Applications of SPECT and PET Imaging for the Physiological Evaluation of Lower Extremity Peripheral Artery Disease
Abstract
:1. Introduction
2. SPECT/CT Imaging
2.1. Skeletal Muscle Perfusion
2.2. Lower Extremity Osteomyelitis and Infection
3. PET/CT Imaging
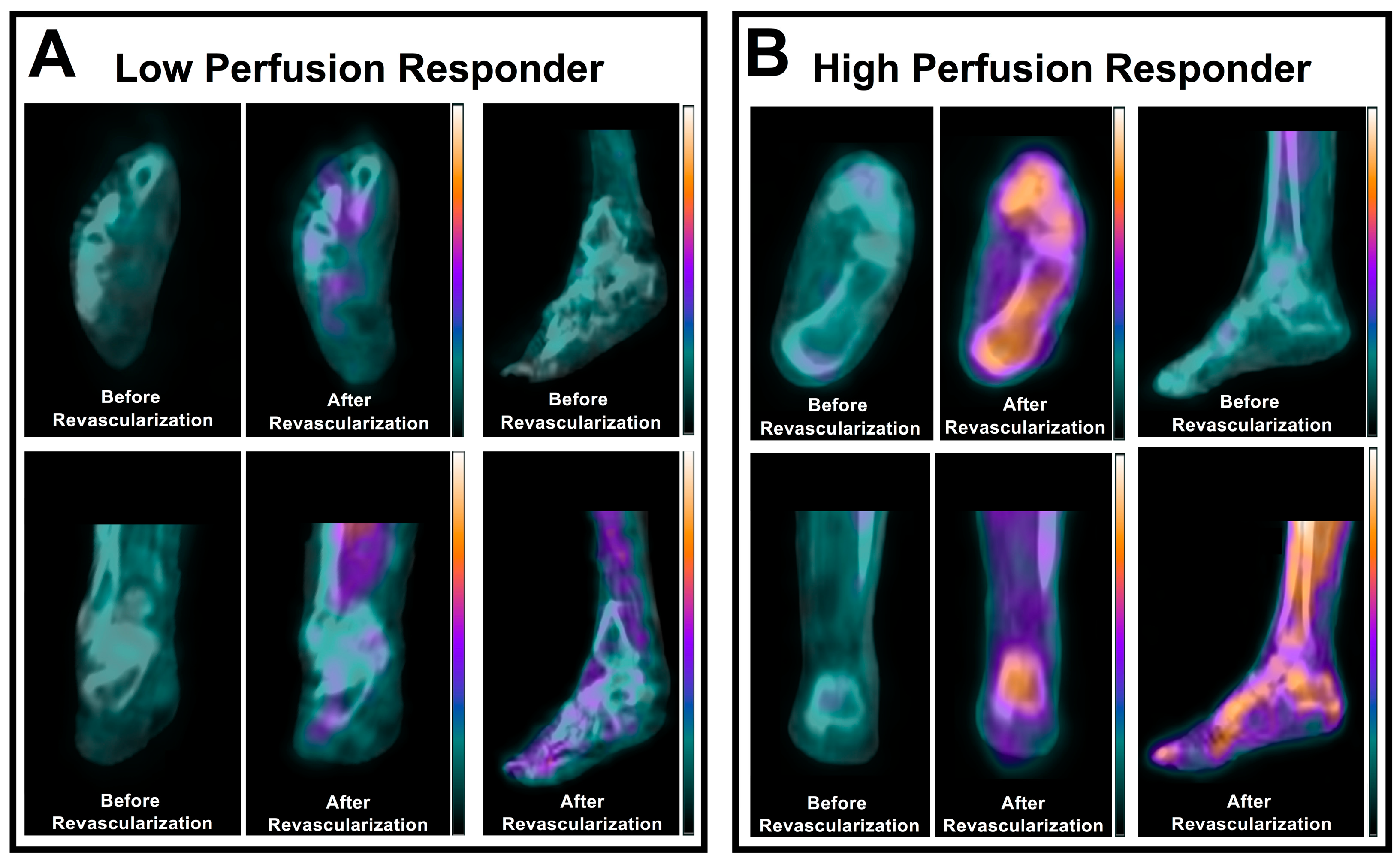
3.1. Skeletal Muscle Perfusion
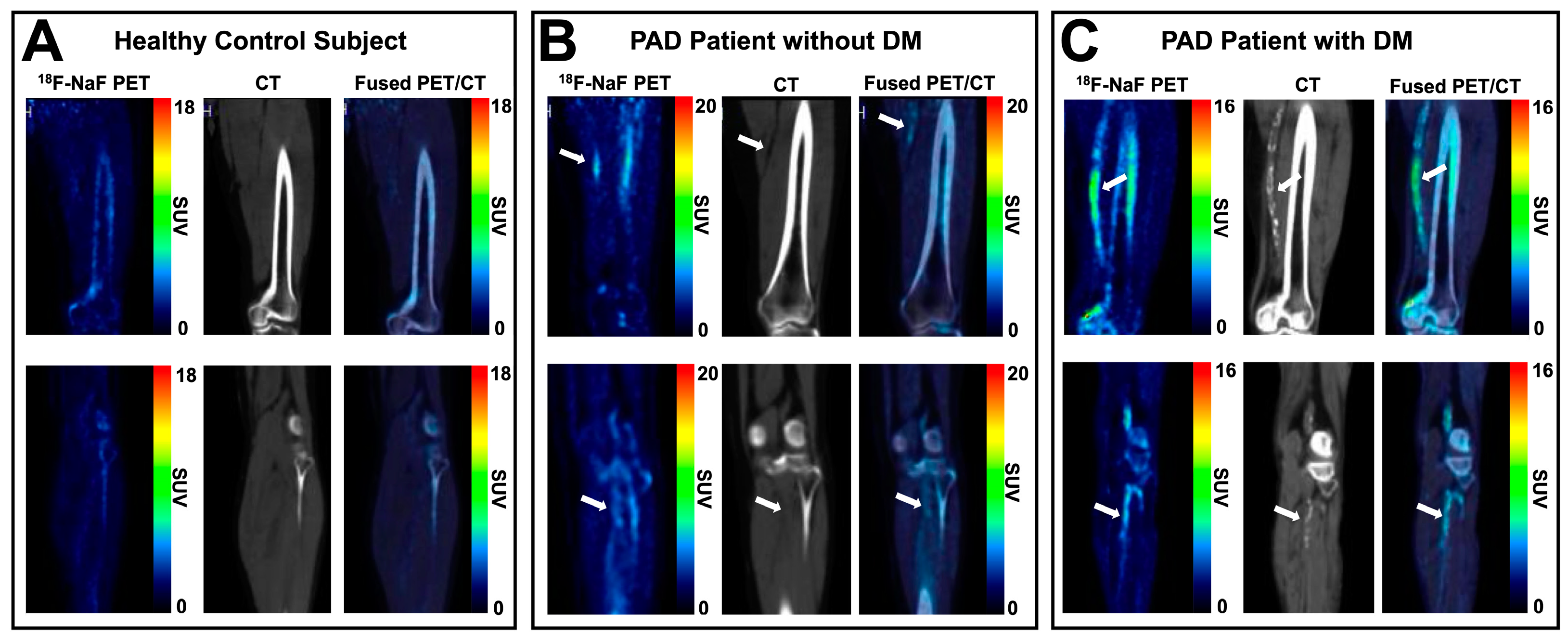
3.2. Skeletal Muscle Metabolism and Peripheral Atherosclerosis
4. Future Directions and Conclusions
Angiogenesis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chou, T.H.; Stacy, M.R. Clinical Applications for Radiotracer Imaging of Lower Extremity Peripheral Arterial Disease and Critical Limb Ischemia. Mol. Imaging Biol. 2020, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Bruzelius, M.; Damrauer, S.M.; Håkansson, N.; Wolk, A.; Åkesson, A.; Larsson, S.C. Anti-Inflammatory Diet and Incident Peripheral Artery Disease: Two Prospective Cohort Studies. Clin. Nutr. 2022, 41, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Spring, B.; Tian, L.; Treat-Jacobson, D.; Ferrucci, L.; Lloyd-Jones, D.; Zhao, L.; Polonsky, T.; Kibbe, M.R.; Bazzano, L.; et al. Effect of Low-Intensity vs High-Intensity Home-Based Walking Exercise on Walk Distance in Patients with Peripheral Artery Disease: The LITE Randomized Clinical Trial. JAMA 2021, 325, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Tern, P.J.W.; Kujawiak, I.; Saha, P.; Berrett, T.B.; Chowdhury, M.M.; Coughlin, P.A. Site and Burden of Lower Limb Atherosclerosis Predicts Long-Term Mortality in a Cohort of Patients with Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients with Peripheral Artery Disease (Updating the 2005 Guideline). J. Am. Coll. Cardiol. 2011, 58, 2020–2045. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Peripheral Artery Disease and Its Risk Factors, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2023, 11, e1553–e1565. [CrossRef] [PubMed]
- Cannon, P.J.; Dell, R.B.; Dwyer, E.M. Regional Myocardial Perfusion Rates in Patients with Coronary Artery Disease. J. Clin. Investig. 1972, 51, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, D.; Odori, T.; Maeda, H.; Ishii, Y.; Hayakawa, K.; Torizuka, K. A Quantitative Assessment of Scintigraphy of the Legs Using 201Tl. Eur. J. Nucl. Med. 1984, 9, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Alvelo, J.L.; Papademetris, X.; Mena-Hurtado, C.; Jeon, S.; Sumpio, B.E.; Sinusas, A.J.; Stacy, M.R. Radiotracer Imaging Allows for Noninvasive Detection and Quantification of Abnormalities in Angiosome Foot Perfusion in Diabetic Patients with Critical Limb Ischemia and Nonhealing Wounds. Circ. Cardiovasc. Imaging 2018, 11, e006932. [Google Scholar] [CrossRef]
