Inhibition of Cancer Stem-like Cells by Curcumin and Other Polyphenol Derivatives in MDA-MB-231 TNBC Cells
Abstract
:1. Introduction
2. Results
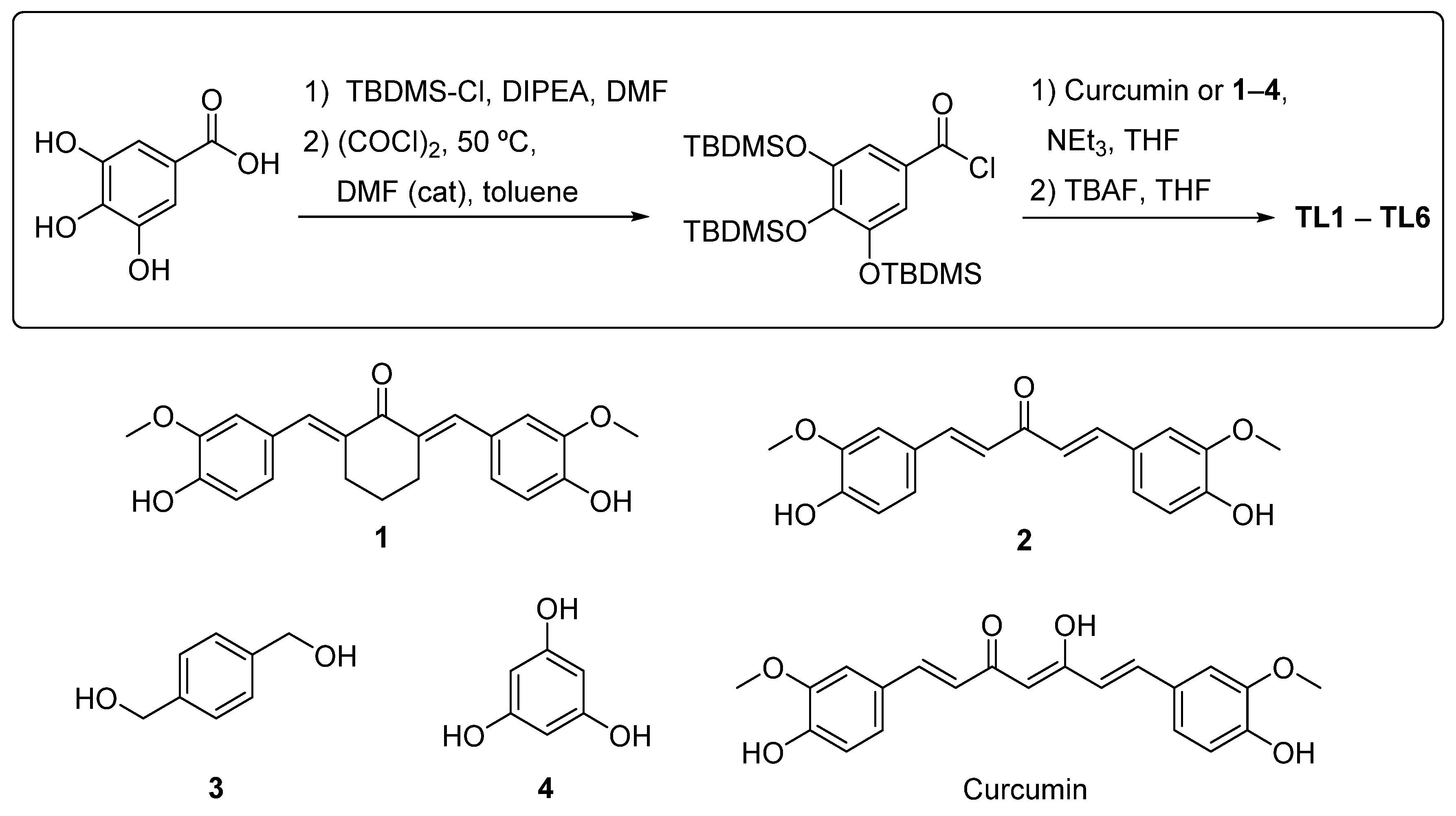
2.1. Design of Polyphenols TL1–TL6
2.2. Synthesis of Polyphenols TL1–TL6
2.3. Analysis of Cytotoxicity and Selectivity of Curcumin and Polyphenols
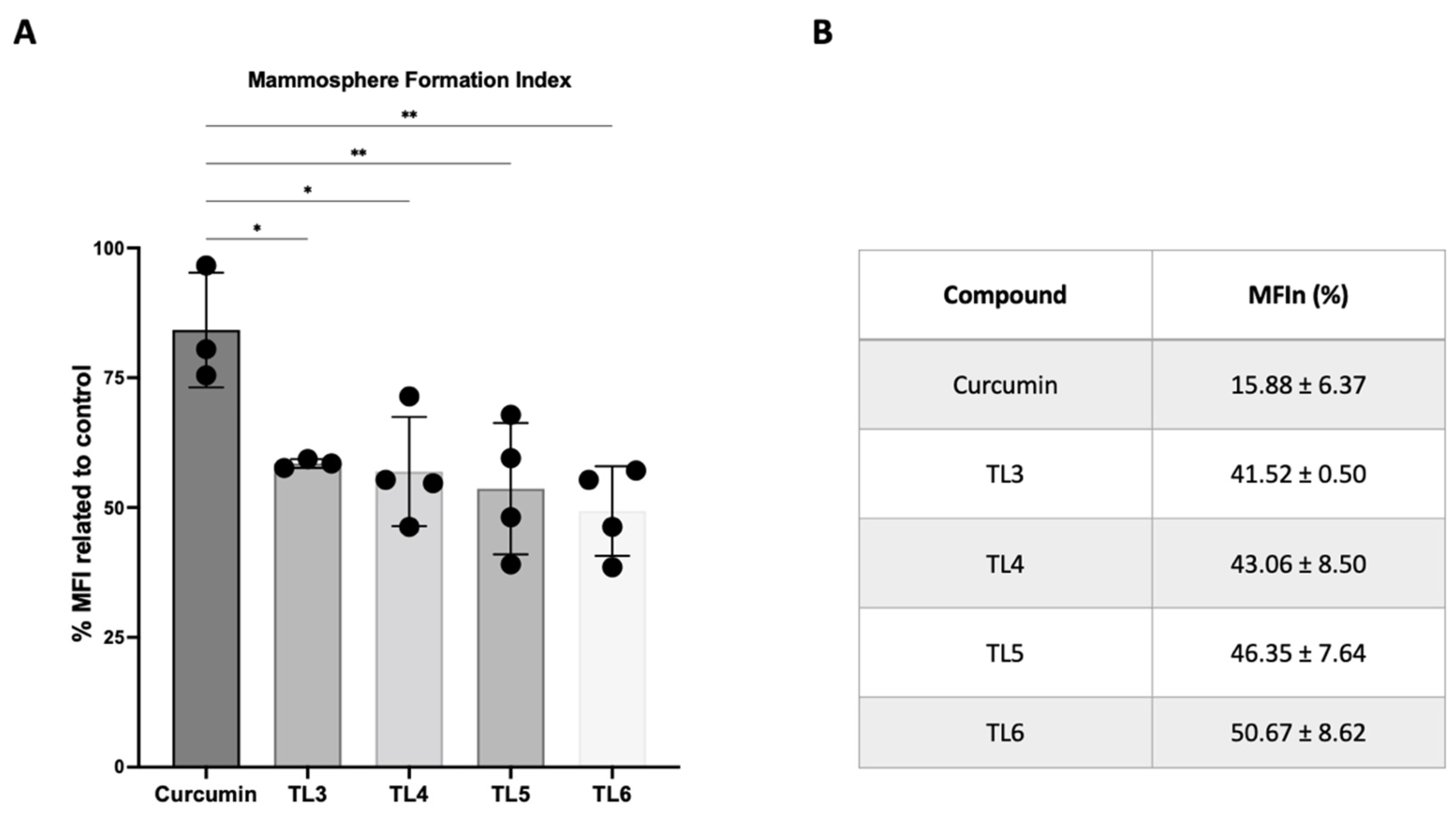
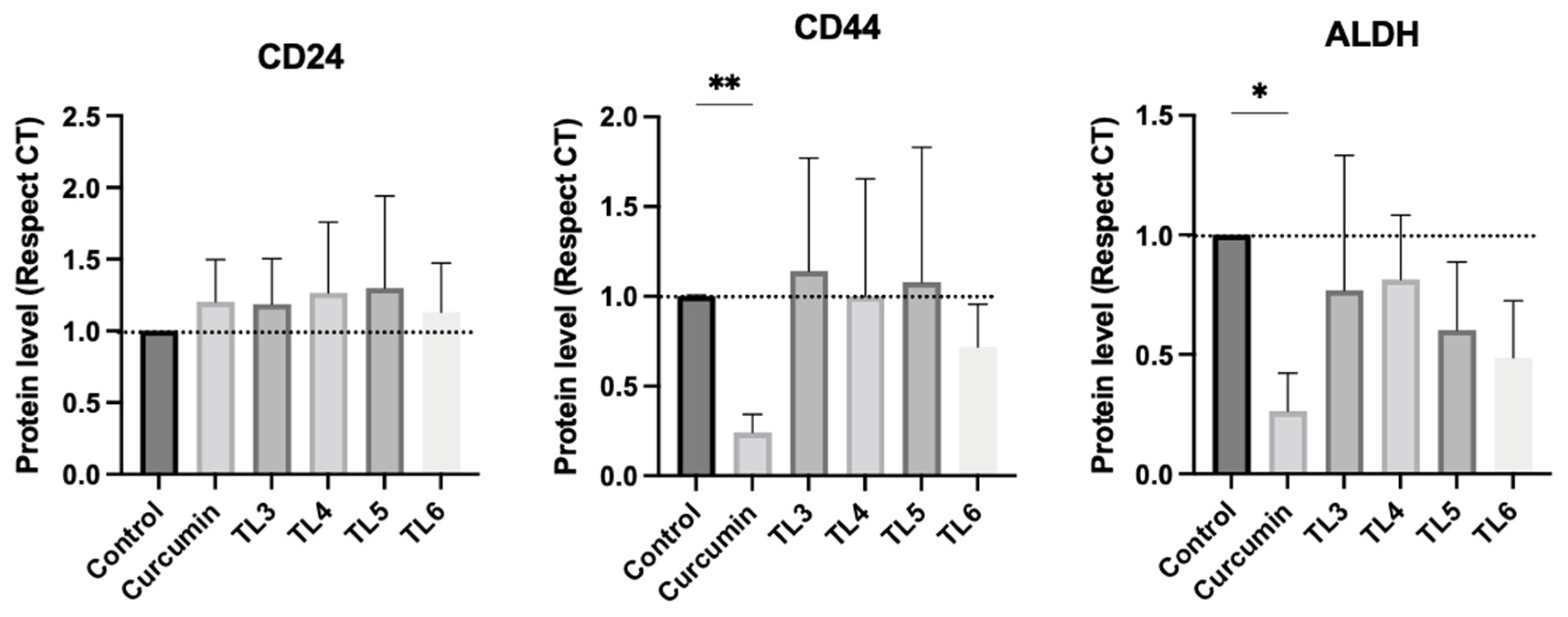
2.4. Effect of Curcumin and Its Derivatives TL3–TL6 on BCSCs
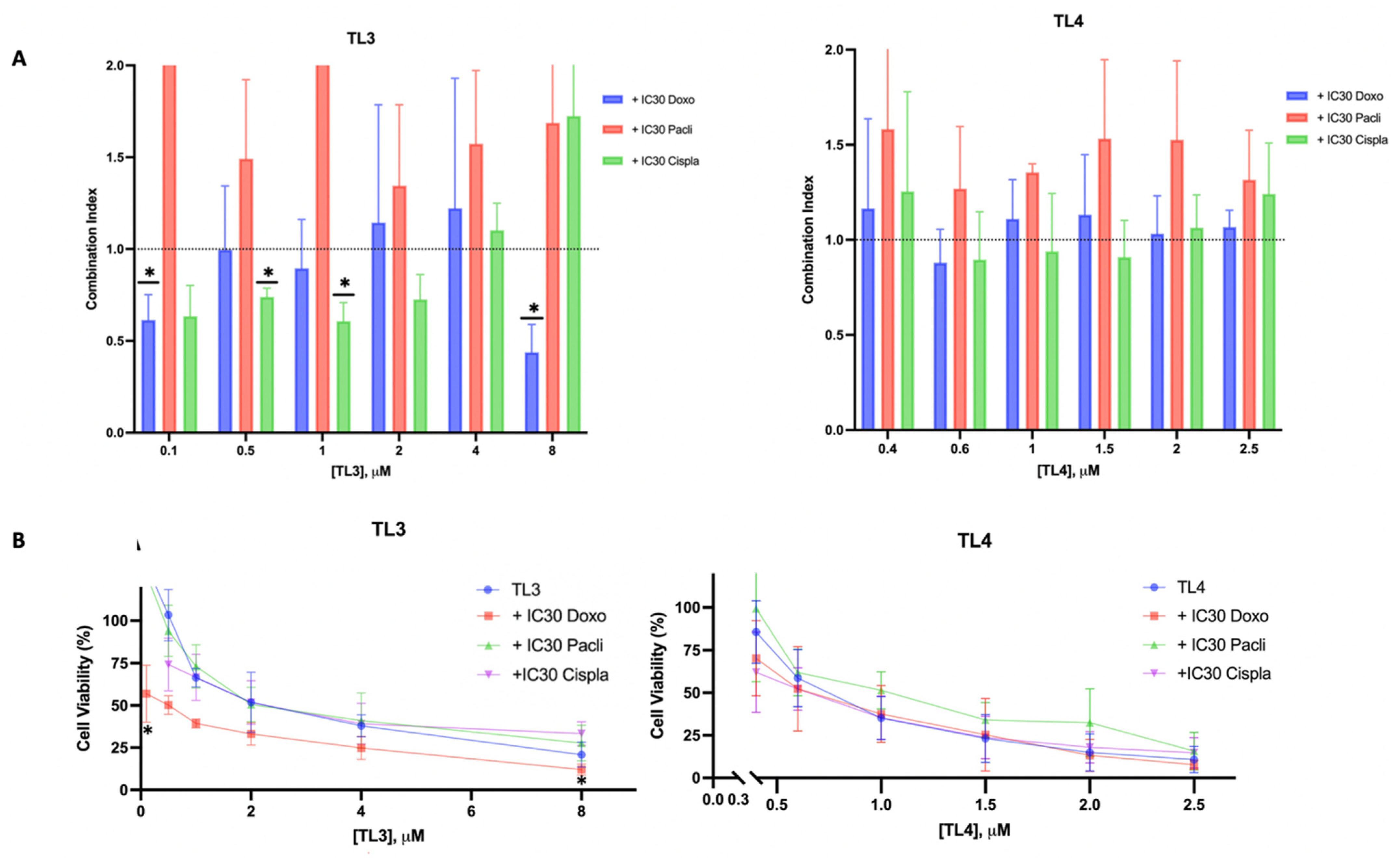
2.5. TL3-doxorubicin/TL3-cisplatin: A Synergetic Combination for TNBC
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Synthesis of the Polyphenols TL1-TL6
4.2.1. Synthesis of 3,4,5-tris(tert-butyldimethylsilyloxy)benzoic Acid [17]
4.2.2. Synthesis of Alcohols 1 and 2
Synthesis of 2,6-bis((E)-4-hydroxy-3-methoxybenzylidene)cyclohexan-1-one (1) [31]
Synthesis of (1E,4E)-1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (2) [32]
4.2.3. General Procedure for the Esterification
((1E,1′E)-(2-Oxocyclohexane-1,3-diylidene)bis(methanylylidene))bis(2-methoxy-4,1-phenylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate]
((1E,4E)-3-Oxopenta-1,4-diene-1,5-diyl)bis(2-methoxy-4,1-phenylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate]
1,4-Phenylenebis(methylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate]
Benzene-1,3,5-triyl tris[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate]
((1E,3Z,6E)-3-Hydroxy-5-oxohepta-1,3,6-triene-1,7-diyliden)bis(2-methoxy-4,1-phenylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate]
4.2.4. General Procedure for the Removal of the tert-Butyldimethylsilyl (TBDMS) Group
1,4-Phenylenebis(methylene) bis(3,4,5-trihydroxybenzoate) (TL1)
Benzene-1,3,5-triyl tris(3,4,5-trihydroxybenzoate) (TL2)
1,4-Phenylenebis(methylene) bis(3,4,5-trihydroxybenzoate) (TL3)
