Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Expression System
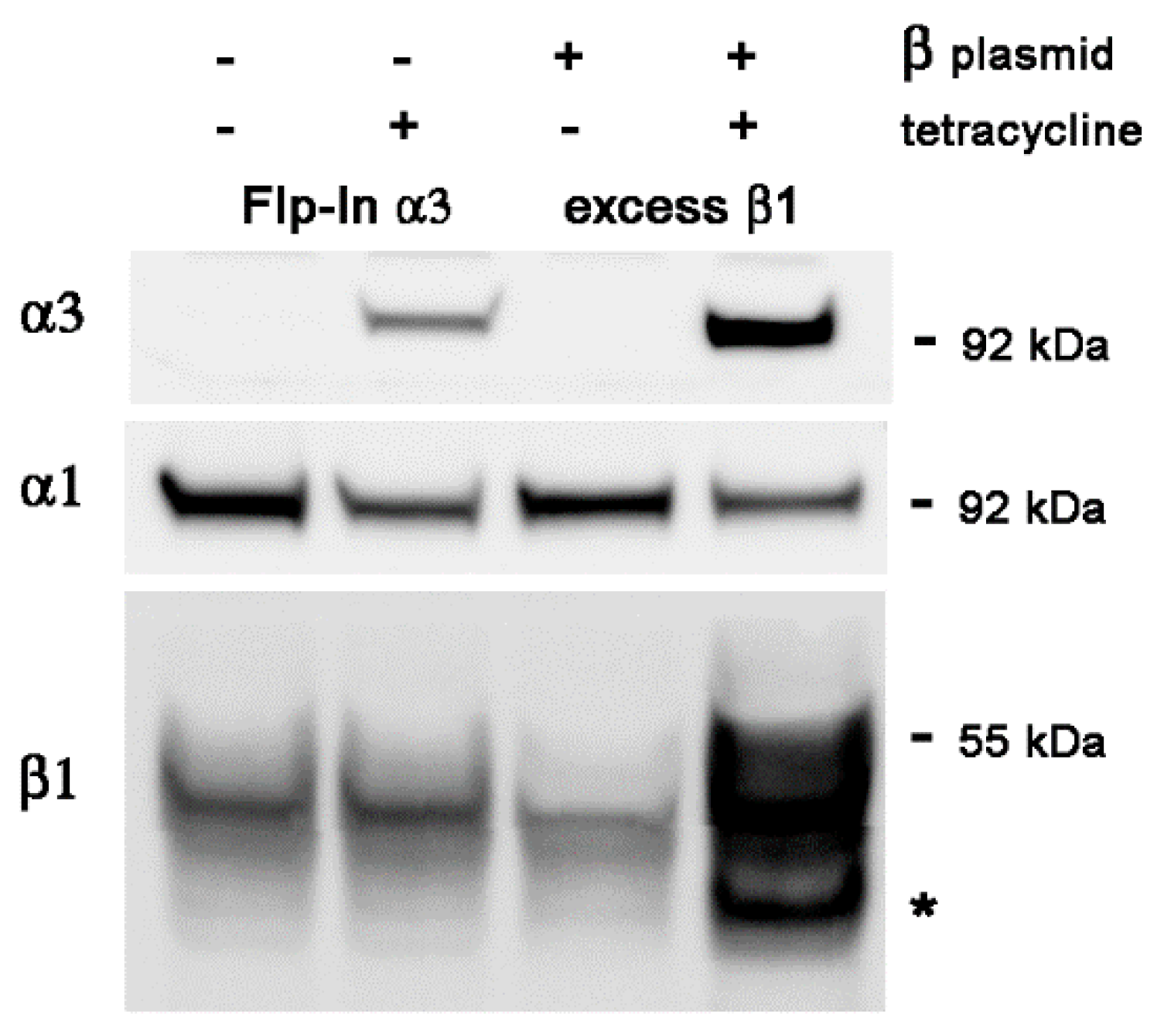
2.2. Transient Transfection of β1
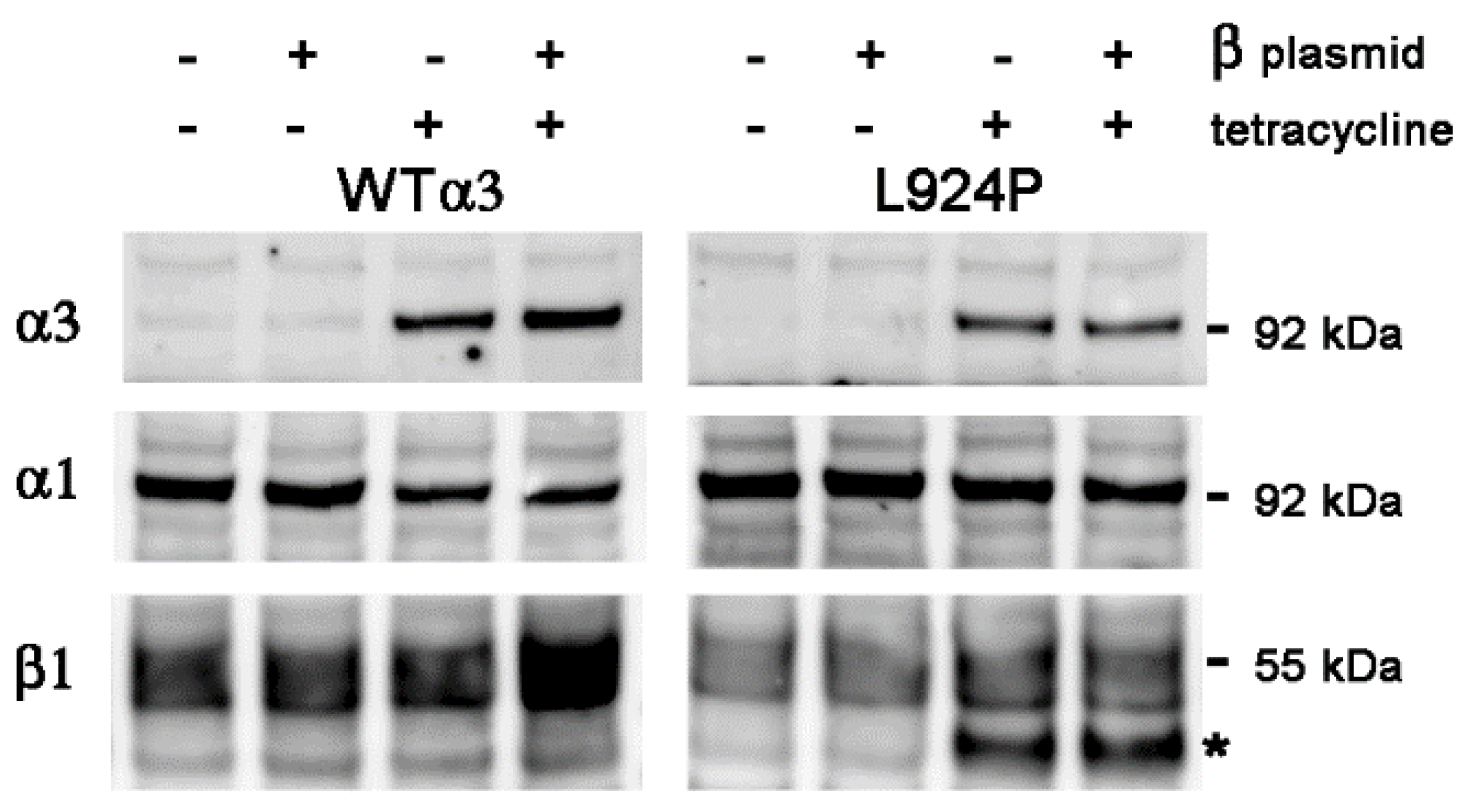
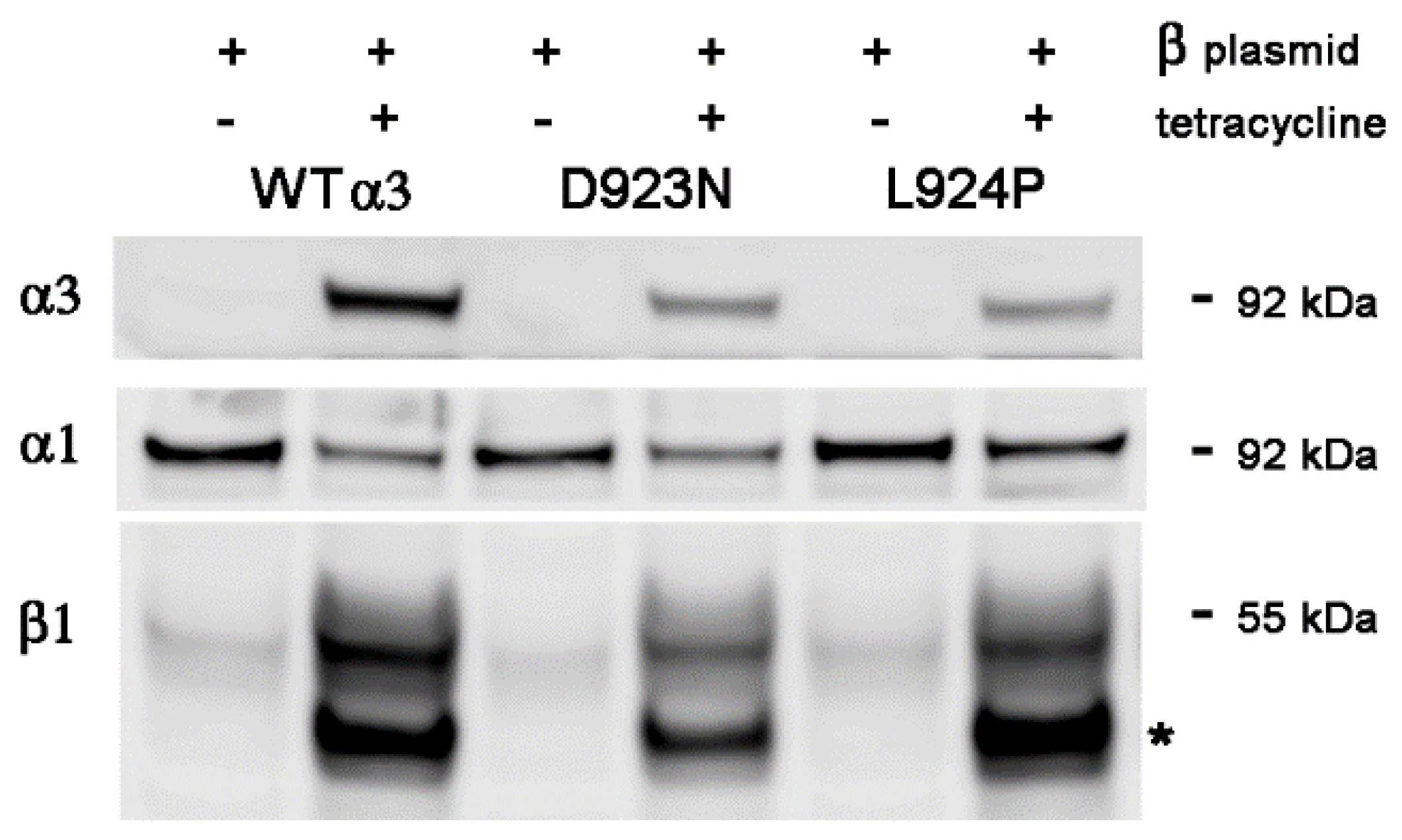
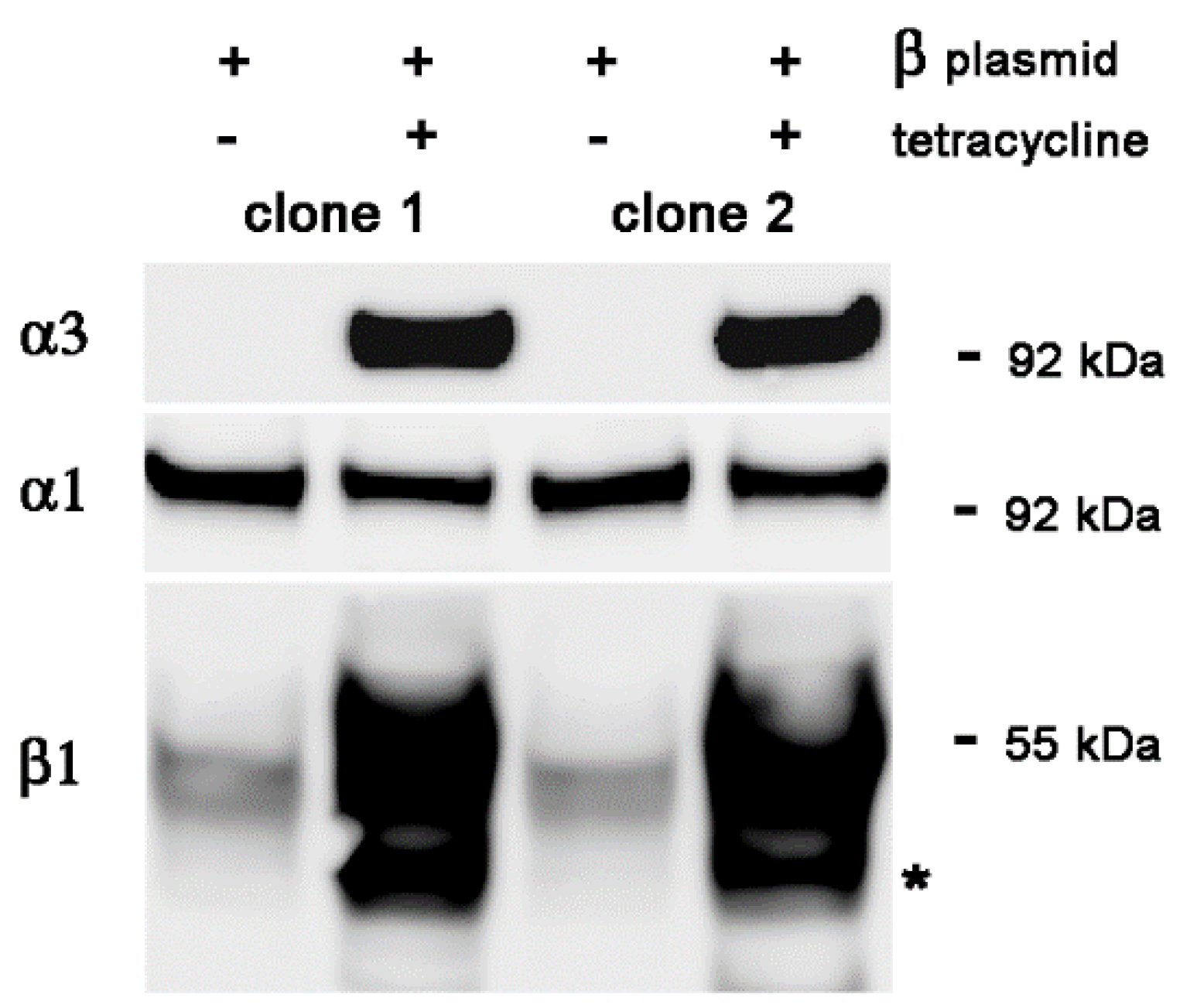
2.3. Stable Transfection of β1 with α3WT
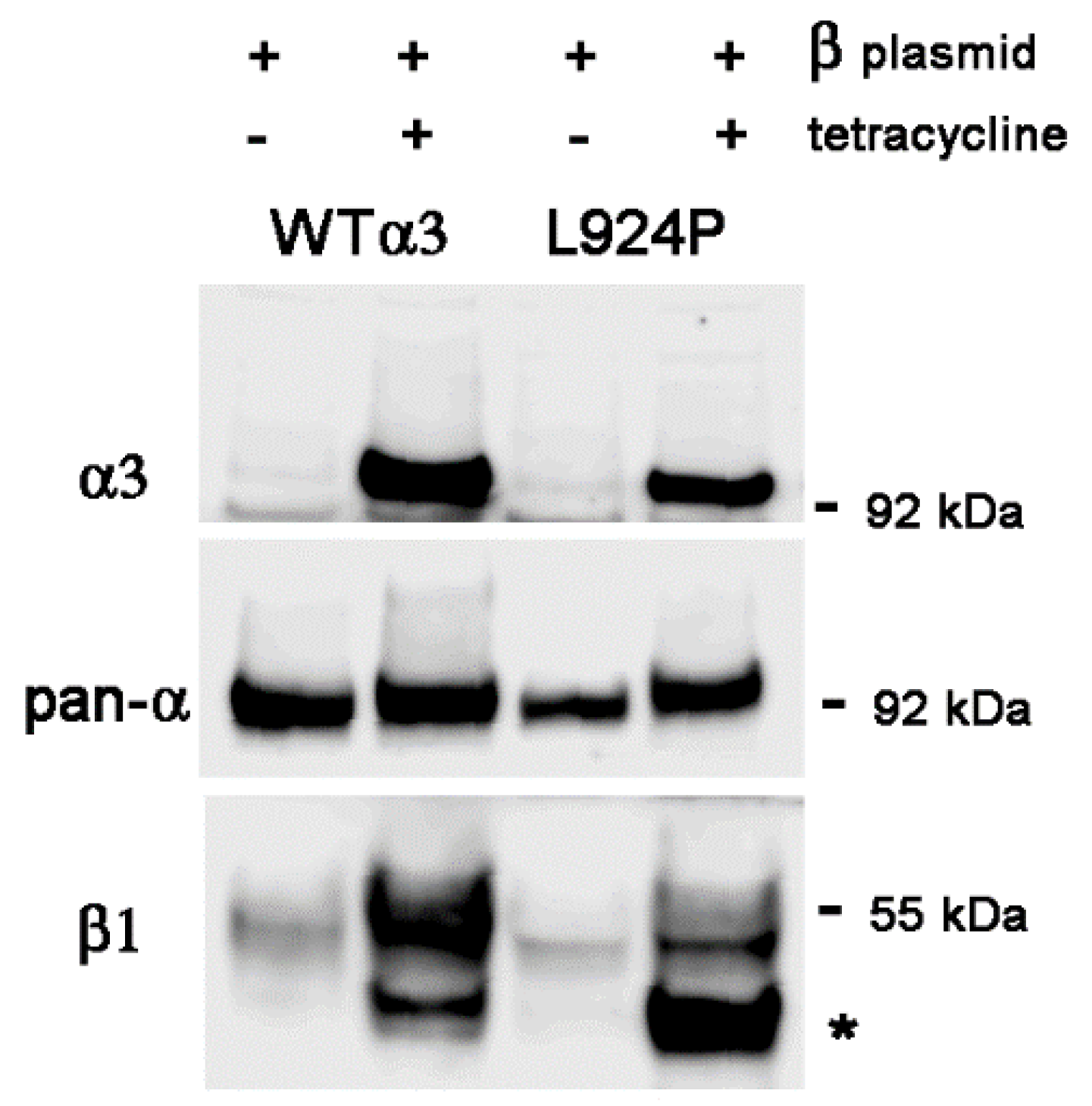
2.4. Stable Transfection of β1 with α3 Mutation L924P
3. Discussion
3.1. Salient Findings
3.2. Related Observations in the Literature
3.3. Unanswered Questions
3.3.1. Involvement of the β3 Subunit
3.3.2. Unknown Regulation of Degradation or Translation
4. Materials and Methods
Detection of Na,K-ATPase Subunits
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Langhans, S.A. Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Devel. Biol. 2015, 3, 66. [Google Scholar] [CrossRef]
- Kryvenko, V.; Vagin, O.; Dada, L.A.; Sznajder, J.I.; Vadasz, I. Maturation of the Na,K-ATPase in the endoplasmic reticulum in health and disease. J. Membr. Biol. 2021, 254, 447–457. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Dyla, M.; Kjaergaard, M.; Poulsen, H.; Nissen, P. Structure and mechanism of P-Type ATPase ion pumps. Annu. Rev. Biochem. 2020, 89, 583–603. [Google Scholar] [CrossRef]
- Dobretsov, M.; Stimers, J.R. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 2005, 10, 2373–2396. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.V.; Nissen, P.; Poulsen, H. The α4 isoform of the Na+,K+-ATPase is tuned for changing extracellular environments. FEBS J. 2016, 283, 282–293. [Google Scholar] [CrossRef]
- Arystarkhova, E.; Haq, I.U.; Luebbert, T.; Mochel, F.; Saunders-Pullman, R.; Bressman, S.B.; Feschenko, P.; Salazar, C.; Cook, J.F.; Demarest, S.; et al. Factors in the disease severity of ATP1A3 mutations: Impairment, misfolding, and allele competition. Neurobiol. Dis. 2019, 132, 104577. [Google Scholar] [CrossRef] [PubMed]
