS-Scavenging, Electroactive, and Osteoinductive Scaffold Based on Electrochemical-Driven In Situ Nanoassembly. Small 2019, 15, e1805440. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.-W.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Transl. 2017, 9, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Sarvari, M.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Gilany, K.; Mehrdad, N.; Larijani, B. Prospect of Stem Cell Therapy and Regenerative Medicine in Osteoporosis. Front. Endocrinol. 2020, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, H.; Li, H.; Dai, K.; Wang, J.; Zhang, X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar] [CrossRef]
- Bartold, M.; Gronthos, S.; Haynes, D.; Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J. Clin. Periodontol. 2019, 46, 12–32. [Google Scholar] [CrossRef]
- Muiños-López, E.; Delgado, D.; Sánchez, P.; Paiva, B.; Anitua, E.; Fiz, N.; Aizpurua, B.; Guadilla, J.; Padilla, S.; Granero-Moltó, F.; et al. Modulation of Synovial Fluid-Derived Mesenchymal Stem Cells by Intra-Articular and Intraosseous Platelet Rich Plasma Administration. Stem Cells Int. 2016, 2016, 1247950. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Best, T.M.; Huard, J.; Philippon, M.; Hornicek, F.; Duan, Z.; Griswold, A.J.; Kaplan, L.D.; Hare, J.M.; Kouroupis, D. Therapeutic Perspectives for Inflammation and Senescence in Osteoarthritis Using Mesenchymal Stem Cells, Mesenchymal Stem Cell-Derived Extracellular Vesicles and Senolytic Agents. Cells 2023, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, J.; Liu, G.-H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Silkstone, D.; Vi, L.; Nadesan, P.; Amani, Y.; Whetstone, H.; Wei, Q.; Alman, B.A. Erratum: Exposure to a youthful circulation rejuvenates bone repair through modulation of β-catenin. Nat. Commun. 2015, 6, 7761. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Fiz, N.; Beitia, M.; Owston, H.E.; Delgado, D.; Jones, E.; Sánchez, M. Effect of Combined Intraosseous and Intraarticular Infiltrations of Autologous Platelet-Rich Plasma on Subchondral Bone Marrow Mesenchymal Stromal Cells from Patients with Hip Osteoarthritis. J. Clin. Med. 2022, 11, 3891. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef] [PubMed]
- Philippart, P.; Meuleman, N.; Stamatopoulos, B.; Najar, M.; Pieters, K.; De Bruyn, C.; Bron, D.; Lagneaux, L. In Vivo Production of Mesenchymal Stromal Cells After Injection of Autologous Platelet-Rich Plasma Activated by Recombinant Human Soluble Tissue Factor in the Bone Marrow of Healthy Volunteers. Tissue Eng. Part A 2014, 20, 160–170. [Google Scholar] [CrossRef]
- Su, K.; Bai, Y.; Wang, J.; Zhang, H.; Liu, H.; Ma, S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin. Rheumatol. 2018, 37, 1341–1350. [Google Scholar] [CrossRef]
- Cole, B.J.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2016, 45, 339–346. [Google Scholar] [CrossRef]
- Zhang, J.; Middleton, K.K.; Fu, F.H.; Im, H.-J.; Wang, J.H.-C. HGF Mediates the Anti-inflammatory Effects of PRP on Injured Tendons. PLoS ONE 2013, 8, e67303. [Google Scholar] [CrossRef]
- Delgado, D.; Garate, A.; Sánchez, P.; Bilbao, A.M.; del Caño, G.G.; Salles, J.; Sánchez, M. Biological and structural effects after intraosseous infiltrations of age-dependent platelet-rich plasma: An in vivo study. J. Orthop. Res. 2020, 38, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2020, 13, 364S–375S. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.V.; Lenza, M.; Irrgang, J.J.; Fu, F.H.; Ferretti, M. How Does Platelet-Rich Plasma Compare Clinically to Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022, 51, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Kwan, M.; Woo, J. Healthy Diet for Healthy Aging. Nutrients 2021, 13, 4310. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fazekas-Pongor, V.; Csiszar, A.; Kunutsor, S.K. The multifaceted benefits of walking for healthy aging: From Blue Zones to molecular mechanisms. GeroScience 2023, 45, 3211–3239. [Google Scholar] [CrossRef] [PubMed]
- Erlangga, Z.; Ghashang, S.K.; Hamdan, I.; Melk, A.; Gutenbrunner, C.; Nugraha, B. The effect of prolonged intermittent fasting on autophagy, inflammasome and senescence genes expressions: An exploratory study in healthy young males. Hum. Nutr. Metab. 2023, 32, 200189. [Google Scholar] [CrossRef]
- Fastame, M.C. Well-being, food habits, and lifestyle for longevity. Preliminary evidence from the sardinian centenarians and long-lived people of the Blue Zone. Psychol. Health Med. 2022, 27, 728–733. [Google Scholar] [CrossRef]