((1E,1′E)-(2-Oxocyclohexane-1,3-diylidene)bis(methanylylidene))bis(2-methoxy-4,1-phenylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate] (TL4) and ((1E,1′Z)-(2-oxocyclohexane-1,3-diylidene)bis(methanylylidene))bis(2-methoxy-4,1-phenylene) bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate] (TL5)
((1E,4E)-3-Oxopenta-1,4-diene-1,5-diyl)bis(2-methoxy-4,1-phenylene) Bis[3,4,5-tris((tert-butyldimethylsilyl)oxy)benzoate] (TL6)
4.3. Biological Evaluation
4.3.1. Cell Culture
4.3.2. Dilution of Polyphenolic Compounds
4.3.3. Cytotoxicity and Drug Combination Assay
4.3.4. Mammosphere-Forming Assay
4.3.5. Western Blot
4.3.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Chen, T.; González, I.; Cao, D.; Peng, Y. Cancer Stem Cells in Triple-Negative Breast Cancer: A Potential Target and Prognostic Marker. Biomark. Med. 2018, 12, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Park, C. Nam Targeting Cancer Stem Cells in Triple-Negative Breast Cancer. Cancers. 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Singleton, C.S.; Chan, L.L.; McCulley, K.J.; Kessel, S.L.; Del Valle, L.; Crabtree, J.S. ER+ Breast Cancer Mammosphere Formation and Analysis. Methods Mol. Biol. 2022, 2422, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Chen, Y.; Liu, P.; Huang, S.; Zhang, Y.; Sun, Y.; Wu, Z.; Hu, M.; Wu, Q.; et al. ALDH1: A Potential Therapeutic Target for Cancer Stem Cells in Solid Tumors. Front. Oncol. 2022, 12, 1026278. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.B.; DE Amorim, Í.S.S.; Souza, L.D.E.; Rodrigues, J.A.; Mencalha, A.L. Targeting Cellular Signaling Pathways in Breast Cancer Stem Cells and Its Implication for Cancer Treatment. Anticancer Res. 2016, 36, 5681–5691. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast Cancer Stem Cells, Heterogeneity, Targeting Therapies and Therapeutic Implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling Pathways Governing Breast Cancer Stem Cells Behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Puig, T.; Turrado, C.; Benhamú, B.; Aguilar, H.; Relat, J.; Ortega-Gutiérrez, S.; Casals, G.; Marrero, P.F.; Urruticoechea, A.; Haro, D.; et al. Novel Inhibitors of Fatty Acid Synthase with Anticancer Activity. Clin. Cancer Res. 2009, 15, 7608–7615. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Current Shreds of Evidence on the Anticancer Role of EGCG in Triple Negative Breast Cancer: An Update of the Current State of Knowledge. Infect Agent. Cancer 2020, 15, 2. [Google Scholar] [CrossRef]
- Oliveras, G.; Blancafort, A.; Urruticoechea, A.; Campuzano, O.; Gómez-Cabello, D.; Brugada, R.; López-Rodríguez, M.L.; Colomer, R.; Puig, T. Novel Anti-Fatty Acid Synthase Compounds with Anti-Cancer Activity in HER2+ Breast Cancer. Ann. N. Y. Acad. Sci. 2010, 1210, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Turrado, C.; Puig, T.; García-Cárceles, J.; Artola, M.; Benhamú, B.; Ortega-Gutiérrez, S.; Relat, J.; Oliveras, G.; Blancafort, A.; Haro, D.; et al. New Synthetic Inhibitors of Fatty Acid Synthase with Anticancer Activity. J. Med. Chem. 2012, 55, 5013–5023. [Google Scholar] [CrossRef] [PubMed]
- Crous-Masó, J.; Palomeras, S.; Relat, J.; Camó, C.; Martínez-Garza, Ú.; Planas, M.; Feliu, L.; Puig, T. (−)-Epigallocatechin 3-Gallate Synthetic Analogues Inhibit Fatty Acid Synthase and Show Anticancer Activity in Triple Negative Breast Cancer. Molecules 2018, 23, 1160. [Google Scholar] [CrossRef] [PubMed]
