Lysophosphatidic Acid (LPA) and Its Receptors in Mood Regulation: A Systematic Review of the Molecular Mechanisms and Therapeutic Potential
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chiurchiù, V.; Maccarrone, M. Bioactive lipids as modulators of immunity, inflammation and emotions. Curr. Opin. Pharmacol. 2016, 29, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Twenge, J.M.; Cooper, A.B.; Joiner, T.E.; Duffy, M.E.; Binau, S.G. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. J. Abnorm. Psychol. 2019, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Akirav, I.; Maroun, M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007, 2007, 30873. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Maitra, M.; Tanti, A.; Suderman, M.; Théroux, J.F.; Davoli, M.A.; Perlman, K.; Yerko, V.; Wang, Y.C.; Tripathy, S.J.; et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 2020, 23, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Goodwin, R.; Olfson, M. Treatment of panic attack and risk of major depressive disorder in the community. Am. J. Psychiatry 2001, 158, 1146–1148. [Google Scholar] [CrossRef]
- Davies, M.R.; Glen, K.; Mundy, J.; Ter Kuile, A.R.; Adey, B.N.; Armour, C.; Assary, E.; Coleman, J.R.I.; Goldsmith, K.A.; Hirsch, C.R.; et al. Factors associated with anxiety disorder comorbidity. J. Affect. Disord. 2023, 323, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Taira, A.; Takanezawa, Y.; Kishi, Y.; Hama, K.; Kishimoto, T.; Mizuno, K.; Saku, K.; Taguchi, R.; Arai, H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002, 277, 48737–48744. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Malchinkhuu, E.; Muraki, T.; Ishikawa, K.; Hayashi, K.; Tosaka, M.; Mochiduki, A.; Inoue, K.; Tomura, H.; Mogi, C.; et al. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J. Neurochem. 2005, 92, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ishii, I.; Kingsbury, M.A.; Chun, J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta 2002, 1585, 108–113. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.W.; Lee, B.H.; Kim, H.J.; Hwang, S.H.; Kim, H.K.; Nah, S.Y. Ginseng pharmacology: A new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front. Pharmacol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Racké, K.; Schwörer, H. Characterization of the role of calcium and sodium channels in the stimulus secretion coupling of 5-hydroxytryptamine release from porcine enterochromaffin cells. Naunyn Schmiedebergs Arch. Pharmacol. 1993, 347, 1–8. [Google Scholar] [CrossRef]
- Andrews, P.L.; Horn, C.C. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. 2006, 125, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Minami, M.; Hirafuji, M.; Ogawa, T.; Akita, K.; Nemoto, M.; Saito, H.; Yoshioka, M.; Parvez, S.H. Neurochemistry and neuropharmacology of emesis—The role of serotonin. Toxicology 2000, 153, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Montes, L.G.; Valles-Sanchez, V.; Moreno-Aguilar, J.; Chavez-Balderas, R.A.; García-Marín, J.A.; Cortés Sotres, J.F.; Hheinze-Martin, G. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J. Psychiatry Neurosci. 2000, 25, 371–377. [Google Scholar] [PubMed]