- Oshima, M.; Akanabe, H.; Sakuma, S.; Yano, T.; Nishikimi, N.; Shionoya, S. Quantification of Leg Muscle Perfusion Using Thallium-201 Single Photon Emission Computed Tomography. J. Nucl. Med. 1989, 30, 458–465. [Google Scholar]
- Boschi, A.; Uccelli, L.; Marvelli, L.; Cittanti, C.; Giganti, M.; Martini, P. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules 2022, 27, 1188. [Google Scholar] [CrossRef]
- Stacy, M.R.; Sinusas, A.J. Novel Applications of Radionuclide Imaging in Peripheral Vascular Disease. Cardiol. Clin. 2016, 34, 167–177. [Google Scholar] [CrossRef]
- Chou, T.H.; Atway, S.A.; Bobbey, A.J.; Sarac, T.P.; Go, M.R.; Stacy, M.R. SPECT/CT Imaging: A Noninvasive Approach for Evaluating Serial Changes in Angiosome Foot Perfusion in Critical Limb Ischemia. Adv. Wound Care 2020, 9, 103–110. [Google Scholar] [CrossRef]
- Hashimoto, H.; Fukushima, Y.; Kumita, S.-I.; Miyamoto, M.; Takagi, G.; Yamazaki, J.; Ikeda, T. Prognostic Value of Lower Limb Perfusion Single-Photon Emission Computed Tomography-Computed Tomography in Patients with Lower Limb Atherosclerotic Peripheral Artery Disease. Jpn. J. Radiol. 2017, 35, 68–77. [Google Scholar] [CrossRef]
- Chou, T.H.; Alvelo, J.L.; Janse, S.; Papademetris, X.; Sumpio, B.E.; Mena-Hurtado, C.; Sinusas, A.J.; Stacy, M.R. Prognostic Value of Radiotracer-Based Perfusion Imaging in Critical Limb Ischemia Patients Undergoing Lower Extremity Revascularization. JACC Cardiovasc. Imaging 2021, 14, 1614–1624. [Google Scholar] [CrossRef]
- Chou, T.H.; Tram, N.K.; Eisert, S.N.; Bobbey, A.J.; Atway, S.A.; Go, M.R.; Stacy, M.R. Dual Assessment of Abnormal Microvascular Foot Perfusion and Lower Extremity Calcium Burden in a Patient with Critical Limb Ischemia Using Hybrid SPECT/CT Imaging. Vasc. Med. 2021, 26, 225–227. [Google Scholar] [CrossRef]
- Palestro, C.J.; Love, C.; Bhargava, K.K. Labeled Leukocyte Imaging: Current Status and Future Directions. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 105–123. [Google Scholar]
- Glaudemans, A.W.J.M.; Uçkay, I.; Lipsky, B.A. Challenges in Diagnosing Infection in the Diabetic Foot. Diabet. Med. 2015, 32, 748–759. [Google Scholar] [CrossRef]
- Heiba, S.I.; Kolker, D.; Mocherla, B.; Kapoor, K.; Jiang, M.; Son, H.; Rangaswamy, B.; Kostakoglu, L.; Savitch, I.; DaCosta, M.; et al. The Optimized Evaluation of Diabetic Foot Infection by Dual Isotope SPECT/CT Imaging Protocol. J. Foot Ankle Surg. 2010, 49, 529–536. [Google Scholar] [CrossRef]
- Horger, M.; Eschmann, S.M.; Pfannenberg, C.; Storek, D.; Dammann, F.; Vonthein, R.; Claussen, C.D.; Bares, R. The Value of SPET/CT in Chronic Osteomyelitis. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1665–1673. [Google Scholar] [CrossRef]