- Giró-Perafita, A.; Rabionet, M.; Planas, M.; Feliu, L.; Ciurana, J.; Ruiz-Martínez, S.; Puig, T. EGCG-Derivative G28 Shows High Efficacy Inhibiting the Mammosphere-Forming Capacity of Sensitive and Resistant TNBC Models. Molecules 2019, 24, 1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin Activates a ROS/KEAP1/NRF2/MiR-34a/b/c Cascade to Suppress Colorectal Cancer Metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Tommonaro Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Liu, H.T.; Ho, Y.S. Anticancer Effect of Curcumin on Breast Cancer and Stem Cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Cridge, B.J.; Larsen, L.; Rosengren, R.J. Curcumin and Its Derivatives in Breast Cancer: Current Developments and Potential for the Treatment of Drug-Resistant Cancers. Oncol. Discov. 2013, 1, 6. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Pal, K.; Roy, S.; Parida, P.K.; Dutta, A.; Bardhan, S.; Das, S.; Jana, K.; Karmakar, P. Folic Acid Conjugated Curcumin Loaded Biopolymeric Gum Acacia Microsphere for Triple Negative Breast Cancer Therapy in Invitro and Invivo Model. Mater. Sci. Eng. C 2019, 95, 204–216. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.S.; Ayob, A.Z.; Myint, H.H.L.; Thiagarajah, S.; Amini, F. Targeting Colorectal Cancer Stem Cells Using Curcumin and Curcumin Analogues: Insights into the Mechanism of the Therapeutic Efficacy. Cancer Cell Int. 2015, 15, 96. [Google Scholar] [CrossRef]
- Razak, N.A.; Akhtar, M.N.; Abu, N.; Ho, W.Y.; Tan, S.W.; Zareen, S.; Taj-Ud-Din, S.N.B.; Long, K.; Alitheen, N.B.; Yeap, S.K. The: In Vivo Anti-Tumor Effect of Curcumin Derivative (2 E,6 E)-2,6-Bis(4-Hydroxy-3-Methoxybenzylidene)Cyclohexanone (BHMC) on 4T1 Breast Cancer Cells. RSC Adv. 2017, 7, 36185–36192. [Google Scholar] [CrossRef]
- Lin, L.; Hutzen, B.; Ball, S.; Foust, E.; Sobo, M.; Deangelis, S.; Pandit, B.; Friedman, L.; Li, C.; Li, P.K.; et al. New Curcumin Analogues Exhibit Enhanced Growth-Suppressive Activity and Inhibit AKT and Signal Transducer and Activator of Transcription 3 Phosphorylation in Breast and Prostate Cancer Cells. Cancer Sci. 2009, 100, 1719–1727. [Google Scholar] [CrossRef]