- Patkar, A.A.; Gopalakrishnan, R.; Naik, P.C.; Murray, H.W.; Vergare, M.J.; Marsden, C.A. Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal. Alcohol. Alcohol. 2003, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Santin, L.J.; Bilbao, A.; Pedraza, C.; Matas-Rico, E.; López-Barroso, D.; Castilla-Ortega, E.; Sánchez-López, J.; Riquelme, R.; Varela-Nieto, I.; de la Villa, P.; et al. Behavioral phenotype of maLPA1-null mice: Increased anxiety-like behavior and spatial memory deficits. Genes. Brain Behav. 2009, 8, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Sánchez-López, J.; Hoyo-Becerra, C.; Matas-Rico, E.; Zambrana-Infantes, E.; Chun, J.; De Fonseca, F.R.; Pedraza, C.; Estivill-Torrús, G.; Santin, L.J. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol. Learn. Mem. 2010, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Callaerts-Vegh, Z.; Leo, S.; Vermaercke, B.; Meert, T.; D’Hooge, R. LPA5 receptor plays a role in pain sensitivity, emotional exploration and reversal learning. Genes. Brain Behav. 2012, 11, 1009–1019. [Google Scholar] [CrossRef]
- Pedraza, C.; Sánchez-López, J.; Castilla-Ortega, E.; Rosell-Valle, C.; Zambrana-Infantes, E.; García-Fernández, M.; Rodriguez de Fonseca, F.; Chun, J.; Santín, L.J.; Estivill-Torrús, G. Fear extinction and acute stress reactivity reveal a role of LPA(1) receptor in regulating emotional-like behaviors. Brain Struct. Funct. 2014, 219, 1659–1672. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Escuredo, L.; Bilbao, A.; Pedraza, C.; Orio, L.; Estivill-Torrús, G.; Santín, L.J.; de Fonseca, F.R.; Pavón, F.J. 1-Oleoyl lysophosphatidic acid: A new mediator of emotional behavior in rats. PLoS ONE 2014, 9, e85348. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukagoshi, M.; Hashimoto, T.; Oka, J.; Saitoh, A.; Yamada, M. Lysophosphatidic acid induces anxiety-like behavior via its receptors in mice. J. Neural Transm. 2015, 122, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Pavón, F.J.; Sánchez-Marín, L.; Estivill-Torrús, G.; Pedraza, C.; Blanco, E.; Suárez, J.; Santín, L.; Rodríguez de Fonseca, F.; Serrano, A. Both genetic deletion and pharmacological blockade of lysophosphatidic acid LPA1 receptor results in increased alcohol consumption. Neuropharmacology 2016, 103, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernández, R.D.; Pérez-Martín, M.; Castilla-Ortega, E.; Rosell Del Valle, C.; García-Fernández, M.I.; Chun, J.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pedraza, C. maLPA1-null mice as an endophenotype of anxious depression. Transl. Psychiatry 2017, 7, e1077. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, S.D.; Lee, R.M.; Lee, B.H.; Choi, S.H.; Hwang, S.H.; Rhim, H.; Kim, H.C.; Nah, S.Y. Gintonin attenuates depressive-like behaviors associated with alcohol withdrawal in mice. J. Affect. Disord. 2017, 215, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernández, R.D.; Nieto-Quero, A.; Gómez-Salas, F.J.; Chun, J.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pérez-Martín, M.; Pedraza, C. Effects of genetic deletion versus pharmacological blockade of the LPA(1) receptor on depression-like behaviour and related brain functional activity. Dis. Model. Mech. 2018, 11, dmm035519. