Sws2 Gene Positively Regulates Melanin Production in Plectropomus leopardus Skin via Direct Regulation of the Synthesis of Retinoic Acid
Abstract
1. Introduction
2. Results
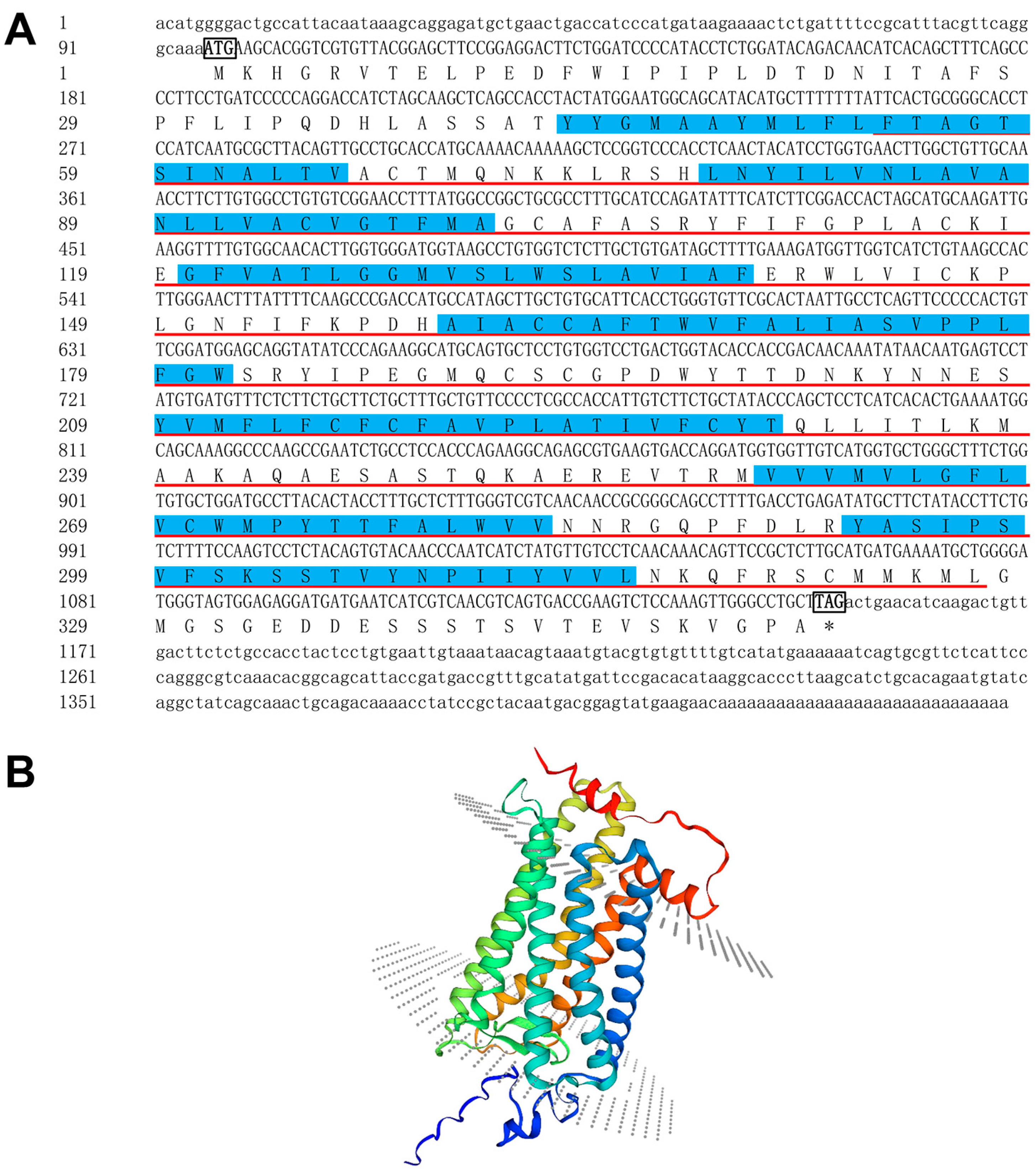