- Sardjiman, S.S.; Reksohadiprodjo, M.S.; Hakim, L.; van der Goot, H.; Timmerman, H. 1,5-Diphenyl-1,4-Pentadiene-3-Ones and Cyclic Analogues as Antioxidative Agents. Synthesis and Structure-Activity Relationship. Eur. J. Med. Chem. 1997, 32, 625–630. [Google Scholar] [CrossRef]
- Quincoces Suarez, J.A.; Rando, D.G.; Santos, R.P.; Gonalves, C.P.; Ferreira, E.; De Carvalho, J.E.; Kohn, L.; Maria, D.A.; Faião-Flores, F.; Michalik, D.; et al. New Antitumoral Agents I: In Vitro Anticancer Activity and in Vivo Acute Toxicity of Synthetic 1,5-Bis(4-Hydroxy-3-Methoxyphenyl)-1,4-Pentadien-3-One and Derivatives. Bioorganic Med. Chem. 2010, 18, 6275–6281. [Google Scholar] [CrossRef]
- Yuan, J.-D.; ZhuGe, D.-L.; Tong, M.-Q.; Lin, M.-T.; Xu, X.-F.; Tang, X.; Zhao, Y.-Z.; Xu, H.-L. PH-Sensitive Polymeric Nanoparticles of MPEG-PLGA-PGlu with Hybrid Core for Simultaneous Encapsulation of Curcumin and Doxorubicin to Kill the Heterogeneous Tumour Cells in Breast Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-Metastasis Activity of Curcumin against Breast Cancer via the Inhibition of Stem Cell-like Properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and Its Derivatives in Cancer Therapy: Potentiating Antitumor Activity of Cisplatin and Reducing Side Effects. Phyther. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lv, Q.; Meng, W.; Tan, Q.; Zhang, S.; Mo, X.; Yang, X. Comparison of Mammosphere Formation from Breast Cancer Cell Lines and Primary Breast Tumors. J. Thorac. Dis. 2014, 6, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Fultang, N.; Chakraborty, M.; Peethambaran, B. Regulation of Cancer Stem Cells in Triple Negative Breast Cancer. Cancer Drug Resist. 