- Arystarkhova, E.; Ozelius, L.J.; Brashear, A.; Sweadner, K.J. Misfolding, altered membrane distributions, and the unfolded protein response contribute to pathogenicity differences in Na,K-ATPase ATP1A3 mutations. J. Biol. Chem. 2021, 296, 100019. [Google Scholar] [CrossRef]
- Arystarkhova, E.; Toustrup-Jensen, M.S.; Holm, R.; Ko, J.K.; Lee, K.E.; Feschenko, P.; Ozelius, L.J.; Brashear, A.; Vilsen, B.; Sweadner, K.J. Temperature instability of a mutation at a multidomain junction in Na,K-ATPase isoform ATP1A3 (p.Arg756His) produces a fever-induced neurological syndrome. J. Biol. Chem. 2023, 299, 102758. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; McDonough, A.A.; Edelman, I.S. Assembly of the (Na+ + K+)-adenosine triphosphatase. Post-translational membrane integration of the α subunit. J. Biol. Chem. 1984, 259, 2629–2635. [Google Scholar] [CrossRef]
- Ackermann, U.; Geering, K. Mutual dependence of Na,K-ATPase α and β-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 1990, 269, 105–108. [Google Scholar] [CrossRef]
- Geering, K. The functional role of β subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 2001, 33, 425–438. [Google Scholar] [CrossRef]
- Geering, K.; Meyer, D.I.; Paccolat, M.P.; Kraehenbuhl, J.P.; Rossier, B.C. Membrane insertion of α- and β-subunits of Na+,K+-ATPase. J. Biol. Chem. 1985, 260, 5154–5160. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P.; Hasler, U.; Beggah, A.; Horisberger, J.D.; Geering, K. Membrane integration of Na,K-ATPase α-subunits and β subunit assembly. J. Biol. Chem. 1998, 273, 24921–24931. [Google Scholar] [CrossRef]
- Shinoda, T.; Ogawa, H.; Cornelius, F.; Toyoshima, C. Crystal structure of the sodium-potassium pump at 2.4 Å resolution. Nature 2009, 459, 446–450. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Toustrup-Jensen, M.S.; Sorensen, T.L.