New Insights into Hepatic and Intestinal Microcirculation and Pulmonary Inflammation in a Model of Septic Shock and Veno-Arterial Extracorporeal Membrane Oxygenation in the Rat
Abstract
1. Introduction
2. Results
2.1. Intestinal and Hepatic Microcirculation
2.2. Hemodynamic Parameters
2.3. Blood Gas Analysis
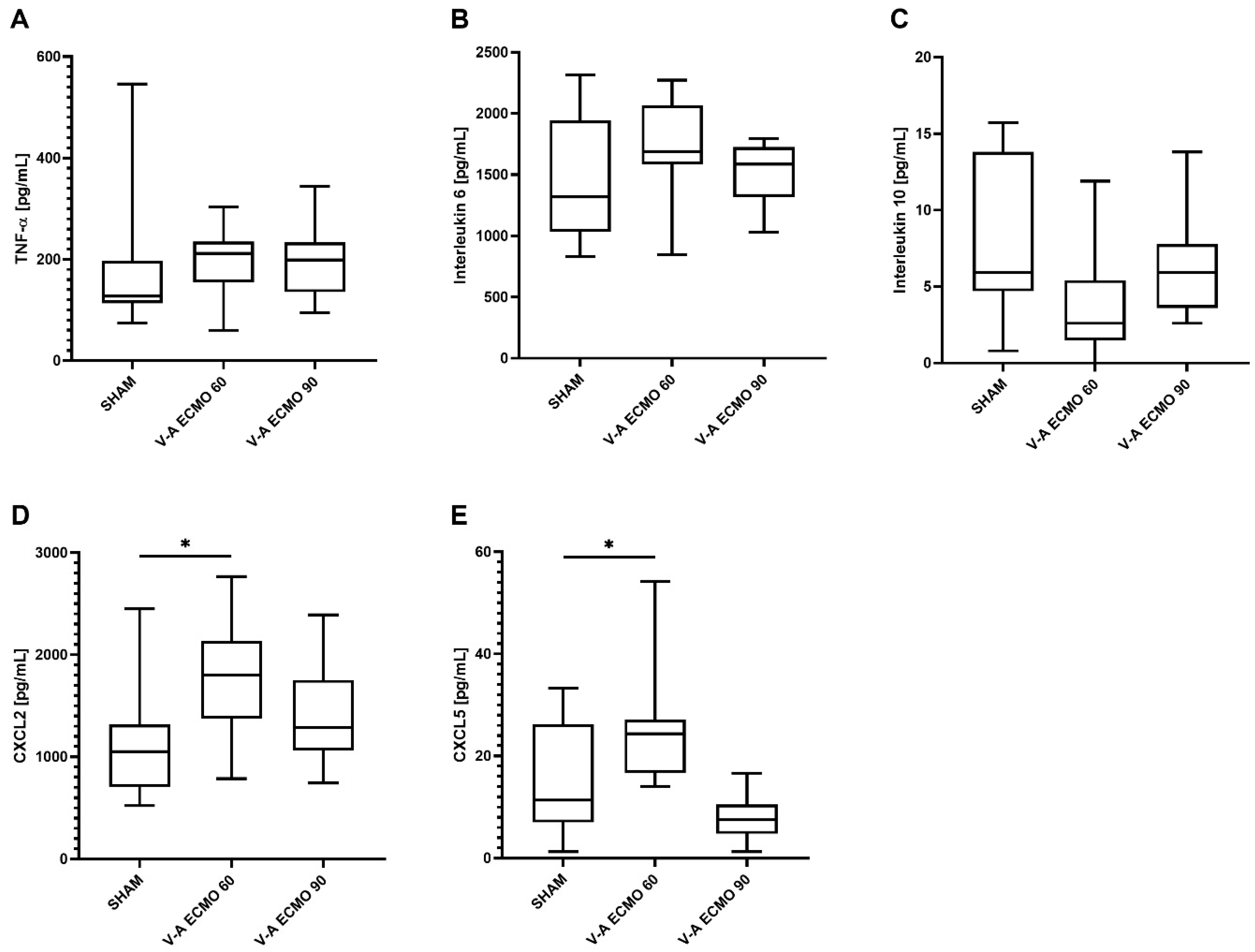
2.4. Inflammatory Parameters
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction and Maintenance of Anesthesia
4.3. Cannulation and Abdominal Laparotomy
4.4. Induction of Septic Shock
4.5. Extracorporeal Membrane Oxygenation
4.6. Intestinal Microcirculation
4.7. Timepoints of Hemodynamic Measurements
4.8. Blood Analyses
4.9. End of Experiments
4.10. Enzyme-Linked Immune Sorbent Assays
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Burgdorff, A.-M.; Bucher, M.; Schumann, J. Vasoplegia in patients with sepsis and septic shock: Pathways and mechanisms. J. Int. Med. Res. 2018, 46, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F.; Aissaoui, N. Septic cardiomyopathy: Diagnosis and management. J. Intensive Med. 2022, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Elbourne, D.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Clemens, F.; Firmin, R.; Hardy, P.; Hibbert, C.; et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol. Assess. 2010, 14, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; McAuley, D.F.; Firmin, R.K.; et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011, 306, 1659–1668. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Poon, W.H.; Tan, C.S.; Brechot, N.; Brodie, D.; Combes, A.; MacLaren, G. Venoarterial extracorporeal membrane oxygenation as mechanical circulatory support in adult septic shock: A systematic review and meta-analysis with individual participant data meta-regression analysis. Crit. Care 2021, 25, 246. [Google Scholar] [CrossRef]
- Sato, R.; Kuriyama, A. Venoarterial Extracorporeal Membranous Oxygenation: Treatment Option for Sepsis-Induced Cardiogenic Shock? A Systematic Review. Crit. Care Med. 2020, 48, e722–e729. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, C.; Orszag, P.; Burgwitz, K.; Marsch, G.; Stumpp, N.; Stiesch, M.; Haverich, A. Microbial adhesion on membrane oxygenators in patients requiring extracorporeal life support detected by a universal rDNA PCR test. ASAIO J. 2013, 59, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kim, Y.H.; Cho, W.H.; Son, B.S.; Yeo, H.J. Biofilm microbiome in extracorporeal membrane oxygenator catheters. PLoS ONE 2021, 16, e0257449. [Google Scholar] [CrossRef] [PubMed]