- Johnson, J.E.; Kennedy, E.J.; Shereff, M.J.; Patel, N.C.; Collier, B.D. Prospective Study of Bone, Indium-111-Labeled White Blood Cell, and Gallium-67 Scanning for the Evaluation of Osteomyelitis in the Diabetic Foot. Foot Ankle Int. 1996, 17, 10–16. [Google Scholar] [CrossRef]
- Ando, A.; Nitta, K.; Ando, I.; Sanada, S.; Katsuda, S.; Tonami, N.; Hiraki, T.; Hisada, K.; Ogawa, H. Mechanism of Gallium 67 Accumulation in Inflammatory Tissue. Eur. J. Nucl. Med. 1990, 17, 21–27. [Google Scholar] [CrossRef]
- Bar-Shalom, R.; Yefremov, N.; Guralnik, L.; Keidar, Z.; Engel, A.; Nitecki, S.; Israel, O. SPECT/CT Using 67 Ga and 111 In-Labeled Leukocyte Scintigraphy for Diagnosis of Infection. J. Nucl. Med. 2006, 47, 587–594. [Google Scholar]
- Aslangul, E.; M’Bemba, J.; Caillat-Vigneron, N.; Coignard, S.; Larger, E.; Boitard, C.; Lipsky, B.A. Diagnosing Diabetic Foot Osteomyelitis in Patients without Signs of Soft Tissue Infection by Coupling Hybrid 67Ga SPECT/CT with Bedside Percutaneous Bone Puncture. Diabet. Care 2013, 36, 2203–2210. [Google Scholar] [CrossRef]
- Scremin, O.U.; Figoni, S.F.; Norman, K.; Scremin, A.M.E.; Kunkel, C.F.; Opava-Rutter, D.; Schmitter, E.D.; Bert, A.; Mandelkern, M. Preamputation Evaluation of Lower-Limb Skeletal Muscle Perfusion with H(2) (15)O Positron Emission Tomography. Am. J. Phys. Med. Rehabil. 2010, 89, 473–486. [Google Scholar] [CrossRef]
- Christensen, N.L.; Sørensen, J.; Bouchelouche, K.; Madsen, M.A.; Buhl, C.S.; Tolbod, L.P. Repeatability of [15O]H2O PET Imaging for Lower Extremity Skeletal Muscle Perfusion: A Test-Retest Study. EJNMMI Res. 2024, 14, 11. [Google Scholar] [CrossRef]
- Depairon, M.; Depresseux, J.C.; Petermans, J.; Zicot, M. Assessment of Flow and Oxygen Delivery to the Lower Extremity in Arterial Insufficiency: A PET-Scan Study Comparison with Other Methods. Angiology 1991, 42, 788–795. [Google Scholar] [CrossRef]
- Burchert, W.; Schellong, S.; van den Hoff, J.; Meyer, G.J.; Alexander, K.; Hundeshagen, H. Oxygen-15-Water PET Assessment of Muscular Blood Flow in Peripheral Vascular Disease. J. Nucl. Med. 1997, 38, 93–98. [Google Scholar]
- Depairon, M.; Depresseux, J.C.; De Landsheere, C.; Merlo, P.; Del Fiore, G.; Quaglia, L.; Peeters, J.M.; Lamotte, D.; Zicot, M. Regional Blood Flow and Oxygen Consumption in the Leg Muscles of Normal Subjects and in Those with Arterial Insufficiency. Study of the Distribution of C15O2 and of 15O2 Using Positron Emission Tomography. J. Mal. Vasc. 1988, 13, 107–115. [Google Scholar]
- Depairon, M.; De Landsheere, C.; Merlo, P.; Del Fiore, G.; Quaglia, L.; Peters, J.M.; Lamotte, D.; Zicot, M. Effect of Exercise on the Leg Distribution of C15O2 and 15O2 in Normals and in Patients with Peripheral Ischemia: A Study Using Positron Tomography. Int. Angiol. 1988, 7, 254–257. [Google Scholar]