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, J.W.; Lee, M.Y.; Kim, S.H.; Mok, H.J.; Ha, K.; Ahn, Y.M.; Kim, K.P. Serum lipidomic analysis for the discovery of biomarkers for major depressive disorder in drug-free patients. Psychiatry Res. 2018, 265, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Tabbai, S.; Moreno-Fernández, R.D.; Zambrana-Infantes, E.; Nieto-Quero, A.; Chun, J.; García-Fernández, M.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Oliveira, T.G.; et al. Effects of the LPA(1) Receptor Deficiency and Stress on the Hippocampal LPA Species in Mice. Front. Mol. Neurosci. 2019, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, L.; Yamada, M.; Hattori, K.; Sasayama, D.; Noda, T.; Yoshida, S.; Kunugi, H.; Yamada, M. Lysophosphatidic acid levels in cerebrospinal fluid and plasma samples in patients with major depressive disorder. Heliyon 2019, 5, e01699. [Google Scholar] [CrossRef]
- Lin, Y.N.; Audira, G.; Malhotra, N.; Ngoc Anh, N.T.; Siregar, P.; Lu, J.H.; Lee, H.; Hsiao, C.D. A Novel Function of the Lysophosphatidic Acid Receptor 3 (LPAR3) Gene in Zebrafish on Modulating Anxiety, Circadian Rhythm Locomotor Activity, and Short-Term Memory. Int. J. Mol. Sci. 2020, 21, 2837. [Google Scholar] [CrossRef]
- Riya, S.; Sultana, S.; Daria, S.; Proma, M.A.; Bhuiyan, M.A.; Haque, M.A.; Islam, M.R. Evaluation of Serum Lysophosphatidic Acid and Lysophosphatidylcholine Levels in Major Depressive Disorder Patients. Cureus 2020, 12, e12388. [Google Scholar] [CrossRef]
- Rosell-Valle, C.; Pedraza, C.; Manuel, I.; Moreno-Rodríguez, M.; Rodríguez-Puertas, R.; Castilla-Ortega, E.; Caramés, J.M.; Gómez Conde, A.I.; Zambrana-Infantes, E.; Ortega-Pinazo, J.; et al. Chronic central modulation of LPA/LPA receptors-signaling pathway in the mouse brain regulates cognition, emotion, and hippocampal neurogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110156. [Google Scholar] [CrossRef]
- Rosell-Valle, C.; Martínez-Losa, M.; Matas-Rico, E.; Castilla-Ortega, E.; Zambrana-Infantes, E.; Gómez-Conde, A.I.; Sánchez-Salido, L.; Ladrón de Guevara-Miranda, D.; Pedraza, C.; Serrano-Castro, P.J.; et al. GABAergic deficits in absence of LPA(1) receptor, associated anxiety-like and coping behaviors, and amelioration by interneuron precursor transplants into the dorsal hippocampus. Brain Struct. Funct. 2021, 226, 1479–1495. [Google Scholar] [CrossRef]
- Omori, W.; Kano, K.; Hattori, K.; Kajitani, N.; Okada-Tsuchioka, M.; Boku, S.; Kunugi, H.; Aoki, J.; Takebayashi, M. Reduced Cerebrospinal Fluid Levels of Lysophosphatidic Acid Docosahexaenoic Acid in Patients with Major Depressive Disorder and Schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 948–955. [Google Scholar] [CrossRef]
- Moreno-Fernández, R.D.; Sampedro-Piquero, P.; Gómez-Salas, F.J.; Nieto-Quero, A.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pedraza, C. Social avoidance and altered hypothalamic-pituitary-adrenal axis in a mouse model of anxious depression: The role of LPA(1) receptor. Behav. Brain Res. 2023, 455, 114681. [Google Scholar] [CrossRef]