-M.; Petersen, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. Crystal structure of the sodium-potassium pump. Nature 2007, 450, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P.; Hasler, U.; Staub, O.; Geering, K. Endoplasmic reticulum quality control of oligomeric membrane proteins: Topogenic determinants involved in the degradation of the unassembled Na,K-ATPase α subunit and in its stabilization by β subunit assembly. Mol. Biol. Cell 2000, 11, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Beggah, A.; Mathews, P.; Beguin, P.; Geering, K. Degradation and endoplasmic reticulum retention of unassembled α and β -subunits of Na,K-ATPase correlate with interaction of BiP. J. Biol. Chem. 1996, 271, 20895–20902. [Google Scholar] [CrossRef]
- Morton, M.J.; Farr, G.A.; Hull, M.; Capendeguy, O.; Horisberger, J.-D.; Caplan, M.J. Association with β-COP regulates the trafficking of the newly synthesized Na,K-ATPase. J. Biol. Chem. 2010, 285, 33737–33746. [Google Scholar] [CrossRef]
- Molinari, M. N-glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 2007, 3, 313–320. [Google Scholar] [CrossRef]
- Malm, M.; Saghaleyni, R.; Lundqvist, M.; Giudici, M.; Chotteau, V.; Field, R.; Varley, P.G.; Hatton, D.; Grassi, L.; Svensson, T.; et al. Evolution from adherent to suspension: Systems biology of HEK293 cell line development. Sci. Rep. 2020, 10, 18996. [Google Scholar] [CrossRef] [PubMed]
- Krshnan, L.; van de Weijer, M.L.; Carvalho, P. Endoplasmic reticulum-associated protein degradation. Cold Spring Harb. Perspect. Biol. 2022, 14, a041247. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Rapoport, T.A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 2018, 53, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Higashi, K.; Kawamura, M. A possible role of the β-subunit of (Na,K)-ATPase in facilitating correct assembly of the α-subunit into the membrane. J. Biol. Chem. 1990, 265, 15991–15995. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Higashi, K.; Kawamura, M. Assembly of the alpha-subunit of Torpedo californica Na+/K(+)-ATPase with its pre-existing beta-subunit in Xenopus oocytes. Biochim. Biophys. Acta 1990, 1023, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, U.; Geering, K. β1- and β3-subunits can associate with presynthesized α-subunits of Xenopus oocyte Na,K-ATPase. J. Biol. Chem. 1992, 267, 12911–12915. [Google Scholar] [CrossRef] [PubMed]