2021, 4, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Xie, C.; Zhu, J.; Meng, Y.; Chen, Y.; Li, Y.; Jiang, Y.; Yang, X.; Wang, S.; et al. Sonic Hedgehog and Wnt/β-Catenin Pathways Mediate Curcumin Inhibition of Breast Cancer Stem Cells. Anticancer Drugs 2018, 29, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Das, T.P.; Damodaran, C. Silencing NOTCH Signaling Causes Growth Arrest in Both Breast Cancer Stem Cells and Breast Cancer Cells. Br. J. Cancer 2013, 109, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Ponce-Cusi, R.; Abarca-Quinones, J. Effect of Curcumin on the Cell Surface Markers CD44 and CD24 in Breast Cancer. Oncol. Rep. 2018, 39, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wick, N.; Germans, S.K.; Peng, Y. The Role of Breast Cancer Stem Cells in Chemoresistance and Metastasis in Triple-Negative Breast Cancer. Cancers 2021, 13, 6209. [Google Scholar] [CrossRef]
- Landeros, N.; Castillo, I.; Pérez-Castro, R. Preclinical and Clinical Trials of New Treatment Strategies Targeting Cancer Stem Cells in Subtypes of Breast Cancer. Cells 2023, 12, 720. [Google Scholar] [CrossRef]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.-J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 136–150.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ye, M.; Lu, Y.; Zhang, H.; Chen, Q.; Huang, S.; Su, S. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-like Breast Cancer Cells. PLoS ONE 2015, 10, e0136694. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin Reverses Doxorubicin Resistance via Inhibition the Efflux Function of ABCB4 in Doxorubicin-resistant Breast Cancer Cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Carrión, F. Curcumin and Paclitaxel Induce Cell Death in Breast Cancer Cell Lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic Interactions of Curcuminoids with Conventional Drugs: A Review. J. Ethnopharmacol. 2017, 209, 1–12. [Google Scholar] [CrossRef]