- Nagata, W.; Koizumi, A.; Nakagawa, K.; Takahashi, S.; Gotoh, M.; Satoh, Y.; Ishizuka, T. Treatment with lysophosphatidic acid prevents microglial activation and depression-like behaviours in a murine model of neuropsychiatric systemic lupus erythematosus. Clin. Exp. Immunol. 2023, 212, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, N.; Okada-Tsuchioka, M.; Inoue, A.; Miyano, K.; Masuda, T.; Boku, S.; Iwamoto, K.; Ohtsuki, S.; Uezono, Y.; Aoki, J.; et al. G protein-biased LPAR1 agonism of prototypic antidepressants: Implication in the identification of novel therapeutic target for depression. Neuropsychopharmacology 2024, 49, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Wellman, C.L.; Izquierdo, A.; Garrett, J.E.; Martin, K.P.; Carroll, J.; Millstein, R.; Lesch, K.P.; Murphy, D.L.; Holmes, A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 2007, 27, 684–691. [Google Scholar] [CrossRef]
- Hartley, C.A.; Phelps, E.A. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology 2010, 35, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Shepard, J.D.; Barron, K.W.; Myers, D.A. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000, 861, 288–295. [Google Scholar] [CrossRef]
- Shepard, J.D.; Barron, K.W.; Myers, D.A. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003, 963, 203–213. [Google Scholar] [CrossRef]
- Davis, M.; Rainnie, D.; Cassell, M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994, 17, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Buckholtz, J.W.; Kolachana, B.; Hariri, A.R.; Pezawas, L.; Blasi, G.; Wabnitz, A.; Honea, R.; Verchinski, B.; Callicott, J.H.; et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.J.; Mozhui, K.; Karlsson, R.M.; Cameron, H.A.; Williams, R.W.; Holmes, A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology 2008, 33, 2595–2604. [Google Scholar] [CrossRef]
- Estivill-Torrús, G.; Llebrez-Zayas, P.; Matas-Rico, E.; Santín, L.; Pedraza, C.; De Diego, I.; Del Arco, I.; Fernández-Llebrez, P.; Chun, J.; De Fonseca, F.R. Absence of LPA1 signaling results in defective cortical development. Cereb. Cortex 2008, 18, 938–950. [Google Scholar] [CrossRef]
- Rodríguez Manzanares, P.A.; Isoardi, N.A.; Carrer, H.F.; Molina, V.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 2005, 25, 8725–8734. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Hamada, A.; Matsuda, H.; Takagi, A.; Tanaka, M.; Aoki, J.; Arai, H.; Noji, S. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev. Dyn. 2008, 237, 3280–3294. [Google Scholar] [CrossRef]
- Li, C.R.; Huang, G.B.; Sui, Z.Y.; Han, E.H.; Chung, Y.C. Effects of 6-hydroxydopamine lesioning of the medial prefrontal cortex on social interactions in adolescent and adult rats. Brain Res. 2010, 1346, 183–189. [Google Scholar] [CrossRef]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef]