- Pralong Zamofing, D.; Yi, Q.H.; Schmalzing, G.; Good, P.; Geering, K. Regulation of alpha 1-beta 3-Na(+)-K(+)-ATPase isozyme during meiotic maturation of Xenopus laevis oocytes. Am. J. Physiol. 1992, 262, C1520–C1530. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Sachs, G.; Vagin, O. Diverse pathways for maturation of the Na,K-ATPase β1 and β2 subunits in the endoplasmic reticulum of Madin-Darby canine kidney cells. J. Biol. Chem. 2010, 285, 39289–39302. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Munson, K.; Sachs, G.; Vagin, O. N-glycan-dependent quality control of the Na,K-ATPase β2 subunit. Biochemistry 2010, 49, 3116–3128. [Google Scholar] [CrossRef][Green Version]
- Gottardi, C.J.; Caplan, M.J. Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly. J. Biol. Chem. 1993, 268, 14342–14347. [Google Scholar] [CrossRef]
- Gatto, C.; McLoud, S.M.; Kaplan, J.H. Heterologous expression of Na+-K+-ATPase in insect cells: Intracellular distribution of pump subunits. Am. J. Physiol. 2001, 281, C982–C992. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Denevich, S.; Sachs, G. Plasma membrane delivery of the gastric H,K-ATPase: The role of β-subunit glycosylation. Am. J. Physiol. Cell Physiol. 2003, 285, C968–C976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laughery, M.D.; Clifford, R.J.; Chi, Y.; Kaplan, J.H. Selective basolateral localization of overexpressed Na-K-ATPase β1- and β2- subunits is disrupted by butryate treatment of MDCK cells. Am. J. Physiol. Renal Physiol. 2007, 292, F1718–F1725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clifford, R.J.; Kaplan, J.H. β-Subunit overexpression alters the stoicheometry of assembled Na-K-ATPase subunits in MDCK cells. Am. J. Physiol. Renal Physiol. 2008, 295, F1314–F1323. [Google Scholar] [CrossRef]