- Schmidt, M.A.; Chakrabarti, A.; Shamim-Uzzaman, Q.; Kaciroti, N.; Koeppe, R.A.; Rajagopalan, S. Calf Flow Reserve with H(2)(15)O PET as a Quantifiable Index of Lower Extremity Flow. J. Nucl. Med. 2003, 44, 915–919. [Google Scholar]
- Myers, K.S.; Rudd, J.H.F.; Hailman, E.P.; Bolognese, J.A.; Burke, J.; Pinto, C.A.; Klimas, M.; Hargreaves, R.; Dansky, H.M.; Fayad, Z.A. Correlation between Arterial FDG Uptake and Biomarkers in Peripheral Artery Disease. JACC Cardiovasc. Imaging 2012, 5, 38–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, J.; Li, Y.; Wu, G.; Wang, Y.; Yang, J.; Wang, M.; Cao, Z.; Li, Q.; Wang, H.; et al. Rapid Reduction in Plaque Inflammation by Sonodynamic Therapy Inpatients with Symptomatic Femoropopliteal Peripheral Artery Disease:A Randomized Controlled Trial. Int. J. Cardiol. 2021, 325, 132–139. [Google Scholar] [CrossRef]
- Chowdhury, M.M.; Tarkin, J.M.; Albaghdadi, M.S.; Evans, N.R.; Le, E.P.V.; Berrett, T.B.; Sadat, U.; Joshi, F.R.; Warburton, E.A.; Buscombe, J.R.; et al. Vascular Positron Emission Tomography and Restenosis in Symptomatic Peripheral Arterial Disease: A Prospective Clinical Study. JACC Cardiovasc. Imaging 2020, 13, 1008–1017. [Google Scholar] [CrossRef]
- Leppänen, O.; Björnheden, T.; Evaldsson, M.; Borén, J.; Wiklund, O.; Levin, M. ATP Depletion in Macrophages in the Core of Advanced Rabbit Atherosclerotic Plaques in Vivo. Atherosclerosis 2006, 188, 323–330. [Google Scholar] [CrossRef]
- Yun, M.; Yeh, D.; Araujo, L.I.; Jang, S.; Newberg, A.; Alavi, A. F-18 FDG Uptake in the Large Arteries: A New Observation. Clin. Nucl. Med. 2001, 26, 314–319. [Google Scholar] [CrossRef]
- Bural, G.G.; Torigian, D.A.; Chamroonrat, W.; Houseni, M.; Chen, W.; Basu, S.; Kumar, R.; Alavi, A. FDG-PET Is an Effective Imaging Modality to Detect and Quantify Age-Related Atherosclerosis in Large Arteries. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 562–569. [Google Scholar] [CrossRef]
- Pasha, A.K.; Moghbel, M.; Saboury, B.; Gharavi, M.H.; Blomberg, B.A.; Torigian, D.A.; Kwee, T.C.; Basu, S.; Mohler Iii, E.R.; Alavi, A. Effects of Age and Cardiovascular Risk Factors on (18)F-FDG PET/CT Quantification of Atherosclerosis in the Aorta and Peripheral Arteries. Hell. J. Nucl. Med. 2015, 18, 5–10. [Google Scholar] [CrossRef]
- Bural, G.G.; Torigian, D.; Rubello, D.; Alavi, A. Atherosclerotic 18F-FDG and MDP Uptake in Femoral Arteries, Changes with Age. Nucl. Med. Commun. 2016, 37, 833–836. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Wolpers, S.; Cyran, C.C.; Schmidt, M.; Foerster, S.; Nikolaou, K.; Reiser, M.F.; Bartenstein, P.; Hacker, M. 18F-FDG PET/CT Identifies Patients at Risk for Future Vascular Events in an Otherwise Asymptomatic Cohort with Neoplastic Disease. J. Nucl. Med. 2009, 50, 1611–1620. [Google Scholar] [CrossRef]