- Haussner, F.; Chakraborty, S.; Halbgebauer, R.; Huber-Lang, M. Challenge to the Intestinal Mucosa During Sepsis. Front. Immunol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Payen, D. The gut as a hidden source of sepsis. Minerva Anestesiol. 2020, 86, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Brehm, M.; Kühn, C.; Schäfer, A.; Bauersachs, J. Heart against veno-arterial ECMO: Competition visualized. Int. J. Cardiol. 2015, 187, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Gehron, J.; Schuster, M.; Rindler, F.; Bongert, M.; Böning, A.; Krombach, G.; Fiebich, M.; Grieshaber, P. Watershed phenomena during extracorporeal life support and their clinical impact: A systematic in vitro investigation. ESC Heart Fail. 2020, 7, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Edinger, F.; Schneck, E.; Schulte, C.; Schmidt, G.; Gehron, J.; Sander, M.; Koch, C. Impact of the inspiratory oxygen fraction on the cardiac output during jugulo-femoral venoarterial extracorporeal membrane oxygenation in the rat. BMC Cardiovasc. Disord. 2022, 22, 174. [Google Scholar] [CrossRef]
- Nahum, E.; Skippen, P.W.; Gagnon, R.E.; Macnab, A.J.; Skarsgard, E.D. Correlation of near-infrared spectroscopy with perfusion parameters at the hepatic and systemic levels in an endotoxemic shock model. Med. Sci. Monit. 2006, 12, BR313–BR317. [Google Scholar]
- Albuszies, G.; Radermacher, P.; Vogt, J.; Wachter, U.; Weber, S.; Schoaff, M.; Georgieff, M.; Barth, E. Effect of increased cardiac output on hepatic and intestinal microcirculatory blood flow, oxygenation, and metabolism in hyperdynamic murine septic shock. Crit. Care Med. 2005, 33, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Thorén, A.; Elam, M.; Ricksten, S.E. Jejunal mucosal perfusion is well maintained during mild hypothermic cardiopulmonary bypass in humans. Anesth. Analg. 2001, 92, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Edinger, F.; Schneck, E.; Schulte, C.; Gehron, J.; Mueller, S.; Sander, M.; Koch, C. Comparison of the effect of membrane sizes and fibre arrangements of two membrane oxygenators on the inflammatory response, oxygenation and decarboxylation in a rat model of extracorporeal membrane oxygenation. BMC Cardiovasc. Disord. 2020, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Edinger, F.; Schmitt, C.; Koch, C.; McIntosh, J.M.; Janciauskiene, S.; Markmann, M.; Sander, M.; Padberg, W.; Grau, V. Application of alpha1-antitrypsin in a rat model of veno-arterial extracorporeal membrane oxygenation. Sci. Rep. 2021, 11, 15849. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, B.; ShamsEldeen, A.M.; Kamar, S.S.; Haidara, M.A.; Al-Hashem, F.; Alshahrani, M.Y.; Al-Hakami, A.M.; Kader, D.H.A.; Maarouf, A. Lipopolysaccharide induces acute lung injury and alveolar haemorrhage in association with the cytokine storm, coagulopathy and AT1R/JAK/STAT augmentation in a rat model that mimics moderate and severe Covid-19 pathology. Clin. Exp. Pharmacol. Physiol. 2022, 49, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.L.; Scholle, J.L.; Shepherd, A.P.; Ward, W.F. Effects of hematocrit on oxygenation of the isolated perfused rat liver. Am. J. Physiol. 1983, 245, G769–G774. [Google Scholar] [CrossRef] [PubMed]
- Thorén, A.; Nygren, A.; Houltz, E.; Ricksten, S.-E. Cardiopulmonary bypass in humans--jejunal mucosal perfusion increases in parallel with well-maintained microvascular hematocrit. Acta Anaesthesiol. Scand. 2005, 49, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Gu, J.; Luo, Y.; Tao, Y.; Chou, Y.; He, Y.; Qin, H.; Chen, T.; Fu, X.; Chen, M.; et al. Impact of hyperoxia on the gut during critical illnesses. Crit. Care 2024, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Chou, H.-C.; Chen, C.-M. Molecular Mechanisms of Hyperoxia-Induced Neonatal Intestinal Injury. Int. J. Mol. Sci. 2023, 24, 4366. [Google Scholar] [CrossRef]
- Chen, C.-M.; Chou, H.-C. Hyperoxia disrupts the intestinal barrier in newborn rats. Exp. Mol. Pathol. 2016, 101, 44–49. [Google Scholar] [CrossRef]
- Schmidt, G.; Pitz, L.; Markmann, M.; Schneck, E.; Sander, M.; Koch, C.; Edinger, F. Micro-lightguide spectrophotometry assessment of hepatic and intestinal microcirculation in endotoxemic rats during intravenous treatment with angiotensin II. Eur. J. Pharm. Sci. 2023, 191, 106588. [Google Scholar] [CrossRef] [PubMed]




| 0 min | 30 min | 60 min | 90 min | 120 min | ||
|---|---|---|---|---|---|---|
| SaO2 *** | sham | 100 (99–100) | 97 (96–98) | 95 (94–96) | 94 (92–95) | 93 (92–95) |
| ### | V-A 60 | 100 (100–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) |
| (%) | V-A 90 | 99 (97–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) |
| ScvO2 | sham | 80 (77–85) | 80 (74–86) | 74 (69–79) | 74 (70–75) | 69 (64–71) |
| V-A 60 | 79 (74–86) | 83 (80–89) | 80 (78–85) | 76 (73–81) | 70 (65–76) | |
| (%) | V-A 90 | 78 (73–83) | 82 (66–85) | 78 (71–82) | 69 (61–74) | 62 (56–70) |
| avDO2 | sham | 4.4 (3.4–5.2) | 3.3 (2.2–4.4) | 3.6 (2.9–4.6) | 3.3 (2.7–3.9) | 3.8 (3.4–4.1) |
| V-A 60 | 4.8 (3.0–5.8) | 2.5 (1.8–3.0) | 2.8 (2.1–2.9) | 3.1 (2.6–3.3) | 3.6 (2.9–4.1) | |
| (mL/dL) | V-A 90 | 4.5 (3.7–6.2) | 2.9 (2.1–4.4) | 3.1 (2.6–3.5) | 3.6 (3.3–3.8) | 4.3 (3.4–4.9) |