- Jaunin, P.; Jaisser, F.; Beggah, A.T.; Takeyasu, K.; Mangeat, P.; Rossier, B.C.; Horisberger, J.-D.; Geering, K. Role of the transmembrane and extracytoplasmic domain of β subunits in subunit assembly, intracellular transport, and functional expression of Na,K-pumps. J. Cell Biol. 1993, 123, 1751–1759. [Google Scholar] [CrossRef]
- Shanbaky, N.M.; Pressley, T.A. Transfection of Na,K-ATPase α-subunit: Regulation of enzyme abundance. Biochem. Cell Biol. 1995, 73, 261–268. [Google Scholar] [CrossRef]
- Jewell, E.A.; Lingrel, J.B. Comparison of the substrate dependence properties of the rat Na, K-ATPase α1, α2, and α3 isoforms expressed in HeLa cells. J. Biol. Chem. 1991, 266, 16925–16930. [Google Scholar] [CrossRef] [PubMed]
- Zahler, R.; Zhang, Z.-T.; Manor, M.; Boron, W.F. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 1997, 110, 201–213. [Google Scholar] [CrossRef]
- Clifford, R.J.; Kaplan, J.H. Regulation of Na,K-ATPase subunit abundance by translational repression. J. Biol. Chem. 2009, 284, 22905–22915. [Google Scholar] [CrossRef]
- Tokhtaeva, E.; Sachs, G.; Vagin, O. Assembly with the Na,K-ATPase α1 subunit is required for export of β1 and β2 subunits from the endoplasmic reticulum. Biochemistry 2009, 48, 11421–11431. [Google Scholar] [CrossRef]
- Lubarski-Gotliv, I.; Dey, K.; Kuznetsov, Y.; Kalchenco, V.; Asher, C.; Garty, H. FXYD5 (dysadherin) may mediate metastatic progression through regulation of the β-Na(+)-K(+)-ATPase subunit in the 4T1 mouse breast cancer model. Am. J. Physiol. Cell Physiol. 2017, 313, C108–C117. [Google Scholar] [CrossRef] [PubMed]
- Bernhem, K.; Blom, H.; Brismar, H. Quantification of endogenous and exogenous protein expressions of Na,K-ATPase with super-resolution PALM/STORM imaging. PLoS ONE 2018, 13, e0195825. [Google Scholar] [CrossRef] [PubMed]