- de Boer, S.A.; Hovinga-de Boer, M.C.; Heerspink, H.J.L.; Lefrandt, J.D.; van Roon, A.M.; Lutgers, H.L.; Glaudemans, A.W.J.M.; Kamphuisen, P.W.; Slart, R.H.J.A.; Mulder, D.J. Arterial Stiffness Is Positively Associated with 18F-Fluorodeoxyglucose Positron Emission Tomography-Assessed Subclinical Vascular Inflammation in People with Early Type 2 Diabetes. Diabet. Care 2016, 39, 1440–1447. [Google Scholar] [CrossRef]
- Hetterich, H.; Rominger, A.; Walter, L.; Habs, M.; Volpers, S.; Hacker, M.; Reiser, M.F.; Bartenstein, P.; Saam, T. Natural History of Atherosclerotic Disease Progression as Assessed by (18)F-FDG PET/CT. Int. J. Cardiovasc. Imaging 2016, 32, 49–59. [Google Scholar] [CrossRef]
- Ishii, H.; Nishio, M.; Takahashi, H.; Aoyama, T.; Tanaka, M.; Toriyama, T.; Tamaki, T.; Yoshikawa, D.; Hayashi, M.; Amano, T.; et al. Comparison of Atorvastatin 5 and 20 Mg/d for Reducing F-18 Fluorodeoxyglucose Uptake in Atherosclerotic Plaques on Positron Emission Tomography/Computed Tomography: A Randomized, Investigator-Blinded, Open-Label, 6-Month Study in Japanese Adults Scheduled for Percutaneous Coronary Intervention. Clin. Ther. 2010, 32, 2337–2347. [Google Scholar] [CrossRef]
- Asadollahi, S.; Rojulpote, C.; Bhattaru, A.; Patil, S.; Gonuguntla, K.; Karambelkar, P.; Borja, A.J.; Vuthaluru, K.; Seraj, S.M.; Zhang, V.; et al. Comparison of Atherosclerotic Burden in Non-Lower Extremity Arteries in Patients with and without Peripheral Artery Disease Using 18 F-NaF-PET/CT Imaging. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 272–278. [Google Scholar]
- Chou, T.H.; Wynveen, M.K.; Rimmerman, E.T.; Patel, S.; Go, M.R.; Stacy, M.R. Detection of Multivessel Calcific Disease Progression in a Patient with Chronic Limb-Threatening Ischemia Using Fluorine-18 Sodium Fluoride Positron Emission Tomography Imaging. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101137. [Google Scholar] [CrossRef]
- Chou, T.H.; Rimmerman, E.T.; Patel, S.; Wynveen, M.K.; Eisert, S.N.; Musini, K.N.; Janse, S.A.; Bobbey, A.J.; Sarac, T.P.; Atway, S.A.; et al. Vessel-by-Vessel Analysis of Lower Extremity 18F-NaF PET/CT Imaging Quantifies Diabetes- and Chronic Kidney Disease-Induced Active Microcalcification in Patients with Peripheral Arterial Disease. EJNMMI Res. 2023, 13, 3. [Google Scholar] [CrossRef]
- Chou, T.-H.; Nabavinia, M.; Tram, N.K.; Rimmerman, E.T.; Patel, S.; Musini, K.N.; Eisert, S.N.; Wolfe, T.; Wynveen, M.K.; Matsuzaki, Y.; et al. Quantification of Skeletal Muscle Perfusion in Peripheral Artery Disease Using 18F-Sodium Fluoride Positron Emission Tomography Imaging. JAHA 2024, 13, e031823. [Google Scholar] [CrossRef]