- Polonio-Alcalá, E.; Palomeras, S.; Torres-Oteros, D.; Relat, J.; Planas, M.; Feliu, L.; Ciurana, J.; Ruiz-Martínez, S.; Puig, T. Fatty Acid Synthase Inhibitor G28 Shows Anticancer Activity in EGFR Tyrosine Kinase Inhibitor Resistant Lung Adenocarcinoma Models. Cancers 2020, 12, 1283. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]



| Compound | IC50 (μM) | IC30 (μM) |
|---|---|---|
| G28 | 34.77 ± 0.70 | 19.48 ± 2.02 |
| Curcumin | 17.36 ± 0.33 | 14.45 ± 0.01 |
| TL1 | 18.02 ± 0.74 | 8.02 ± 1.23 |
| TL2 | 32.03 ± 2.26 | 20.87 ± 1.12 |
| TL3 | 0.93 ± 0.03 (***) | 0.76 ± 0.03 |
| TL4 | 0.86 ± 0.08 (***) | 0.65 ± 0.09 |
| TL5 | 1.31 ± 0.17 (***) | 0.80 ± 0.07 |
| TL5 | 3.58 ± 0.29 (***) | 1.86 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros, M.; Riesco-Llach, G.; Polonio-Alcalá, E.; Morla-Barcelo, P.M.; Ruiz-Martínez, S.; Feliu, L.; Planas, M.; Puig, T. Inhibition of Cancer Stem-like Cells by Curcumin and Other Polyphenol Derivatives in MDA-MB-231 TNBC Cells. Int. J. Mol. Sci. 2024, 25, 7446. https://doi.org/10.3390/ijms25137446
Ros M, Riesco-Llach G, Polonio-Alcalá E, Morla-Barcelo PM, Ruiz-Martínez S, Feliu L, Planas M, Puig T. Inhibition of Cancer Stem-like Cells by Curcumin and Other Polyphenol Derivatives in MDA-MB-231 TNBC Cells. International Journal of Molecular Sciences. 2024; 25(13):7446. https://doi.org/10.3390/ijms25137446
Chicago/Turabian StyleRos, Maria, Gerard Riesco-Llach, Emma Polonio-Alcalá, Pere Miquel Morla-Barcelo, Santiago Ruiz-Martínez, Lidia Feliu, Marta Planas, and Teresa Puig. 2024. "Inhibition of Cancer Stem-like Cells by Curcumin and Other Polyphenol Derivatives in MDA-MB-231 TNBC Cells" International Journal of Molecular Sciences 25, no. 13: 7446. https://doi.org/10.3390/ijms25137446