- Cherniavsky-Lev, M.; Karlish, S.J.; Garty, H. Cardiac glycosides induced toxicity in human cells expressing α1-, α2-, or α3-isoforms of Na-K-ATPase. Am. J. Physiol. Cell Physiol. 2015, 309, C126–C135. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.; Williams, M.T.; Schaefer, T.L.; Bohanan, C.S.; Neumann, J.C.; Behbehani, M.M.; Vorhees, C.V.; Lingrel, J.B. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity and anxiety in mice. J. Neurosci. 2007, 27, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, L.J.; Hrizo, S.L.; Paul, S.M.; Van Voorhies, W.A.; Beitel, G.J.; Palladino, M.J. Novel mutations affecting the Na, K ATPase α model complex neurological diseases and implicate the sodium pump in increased longevity. Hum. Genet. 2009, 126, 431–447. [Google Scholar] [CrossRef]
- Kutz, L.C.; Mukherji, S.T.; Wang, X.; Bryant, A.; Larre, I.; Heiny, J.A.; Lingrel, J.B.; Pierre, S.V.; Xie, Z. Isoform-specific role of Na/K-ATPase α1 in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E620–E629. [Google Scholar] [CrossRef] [PubMed]
- Flodby, P.; Kim, Y.H.; Beard, L.L.; Gao, D.; Ji, Y.; Kage, H.; Liebler, J.M.; Minoo, P.; Kim, K.J.; Borok, Z.; et al. Knockout Mice Reveal a Major Role for Alveolar Epithelial Type I Cells in Alveolar Fluid Clearance. Am. J. Respir. Cell Mol. Biol. 2016, 55, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Crambert, G.; Hasler, U.; Beggah, A.T.; Yu, C.; Modyanov, N.N.; Horisberger, J.-D.; Lelievre, L.; Geering, K. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem. 2000, 275, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Habeck, M.; Tokhtaeva, E.; Nadav, Y.; Ben, Z.E.; Ferris, S.P.; Kaufman, R.J.; Bab-Dinitz, E.; Kaplan, J.H.; Dada, L.A.; Farfel, Z.; et al. Selective assembly of Na,K-ATPase α2 β2 heterodimers in the heart: Distinct functional properties and isoform-selective inhibitors. J. Biol. Chem. 2016, 291, 23159–23174. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef]