- Derlin, T.; Richter, U.; Bannas, P.; Begemann, P.; Buchert, R.; Mester, J.; Klutmann, S. Feasibility of 18F-Sodium Fluoride PET/CT for Imaging of Atherosclerotic Plaque. J. Nucl. Med. 2010, 51, 862–865. [Google Scholar] [CrossRef]
- Janssen, T.; Bannas, P.; Herrmann, J.; Veldhoen, S.; Busch, J.D.; Treszl, A.; Münster, S.; Mester, J.; Derlin, T. Association of Linear 18F-Sodium Fluoride Accumulation in Femoral Arteries as a Measure of Diffuse Calcification with Cardiovascular Risk Factors: A PET/CT Study. J. Nucl. Cardiol. 2013, 20, 569–577. [Google Scholar] [CrossRef]
- Takx, R.A.P.; van Asperen, R.; Bartstra, J.W.; Zwakenberg, S.R.; Wolterink, J.M.; Celeng, C.; de Jong, P.A.; Beulens, J.W. Determinants of 18F-NaF Uptake in Femoral Arteries in Patients with Type 2 Diabetes Mellitus. J. Nucl. Cardiol. 2021, 28, 2700–2705. [Google Scholar] [CrossRef]
- Derlin, T.; Habermann, C.R.; Lengyel, Z.; Busch, J.D.; Wisotzki, C.; Mester, J.; Pávics, L. Feasibility of 11C-Acetate PET/CT for Imaging of Fatty Acid Synthesis in the Atherosclerotic Vessel Wall. J. Nucl. Med. 2011, 52, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Quimby, E.H. The Use of Radioactive Sodium in the Study of Peripheral Vascular Disease. Ann. Surg. 1947, 125, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kety, S.S. Measurement of regional circulation by the local clearance of radioactive sodium. Am. Heart J. 1949, 38, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Wagner, H.N.; Zuidema, G.D. New Method for Studying Peripheral Circulation in Man. Arch. Surg. 1965, 91, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Mutluoglu, M.; Sivrioglu, A.K.; Eroglu, M.; Uzun, G.; Turhan, V.; Ay, H.; Lipsky, B.A. The Implications of the Presence of Osteomyelitis on Outcomes of Infected Diabetic Foot Wounds. Scand. J. Infect. Dis. 2013, 45, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.F. Mechanism of Gallium-67 Accumulation in Inflammatory Lesions. J. Nucl. Med. 1985, 26, 88–92. [Google Scholar] [PubMed]
- Pande, R.L.; Park, M.-A.; Perlstein, T.S.; Desai, A.S.; Doyle, J.; Navarrete, N.; Copeland-Halperin, R.S.; Redline, W.; Di Carli, M.F.; Creager, M.A. Impaired Skeletal Muscle Glucose Uptake by [18F]Fluorodeoxyglucose-Positron Emission Tomography in Patients with Peripheral Artery Disease and Intermittent Claudication. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.R. Molecular Imaging of Lower Extremity Peripheral Arterial Disease: An Emerging Field in Nuclear Medicine. Front. Med. 2022, 8, 793975. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.R.; Paeng, J.C.; Sinusas, A.J. The Role of Molecular Imaging in the Evaluation of Myocardial and Peripheral Angiogenesis. Ann. Nucl. Med. 2015, 29, 217–223. [Google Scholar] [CrossRef]