- Marrone, R.L.; Pray, S.L.; Bridges, C.C. Norepinephrine elicitation of aggressive display responses in Betta splendens. Psychon. Sci. 1966, 5, 207–208. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Harvey, P.O.; Pruessner, J.; Czechowska, Y.; Lepage, M. Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry 2007, 12, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Der-Avakian, A.; Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Malkesman, O.; Scattoni, M.L.; Paredes, D.; Tragon, T.; Pearson, B.; Shaltiel, G.; Chen, G.; Crawley, J.N.; Manji, H.K. The female urine sniffing test: A novel approach for assessing reward-seeking behavior in rodents. Biol. Psychiatry 2010, 67, 864–871. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Reed, G.M.; Hyman, S.E.; Saxena, S. The development of the ICD-11 Clinical Descriptions and Diagnostic Guidelines for Mental and Behavioural Disorders. World Psychiatry 2015, 14, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ma, L.; Li, Y.; Wang, F.; Zheng, G.Y.; Sun, Z.; Jiang, F.; Chen, Y.; Liu, H.; Dang, A.; et al. Genetic and Functional Evidence Supports LPAR1 as a Susceptibility Gene for Hypertension. Hypertension 2015, 66, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Di Daniel, E.; Maycox, P.; Racagni, G.; Popoli, M. Abnormalities in α/β-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int. J. Neuropsychopharmacol. 2011, 14, 941–953. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Hoyo-Becerra, C.; Pedraza, C.; Chun, J.; Rodríguez De Fonseca, F.; Estivill-Torrús, G.; Santín, L.J. Aggravation of chronic stress effects on hippocampal neurogenesis and spatial memory in LPA1 receptor knockout mice. PLoS ONE 2011, 6, e25522. [Google Scholar] [CrossRef]
- Alvarez Dolado, M.; Broccoli, V. GABAergic neuronal precursor grafting: Implications in brain regeneration and plasticity. Neural Plast. 2011, 2011, 384216. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Takahashi, L.K.; Kalin, N.H.; Vanden Burgt, J.A.; Sherman, J.E. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav. Neurosci. 1989, 103, 648–654. [Google Scholar] [CrossRef]
- Murph, M.M.; Scaccia, L.A.; Volpicelli, L.A.; Radhakrishna, H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J. Cell Sci. 2003, 116, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef]
- Ma, L.; Uchida, H.; Nagai, J.; Inoue, M.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: An initiator of nerve injury-induced neuropathic pain. Mol. Pain. 2009, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tong, J.; He, D.; Pendyala, S.; Evgeny, B.; Chun, J.; Sperling, A.I.; Natarajan, V. Role of lysophosphatidic acid receptor LPA2 in the development of allergic airway inflammation in a murine model of asthma. Respir. Res. 2009, 10, 114. [Google Scholar] [CrossRef]
- Tomás, M.; Lázaro-Diéguez, F.; Durán, J.M.; Marín, P.; Renau-Piqueras, J.; Egea, G. Protective effects of lysophosphatidic acid (LPA) on chronic ethanol-induced injuries to the cytoskeleton and on glucose uptake in rat astrocytes. J. Neurochem. 2003, 87, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, M.; Crabb, D.; Xu, Y.; Liangpunsakul, S. Ethanol-induced alterations in fatty acid-related lipids in serum and tissues in mice. Alcohol. Clin. Exp. Res. 2011, 35, 229–234. [Google Scholar] [CrossRef]
- LeMarquand, D.; Pihl, R.O.; Benkelfat, C. Serotonin and alcohol intake, abuse, and dependence: Findings of animal studies. Biol. Psychiatry 1994, 36, 395–421. [Google Scholar] [CrossRef]