- He, B.; Soderlund, D.M. Human embryonic kidney (HEK293) cells express endogenous voltage-gated sodium currents and Na v 1.7 sodium channels. Neurosci. Lett. 2010, 469, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Comm. 2014, 5, 4767. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Canfield, V.; Sanchez-Watts, G.; Watts, A.G.; Scherer, S.; Beatty, B.G.; Gros, P.; Levenson, R. Structural organization and chromosomal localization of the human Na,K-ATPase β3 subunit gene and pseudogene. Mamm. Genome 1998, 9, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Canfield, V.A.; Beckers, M.C.; Gros, P.; Levenson, R. Identification of the mammalian Na,K-ATPase β3 subunit. J. Biol. Chem. 1996, 271, 22754–22758. [Google Scholar] [CrossRef] [PubMed]
- Arystarkhova, E.; Sweadner, K.J. Tissue-specific expression of the Na,K-ATPase β3 subunit: The presence of β3 in lung and liver addresses the problem of the missing subunit. J. Biol. Chem. 1997, 272, 22405–22408. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vasallo, P.; Wetzel, R.K.; Garcia-Segura, L.M.; Molina-Holgado, E.; Arystarkhova, E.; Sweadner, K.J. Oligodendrocytes in brain and optic nerve express the β3 subunit isoform of Na,K-ATPase. Glia 2000, 31, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Wu, Y.; Matsuhisa, K.; Saito, A.; Sakaue, F.; Imaizumi, K.; Kaneko, M. Hypertonicity-responsive ubiquitin ligase RNF183 promotes Na, K-ATPase lysosomal degradation through ubiquitination of its β1 subunit. Biochem. Biophys. Res. Commun. 2020, 521, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Baxter Lowe, L.A.; Yohanan, J.M.; Hokin, L.E. In vitro biosynthesis of the beta-subunit of the Na,K-ATPase in the brine shrimp. Prog. Clin. Biol. Res. 1988, 268, 35–42. [Google Scholar]
- Cayanis, E.; Bayley, H.; Edelman, I.S. Cell-free transcription and translation of Na,K-ATPase α and β subunit cDNAs. J. Biol. Chem. 1990, 265, 10829–10835. [Google Scholar] [CrossRef]
- Devarajan, P.; Gilmore Hebert, M.; Benz, E.J., Jr. Differential translation of the Na,K-ATPase subunit mRNAs. J. Biol. Chem. 1992, 267, 22435–22439. [Google Scholar] [CrossRef]