- Carroll, J.E.; Cole, S.W.; Seeman, T.E.; Breen, E.C.; Witarama, T.; Arevalo, J.M.; Ma, J.; Irwin, M.R. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain, Behav. Immun. 2016, 51, 223–229. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J.; Chen, J.-R. Feeding Blueberry Diets in Early Life Prevent Senescence of Osteoblasts and Bone Loss in Ovariectomized Adult Female Rats. PLoS ONE 2011, 6, e24486. [Google Scholar] [CrossRef]
- Samakkarnthai, P.; Saul, D.; Zhang, L.; Aversa, Z.; Doolittle, M.L.; Sfeir, J.G.; Kaur, J.; Atkinson, E.J.; Edwards, J.R.; Russell, G.G.; et al. In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging 2023, 15, 3331–3355. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; O Johnson, K.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiao, Y.; Wei, B.; Yang, Z.; Wu, J.-F.; Jensen, J.; Jean, W.-H.; Huang, C.-Y.; Kuo, C.-H. Aged cells in human skeletal muscle after resistance exercise. Aging 2018, 10, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, W.; Zhou, Y.; Li, Y.; Cheng, J.; Wei, F. Immunosenescence of T cells: A key player in rheumatoid arthritis. Inflamm. Res. 2022, 71, 1449–1462. [Google Scholar] [CrossRef]
| Study Summary | NGS Methods | Key Findings | Key Genes with Elevated Expression | Reference |
|---|---|---|---|---|
| RNA profile expression in eight experimental models of cellular senescence commonly studied. WI-38 and IMR-90 fibroblasts, HUVECs and HAECs were cultured and utilized for downstream sequencing. | RNA seq, Illumina Hiseq 2500 with 2 × 150-bp strategy | 50 elevated and 18 reduced transcripts and the identification of subsets of transcripts (both coding and non-coding) display shared expression patterns across a range of senescent cell models. | SRPX, PURPL (p53 regulator) | [111] |
| RNA expression profiling of fibroblasts and their senescence induced by 5-aza identified 3 epigenetically silenced pathways. | Illumina HiSeq 2000; strategy not specified | 5-aza-induced senescence has been closely linked to alterations in the interferon/innate immunity pathway’s gene expression, and during immortalization, important regulators of this pathway are muted. | IL-1α and IL-1β | [112] |
| Use of multiple whole-transcriptome datasets, created by the authors or made publicly available, to characterise the heterogeneity of the programmed senescence. | Illumina HiSeq 2000 with 2× 150 bp | Demonstrates that ollowing senescence induction, the senescent phenotype identified for 55 genes at the core of the senescence-associated transcriptome is dynamic, changing at different intervals. | Upregulation of genes associated with G1 DNA damage checkpoint (PLK3 and CCND1) and upregulation of BCL2L2 (negative regulator of apoptosis) | [113] |
| Comprehensive analysis of the transcriptome and senolytic responses in a panel of 13 cancer cell lines rendered senescent by two distinct compounds. | HiSeq 2500; single-end 65 bp | Cell lines that were made senescent by two different substances showed that the senescence trigger has less of an impact on the composition of the SASP. The SENCAN gene expression classifier to detect senescence using machine learning was developed. | IL6 and CXCL8 | [114] |
| Gene set generation (SenMayo) consisting of 125 previously identified senescence/SASP-associated factors. | HiSeq 2000; strategy not specified | Provided a unique gene set (SenMayo) that can be utilized in bulk and scRNA-seq investigations to detect cells expressing high amounts of senescence/SASP genes. SenMayo rises with aging across tissues and species and is responsive to senescent cell clearance. | CDKN1A/P21Cip1 and several SASP markers such as CCL2 and IL6 showed consistent upregulation with aging | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, M.; Goyal, A.; Pathak, S.; Ganguly, P. Cellular Senescence and Inflammaging in the Bone: Pathways, Genetics, Anti-Aging Strategies and Interventions. Int. J. Mol. Sci. 2024, 25, 7411. https://doi.org/10.3390/ijms25137411
Lawrence M, Goyal A, Pathak S, Ganguly P. Cellular Senescence and Inflammaging in the Bone: Pathways, Genetics, Anti-Aging Strategies and Interventions. International Journal of Molecular Sciences. 2024; 25(13):7411. https://doi.org/10.3390/ijms25137411
Chicago/Turabian StyleLawrence, Merin, Abhishek Goyal, Shelly Pathak, and Payal Ganguly. 2024. "Cellular Senescence and Inflammaging in the Bone: Pathways, Genetics, Anti-Aging Strategies and Interventions" International Journal of Molecular Sciences 25, no. 13: 7411. https://doi.org/10.3390/ijms25137411
APA StyleLawrence, M., Goyal, A., Pathak, S., & Ganguly, P. (2024). Cellular Senescence and Inflammaging in the Bone: Pathways, Genetics, Anti-Aging Strategies and Interventions. International Journal of Molecular Sciences, 25(13), 7411. https://doi.org/10.3390/ijms25137411