- Pettinati, H.M.; Oslin, D.; Decker, K. Role of serotonin and serotonin-selective pharmacotherapy in alcohol dependence. CNS Spectr. 2000, 5, 33–46. [Google Scholar] [CrossRef]
- Virkkunen, M.; Goldman, D.; Nielsen, D.A.; Linnoila, M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J. Psychiatry Neurosci. 1995, 20, 271–275. [Google Scholar] [PubMed]
- Lovinger, D.M. Serotonin’s role in alcohol’s effects on the brain. Alcohol. Health Res. World 1997, 21, 114–120. [Google Scholar] [PubMed]
- Lee, N.; Lee, S.H.; Yoo, H.R.; Yoo, H.S. Anti-Fatigue Effects of Enzyme-Modified Ginseng Extract: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Altern. Complement. Med. 2016, 22, 859–864. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.J.; Shin, E.J.; Lee, B.H.; Choi, S.H.; Hwang, S.H.; Rhim, H.; Cho, I.H.; Kim, H.C.; Nah, S.Y. Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer’s disease. Neurochem. Int. 2016, 101, 56–65. [Google Scholar] [CrossRef]
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The Innate Immune Receptors TLR2/4 Mediate Repeated Social Defeat Stress-Induced Social Avoidance through Prefrontal Microglial Activation. Neuron 2018, 99, 464–479.e467. [Google Scholar] [CrossRef]
- Atanasova, D.; Lazarov, N.; Stoyanov, D.S.; Spassov, R.H.; Tonchev, A.B.; Tchekalarova, J. Reduced neuroinflammation and enhanced neurogenesis following chronic agomelatine treatment in rats undergoing chronic constant light. Neuropharmacology 2021, 197, 108706. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef]
- Mateus-Pinheiro, A.; Pinto, L.; Bessa, J.M.; Morais, M.; Alves, N.D.; Monteiro, S.; Patrício, P.; Almeida, O.F.; Sousa, N. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl. Psychiatry 2013, 3, e210. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Noto, D.; Murayama, G.; Chiba, A.; Miyake, S. Unique primed status of microglia under the systemic autoimmune condition of lupus-prone mice. Arthritis Res. Ther. 2019, 21, 303. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Anderson, I.M. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. J. Affect. Disord. 2000, 58, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Salvadore, G.; Quiroz, J.A.; Machado-Vieira, R.; Henter, I.D.; Manji, H.K.; Zarate, C.A., Jr. The neurobiology of the switch process in bipolar disorder: A review. J. Clin. Psychiatry 2010, 71, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Rappley, I.; Myers, D.S.; Milne, S.B.; Ivanova, P.T.; Lavoie, M.J.; Brown, H.A.; Selkoe, D.J. Lipidomic profiling in mouse brain reveals differences between ages and genders, with smaller changes associated with alpha-synuclein genotype. J. Neurochem. 2009, 111, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Bravo, F.V.; Chan, R.B.; Sousa, N.; Di Paolo, G.; Oliveira, T.G. Differential lipid composition and regulation along the hippocampal longitudinal axis. Transl. Psychiatry 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
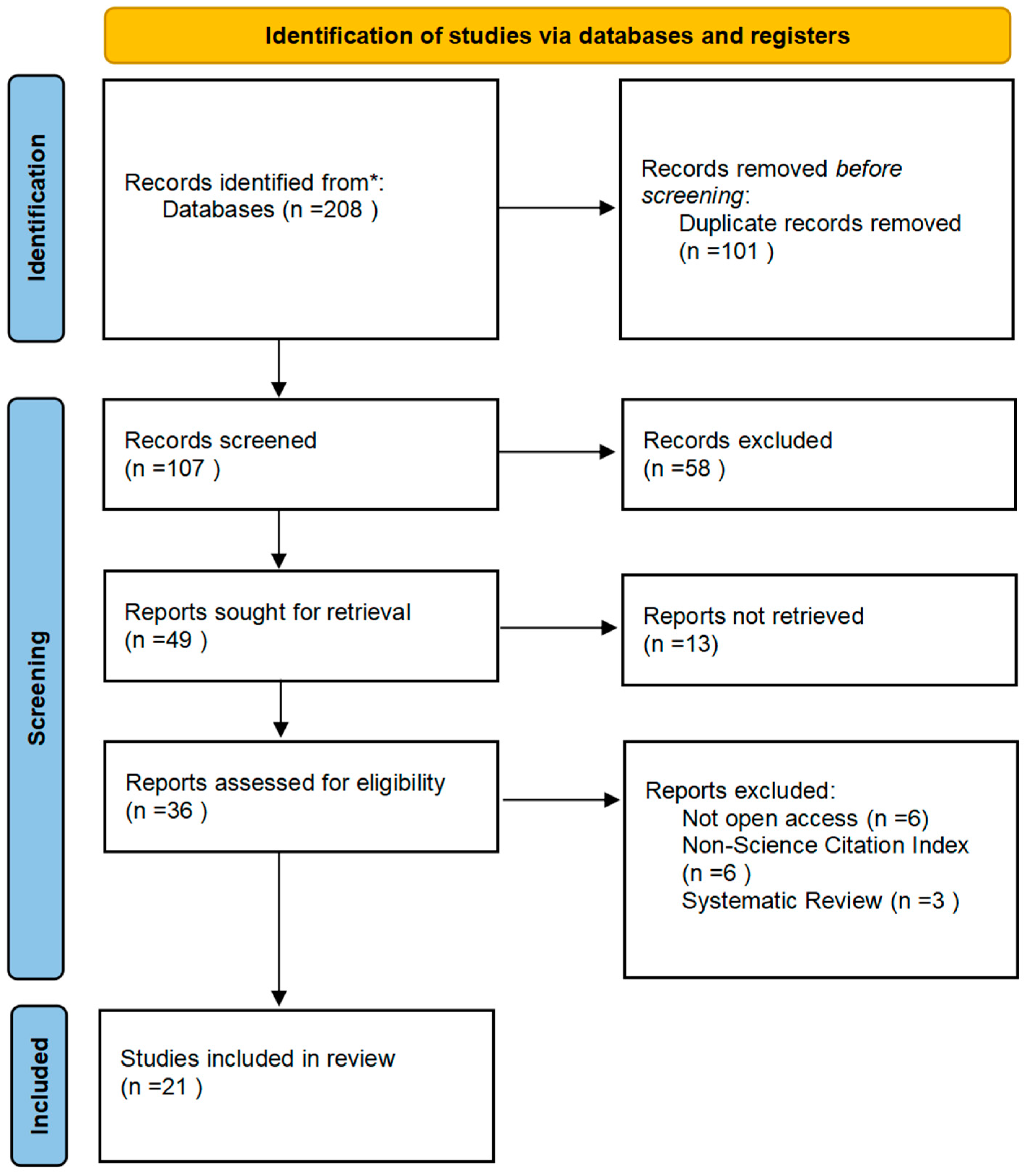
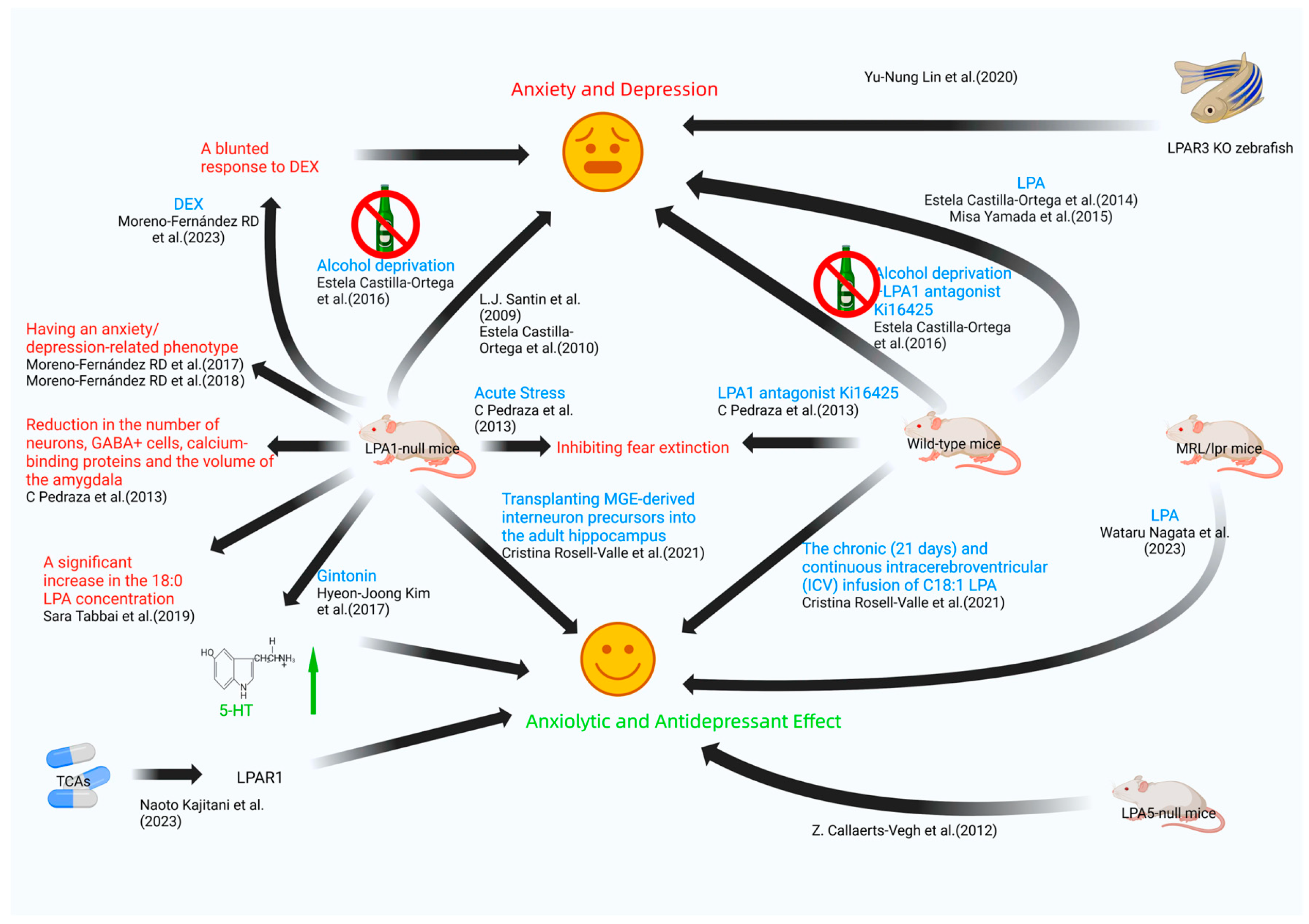
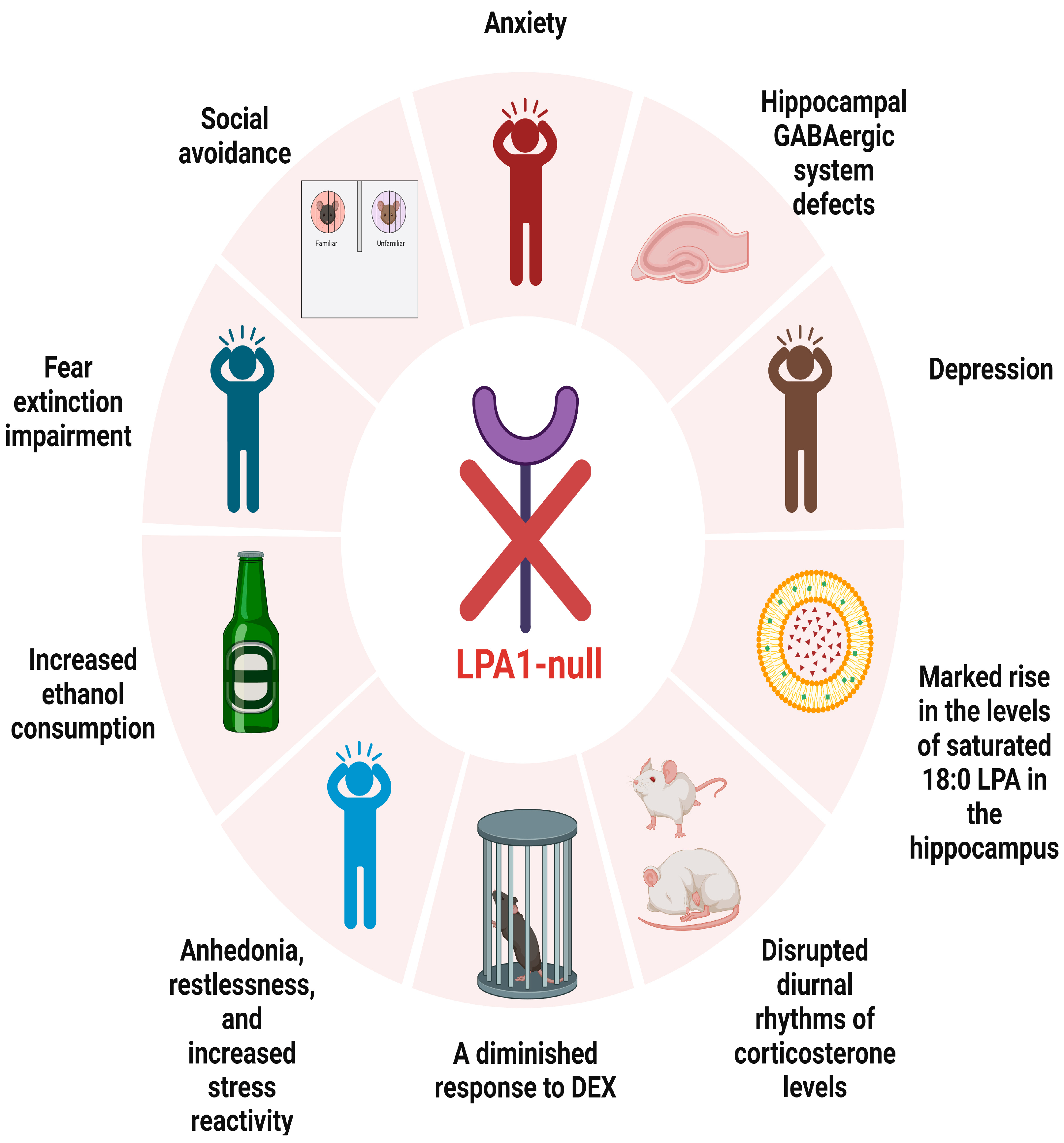
| ID | Search Terms | Results |
|---|---|---|
| #1 | (lysophosphatidic acid[Title/Abstract]) AND (mood[Title/Abstract]) | 32 |