| pO2 *** | sham | 130 (115–140) | 108 (106–120) | 106 (96–110) | 98 (90–100) | 87 (79 – 94) |
| ### | V-A 60 | 146 (134–158) | 202 (192–211) | 193 (181–201) | 187 (171–196) | 178 (153–189) |
| (mmHg) | V-A 90 | 119 (112–141) | 203 (184–238) | 188 (168–214) | 179 (162–214) | 162 (150–196) |
| pCO2 *** | sham | 44 (42–46) | 58 (52–61) | 60 (57–65) | 61 (55–66) | 60 (55–67) |
| ### | V-A 60 | 40 (39–43) | 36 (32–39) | 40 (38–42) | 38 (37–39) | 37 (37–39) |
| (mmHg) | V-A 90 | 51 (43–55) | 37 (35–40) | 36 (32–40) | 39 (35–42) | 37 (35–38) |
| pH *** | sham | 7.37 (7.33–7.39) | 7.25 (7.23–7.28) | 7.21 (7.19–7.23) | 7.23 (7.20–7.26) | 7.25 (7.22–7.27) |
| ### | V-A 60 | 7.39 (7.37–7.42) | 7.42 (7.39–7.47) | 7.38 (7.36–7.40) | 7.41 (7.39–7.44) | 7.42 (7.41–7.45) |
| V-A 90 | 7.31 (7.29–7.38) | 7.41 (7.39–7.44) | 7.41 (7.38–7.45) | 7.41 (7.34–7.44) | 7.42 (7.38–7.46) | |
| BE *** | sham | −1.0 (−2.0–0.7) | −3.9 (−4.8–−2.7) | −4.8 (−6.5–−4.2) | −3.5 (−4.9–−2.0) | −1.8 (−2.9–−1.2) |
| ### | V-A 60 | −0.3 (−1.0–1.6) | −0.6 (−1.3–0.1) | −1.1 (−2.1–−0.9) | 0.0 (−0.5–.3) | 1.2 (−0.6–1.9) |
| V-A 90 | −0.3 (−1.8–0.3) | −0.5 (−2.1–0.3) | −0.9 (−1.7–−0.2) | −0.9 (−1.5–0.1) | −0.5 (−3.0–0.2) | |
| Lac | sham | 1.3 (1.1–1.5) | 1.9 (1.8–2.1) | 2.1 (1.8–2.8) | 1.6 (1.4–2.2) | 1.2 (1.0–1.7) |
| V-A 60 | 1.4 (1.3–1.8) | 2.0 (1.8–2.0) | 2.2 (2.1–2.3) | 2.1 (1.7–2.3) | 1.8 (1.4–1.9) | |
| (mmol/L) | V-A 90 | 1.5 (1.1–1.9) | 2.1 (1.8–2.3) | 2.3 (2.1–2.8) | 2.3 (1.9–2.6) | 2.1 (1.7–2.7) |
| Hb *** | sham | 15.1 (15.0–15.5) | 13.5 (13.2–13.9) | 12.8 (12.3–13.3) | 11.6 (11.2–11.9) | 11.1 (10.3–11.5) |
| ### | V-A 60 | 15.5 (15.1–16.0) | 8.3 (8.2–8.7) | 8.0 (7.9–8.3) | 7.7 (7.5–7.9) | 7.3 (7.1–7.9) |
| (g/dL) | V-A 90 | 15.2 (14.6–15.6) | 8.2 (7.7–8.9) | 7.8 (7.7–8.2) | 7.4 (7.1–7.7) | 7.4 (7.1–7.5) |
| Hct *** | sham | 46.4 (46.0–47.4) | 41.5 (40.6–42.6) | 39.1 (37.9–41.0) | 35.8 (34.6–36.6) | 34.1 (31.8–35.4) |
| ### | V-A 60 | 47.7 (46.4–48.7) | 25.8 (25.4 – 27.1) | 24.9 (24.7–25.8) | 24.0 (23.5–24.5) | 22.7 (22.3–24.5) |
| (%) | V-A 90 | 46.4 (44.5–47.6) | 25.5 (24.0–27.4) | 24.4 (23.8–25.5) | 23.1 (22.0–23.9) | 23.2 (22.1–23.6) |
| Glu | sham | 163 (157–171) | 213 (181–231) | 184 (166–223) | 147 (132–160) | 138 (120–150) |
| V-A 60 | 180 (168–189) | 179 (171–192) | 161 (146–168) | 138 (126–154) | 135 (122–153) | |
| (mg/dL) | V-A 90 | 168 (156–181) | 171 (157–182) | 157 (151–172) | 146 (129–156) | 128 (116–140) |
| Na | sham | 141 (141–142) | 143 (142–143) | 143 (143–144) | 144 (142–145) | 144 (143–144) |
| V-A 60 | 142 (141–142) | 143 (142–145) | 144 (144–145) | 145 (143–145) | 145 (144–145) | |