- Willmann, J.K.; Chen, K.; Wang, H.; Paulmurugan, R.; Rollins, M.; Cai, W.; Wang, D.S.; Chen, I.Y.; Gheysens, O.; Rodriguez-Porcel, M.; et al. Monitoring of the Biological Response to Murine Hindlimb Ischemia with 64Cu-Labeled Vascular Endothelial Growth Factor-121 Positron Emission Tomography. Circulation 2008, 117, 915–922. [Google Scholar] [CrossRef]
- Jeong, J.M.; Hong, M.K.; Chang, Y.S.; Lee, Y.-S.; Kim, Y.J.; Cheon, G.J.; Lee, D.S.; Chung, J.-K.; Lee, M.C. Preparation of a Promising Angiogenesis PET Imaging Agent: 68Ga-Labeled c(RGDyK)-Isothiocyanatobenzyl-1,4,7-Triazacyclononane-1,4,7-Triacetic Acid and Feasibility Studies in Mice. J. Nucl. Med. 2008, 49, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, J.; Konopka, C.J.; Schuh, S.; Bouvin, H.; Cole, J.A.; Huntsman, H.D.; Kilian, K.A.; Dobrucki, I.T.; Boppart, M.D.; Dobrucki, L.W. Multimodal Assessment of Mesenchymal Stem Cell Therapy for Diabetic Vascular Complications. Theranostics 2017, 7, 3876–3888. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Zhang, Y.; Hong, H.; Hacker, T.A.; Valdovinos, H.F.; Zagzebski, J.A.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of Angiogenesis in a Murine Hindlimb Ischemia Model with 64Cu-Labeled TRC105. Mol. Pharm. 2013, 10, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Hong, H.; Koch, J.M.; Valdovinos, H.F.; Hacker, T.A.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Pravastatin Stimulates Angiogenesis in a Murine Hindlimb Ischemia Model: A Positron Emission Tomography Imaging Study with 64 Cu-NOTA-TRC105. Am. J. Transl. Res. 2014, 6, 54–63. [Google Scholar]
- Lareyre, F.; Behrendt, C.A.; Chaudhuri, A.; Lee, R.; Carrier, M.; Adam, C.; Lê, C.D.; Raffort, J. Applications of Artificial Intelligence for Patients with Peripheral Artery Disease. J. Vasc. Surg. 2023, 77, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial Intelligence in Precision Cardiovascular Medicine. JACC 2017, 69, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ara, L.; Ding, H.; Rollins, D.; Motaganahalli, R.; Sawchuk, A.P. Computational Methods to Automate the Initial Interpretation of Lower Extremity Arterial Doppler and Duplex Carotid Ultrasound Studies. J. Vasc. Surg. 2021, 74, 988–996. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, Q.; Zhou, H.; Zhang, H.; Cheng, P.; Ding, M.; Xu, X.; Zhang, X. Deep Learning-Based Classification of Lower Extremity Arterial Stenosis in Computed Tomography Angiography. Eur. J. Radiol. 2021, 136, 109528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Conlin, C.C.; Li, X.; Layec, G.; Chang, K.; Kalpathy-Cramer, J.; Lee, V.S. Exercise-Induced Calf Muscle Hyperemia: Rapid Mapping of Magnetic Resonance Imaging Using Deep Learning Approach. Physiol. Rep. 2020, 8, e14563. [Google Scholar] [CrossRef]