| #2 | (lysophosphatidic acid[Title/Abstract]) AND (emotion[Title/Abstract]) | 12 |
| #3 | (lysophosphatidic acid[Title/Abstract]) AND (depression[Title/Abstract]) | 99 |
| #4 | (lysophosphatidic acid[Title/Abstract]) AND (anxiety[Title/Abstract]) | 65 |
| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Text | Reference Standard | Flow and Timing | Patient Selection | Index Text | Reference Standard | |
| L.J. Santin et al. [26] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Estela Castilla-Ortega et al. [27] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Z. Callaerts-Vegh et al. [28] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| C. Pedraza et al. [29] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Estela Castilla-Ortega et al. [30] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Misa Yamada et al. [31] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Estela Castilla-Ortega et al. [32] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Moreno-Fernández RD et al. [33] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Hyeon-Joong Kim et al. [34] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Moreno-Fernández RD et al. [35] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Eun Young Kim et al. [36] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Sara Tabbai et al. [37] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Leo Gotoh et al. [38] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Yu-Nung Lin et al. [39] | Unclear risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Sumaia Riya et al. [40] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Cristina Rosell-Valle et al. [41] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Cristina Rosell-Valle et al. [42] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Wataru Omori et al. [43] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Moreno-Fernández RD et al. [44] | Low risk | High risk | Unclear risk | Unclear risk | Low risk | High risk | Unclear risk |
| Wataru Nagata et al. [45] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Naoto Kajitani et al. [46] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Authors | Year | Group | Intervention | Results |
|---|---|---|---|---|
| L.J. Santin et al. [26] | 2009 | LPA1-null mice | unknown | anxiety |
| Estela Castilla-Ortega et al. [27] | 2010 | LPA1-null mice | unknown | anxiety |
| Z. Callaerts-Vegh et al. [28] | 2012 | LPA5-null mice | unknown | significantly reduced anxiety levels |
| C. Pedraza et al. [29] | 2013 | LPA1-null mice | unknown | fear extinction impairment |
| wild-type mice | LPA1 antagonist Ki16425 administration | fear extinction impairment | ||
| Estela Castilla-Ortega et al. [30] | 2014 | rats | LPA administration | anxiety and depression |
| Misa Yamada et al. [31] | 2015 | adult mouse | LPA administration | anxiety |
| Estela Castilla-Ortega et al. [32] | 2016 | LPA1-null mice | alcohol withdrawal | anxiety |
| wild-type mice | alcohol withdrawal + LPA1 antagonist Ki16425 administration | increased ethanol consumption | ||