| (mmol/L) | V-A 90 | 142 (141–143) | 144 (143–144) | 144 (143–145) | 145 (144–146) | 145 (144–146) |
| K | sham | 4.5 (4.5–4.7) | 3.9 (3.8–4.2) | 3.9 (3.7–3.9) | 4.2 ( 4.0–4.4) | 4.6 (4.4–4.8) |
| V-A 60 | 4.6 (4.4–4.6) | 4.0 (4.0–4.2) | 3.9 (3.7–3.9) | 4.2 (4.1–4.3) | 4.5 (4.3–4.6) | |
| (mmol/L) | V-A 90 | 4.7 (4.4–4.9) | 4.3 (4.0–4.4) | 4.1 (3.9–4.2) | 4.3 (4.1–4.4) | 4.7 (4.4–4.8) |
| Cl *** | sham | 109 (108–110) | 110 (109–111) | 111 (110–112) | 111 (111–113) | 112 (111–114) |
| ## | V-A 60 | 109 (108–111) | 113 (112–114) | 115 (114–115) | 115 (114–116) | 115 (114–116) |
| (mmol/L) | V-A 90 | 108 (108–110) | 113 (112–114) | 114 (113–114) | 115 (114–116) | 115 (115–116) |
| Ca | sham | 1.51 (1.50–1.53) | 1.47 (1.45–1.49) | 1.46 (1.45–1.50) | 1.46 (1.40–1.48) | 1.43 (1.41–1.44) |
| V-A 60 | 1.48 (1.46–1.53) | 1.46 (1.44–1.49) | 1.44 (1.41–1.47) | 1.41 (1.37–1.42) | 1.38 (1.34–1.40) | |
| (mmol/L) | V-A 90 | 1.51 (1.48–1.54) | 1.47 (1.46–1.49) | 1.42 (1.39–1.42) | 1.40 (1.38–1.44) | 1.41 (1.38–1.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edinger, F.; Holtz, L.; Schmidt, G.; Schneck, E.; Zajonz, T.; Sander, M.; Koch, C. New Insights into Hepatic and Intestinal Microcirculation and Pulmonary Inflammation in a Model of Septic Shock and Veno-Arterial Extracorporeal Membrane Oxygenation in the Rat. Int. J. Mol. Sci. 2024, 25, 7421. https://doi.org/10.3390/ijms25137421
Edinger F, Holtz L, Schmidt G, Schneck E, Zajonz T, Sander M, Koch C. New Insights into Hepatic and Intestinal Microcirculation and Pulmonary Inflammation in a Model of Septic Shock and Veno-Arterial Extracorporeal Membrane Oxygenation in the Rat. International Journal of Molecular Sciences. 2024; 25(13):7421. https://doi.org/10.3390/ijms25137421
Chicago/Turabian StyleEdinger, Fabian, Lena Holtz, Götz Schmidt, Emmanuel Schneck, Thomas Zajonz, Michael Sander, and Christian Koch. 2024. "New Insights into Hepatic and Intestinal Microcirculation and Pulmonary Inflammation in a Model of Septic Shock and Veno-Arterial Extracorporeal Membrane Oxygenation in the Rat" International Journal of Molecular Sciences 25, no. 13: 7421. https://doi.org/10.3390/ijms25137421
APA StyleEdinger, F., Holtz, L., Schmidt, G., Schneck, E., Zajonz, T., Sander, M., & Koch, C. (2024). New Insights into Hepatic and Intestinal Microcirculation and Pulmonary Inflammation in a Model of Septic Shock and Veno-Arterial Extracorporeal Membrane Oxygenation in the Rat. International Journal of Molecular Sciences, 25(13), 7421. https://doi.org/10.3390/ijms25137421