- Gao, J.M.; Ren, Z.H.; Pan, X.; Chen, Y.X.; Zhu, W.; Li, W.; Yang, Y.X.; Fu, G.X. Identifying Peripheral Arterial Disease in the Elderly Patients Using Machine-Learning Algorithms. Aging Clin. Exp. Res. 2022, 34, 679–685. [Google Scholar] [CrossRef]
- Cox, M.; Reid, N.; Panagides, J.C.; Di Capua, J.; DeCarlo, C.; Dua, A.; Kalva, S.; Kalpathy-Cramer, J.; Daye, D. Interpretable Machine Learning for the Prediction of Amputation Risk Following Lower Extremity Infrainguinal Endovascular Interventions for Peripheral Arterial Disease. Cardiovasc. Interv. Radiol. 2022, 45, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Normahani, P.; Sounderajah, V.; Mandic, D.; Jaffer, U. Machine Learning-Based Classification of Arterial Spectral Waveforms for the Diagnosis of Peripheral Artery Disease in the Context of Diabetes: A Proof-of-Concept Study. Vasc. Med. 2022, 27, 450–456. [Google Scholar] [CrossRef] [PubMed]
| Modality | Radioisotope | Physiologic Target | Reference |
|---|---|---|---|
| SPECT | 201Tl | perfusion | [1,8,9,10,11,12] |
| 99mTc-sestamibi | perfusion | [1,11,12] | |
| 99mTc-tetrofosmin | perfusion | [1,9,11,12,13,14,15,16] | |
| 99mTc-diphosphonate + 111In-WBC | osteomyelitis, infection | [17,18,19] | |
| 99mTc-antigranulocyte antibodies | osteomyelitis, infection | [20] | |
| 67Ga-citrate | osteomyelitis, infection | [21,22,23,24] | |
| PET | H215O | perfusion | [25,26,27,28,29,30,31] |
| 15O2 | perfusion | [27,29,30] | |
| C15O2 | perfusion | [29,30] | |
| 18F-FDG | metabolism, atherosclerosis | [32,33,34,35,36,37,38,39,40,41,42,43] | |
| 18F-NaF | perfusion, atherosclerosis | [34,44,45,46,47,48,49,50] | |
| 11C-acetate | atherosclerosis | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimmerman, E.T.; Stacy, M.R. Applications of SPECT and PET Imaging for the Physiological Evaluation of Lower Extremity Peripheral Artery Disease. Int. J. Mol. Sci. 2024, 25, 7474. https://doi.org/10.3390/ijms25137474
Rimmerman ET, Stacy MR. Applications of SPECT and PET Imaging for the Physiological Evaluation of Lower Extremity Peripheral Artery Disease. International Journal of Molecular Sciences. 2024; 25(13):7474. https://doi.org/10.3390/ijms25137474
Chicago/Turabian StyleRimmerman, Eleanor T., and Mitchel R. Stacy. 2024. "Applications of SPECT and PET Imaging for the Physiological Evaluation of Lower Extremity Peripheral Artery Disease" International Journal of Molecular Sciences 25, no. 13: 7474. https://doi.org/10.3390/ijms25137474