| Moreno-Fernández RD et al. [33] | 2017 | LPA1-null mice | unknown | panic-like responses, anhedonia, restlessness, and increased stress reactivity |
| LPA1-null mice | antidepressant treatment | depressive-like behaviors improved | ||
| Hyeon-Joong Kim et al. [34] | 2017 | enterochromaffin cell line BON cells | gintonin administration | release of 5-hydroxytryptamine |
| C57BL/6 mice | gintonin administration | depressive-like behaviors improved | ||
| Moreno-Fernández RD et al. [35] | 2018 | LPA1-null mice | unknown | anxiety and depression |
| Sara Tabbai et al. [37] | 2019 | LPA1-null mice | unknown | marked rise in the levels of saturated 18:0 LPA in the hippocampus |
| mice | acute stress | changes in hippocampal LPA levels | ||
| Yu-Nung Lin et al. [39] | 2020 | LPAR3 knockout (KO) zebrafish | unknown | anxiety |
| Cristina Rosell-Valle et al. [41] | 2021 | mice | 21-day sustained intracerebroventricular (ICV) infusion of C18:1 LPA and LPA1-3 receptor antagonist Ki16425 | reduced anxious tendencies |
| Cristina Rosell-Valle et al. [42] | 2021 | LPA1-null mice | unknown | hippocampal GABAergic system defects |
| LPA1-null mice | GABAergic precursor cell transplantation | normalize or mitigate hippocampal GABAergic system defects | ||
| Moreno-Fernández RD et al. [44] | 2023 | LPA1-null mice | DEX | diminished response to DEX |
| Wataru Nagata et al. [45] | 2023 | MRL/lpr mice | LPA administration | alleviates depressive-like behaviors |
| Naoto Kajitani et al. [46] | 2023 | LPA1 receptor | tricyclic and tetracyclic antidepressant administration | TCAs directly bind to LPA1 receptor and exhibit G-protein-biased agonism |
| Authors | Year | Group | Intervention | Results |
|---|---|---|---|---|
| Eun Young Kim et al. [36] | 2018 | patients with current MDD (cMDD) | unknown | LPA (16:1), TG (44:0), and TG (54:8) differentiated cMDD from healthy controls with 76% accuracy |
| Leo Gotoh et al. [38] | 2019 | patients with MDD | unknown | no significant differences in LPA levels between MDD patients and healthy controls in either CSF or plasma samples |
| Sumaia Riya et al. [40] | 2020 | patients with MDD | unknown | no significant differences in serum LPA and LPC levels between MDD patients and healthy controls |
| Wataru Omori et al. [43] | 2021 | patients with MDD and SCZ | unknown | significantly lower levels of LPA 22:6 in CSF of patients with MDD and SCZ compared to healthy controls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Li, Y. Lysophosphatidic Acid (LPA) and Its Receptors in Mood Regulation: A Systematic Review of the Molecular Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 7440. https://doi.org/10.3390/ijms25137440
Li N, Li Y. Lysophosphatidic Acid (LPA) and Its Receptors in Mood Regulation: A Systematic Review of the Molecular Mechanisms and Therapeutic Potential. International Journal of Molecular Sciences. 2024; 25(13):7440. https://doi.org/10.3390/ijms25137440
Chicago/Turabian StyleLi, Nan, and Yanchun Li. 2024. "Lysophosphatidic Acid (LPA) and Its Receptors in Mood Regulation: A Systematic Review of the Molecular Mechanisms and Therapeutic Potential" International Journal of Molecular Sciences 25, no. 13: 7440. https://doi.org/10.3390/ijms25137440