- Grindstaff, K.K.; Blanco, G.; Mercer, R. Translational regulation of Na,K-ATPase α1 and β1 polypeptide expression in epithelial cells. J. Biol. Chem. 1996, 271, 23211–23221. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ismail-Beigi, F. Control of Na+-K+-ATPase β1-subunit expression: Role of 3′-untranslated region. Am. J. Physiol. 2004, 286, C580–C585. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ismail-Beigi, F. Different Na, K-ATPase mRNA(beta1) species exhibit unique translational efficiencies. Arch. Biochem. Biophys. 2001, 390, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A.; Gopal, J.; Willis, D.; Espineda, C.; Twiss, J.L.; Rajasekaran, A.K. Na,K-ATPase β1-subunit increases the translation efficiency of the α1-subunit in MSV-MDCK cells. Molec. Biol. Cell 2004, 15, 3224–3232. [Google Scholar] [CrossRef]
- Price, E.M.; Rice, D.A.; Lingrel, J.B. Structure-function studies of Na,K-ATPase. Site-directed mutagenesis of the border residues from the H1-H2 extracellular domain of the α subunit. J. Biol. Chem. 1990, 265, 6638–6641. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arystarkhova, E.; Sweadner, K.J. Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence. Int. J. Mol. Sci. 2024, 25, 7414. https://doi.org/10.3390/ijms25137414
Arystarkhova E, Sweadner KJ. Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence. International Journal of Molecular Sciences. 2024; 25(13):7414. https://doi.org/10.3390/ijms25137414
Chicago/Turabian StyleArystarkhova, Elena, and Kathleen J. Sweadner. 2024. "Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence" International Journal of Molecular Sciences 25, no. 13: 7414. https://doi.org/10.3390/ijms25137414
APA StyleArystarkhova, E., & Sweadner, K. J. (2024). Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence. International Journal of Molecular Sciences, 25(13), 7414. https://doi.org/10.3390/ijms25137414