2.1. P. leopardus sws2 cDNA Cloning and Sequence Analysis
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
2.3. Expression Levels of sws2 in Different Tissues
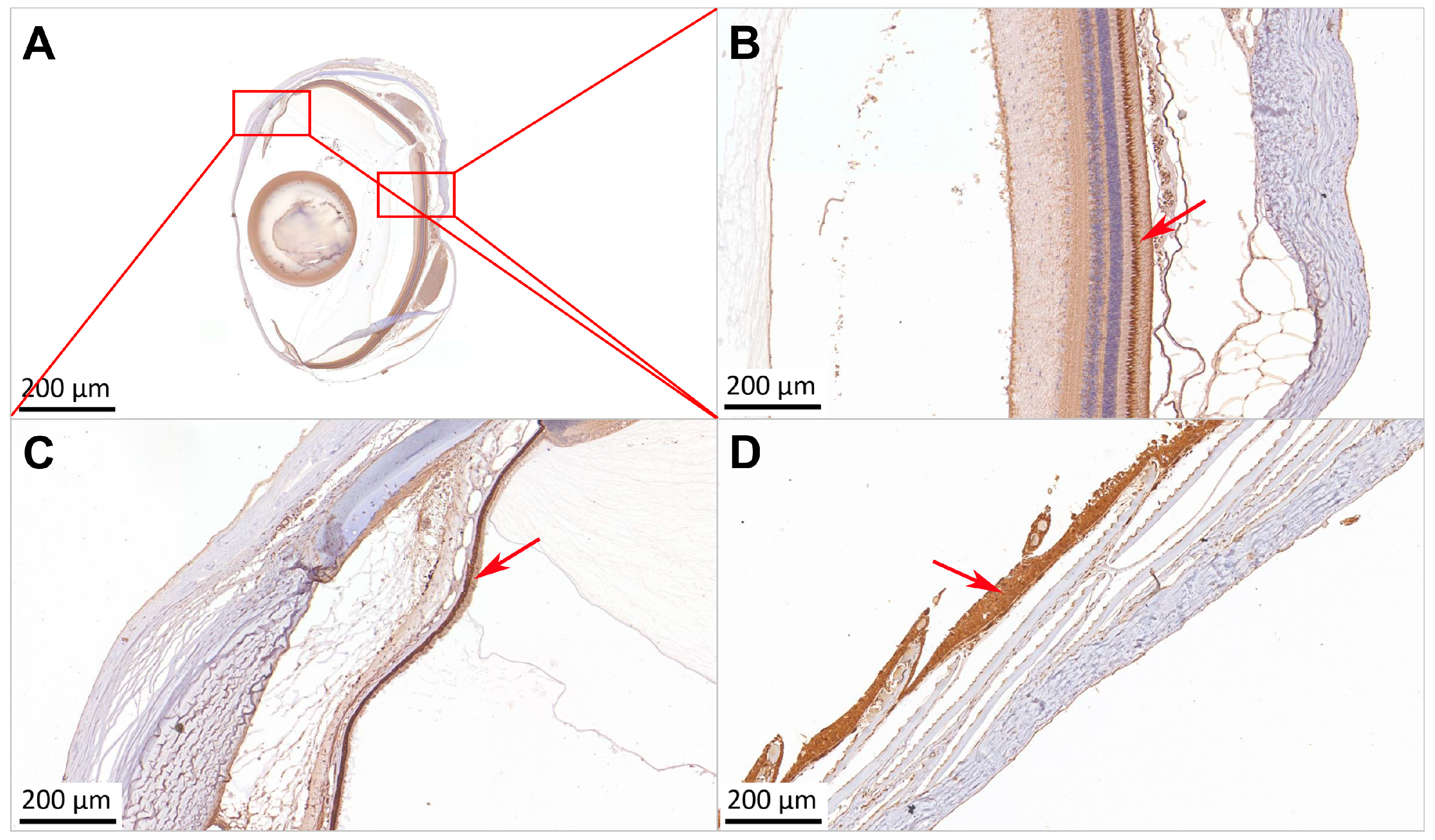
2.4. Localization of Sws2 in the Eye and Skin of P. leopardus
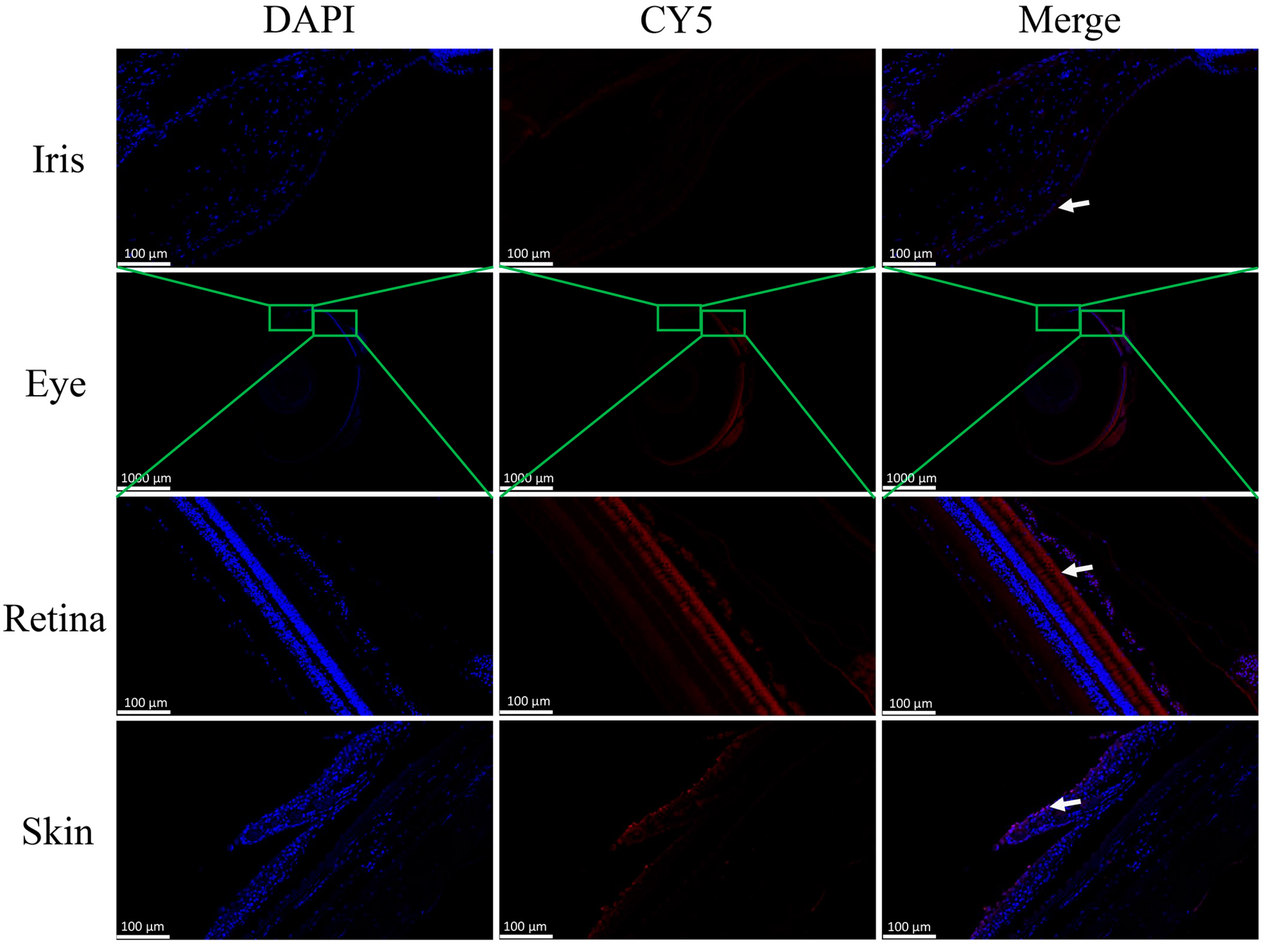
2.5. In Situ Hybridization of sws2 in the Eye and Skin of P. leopardus
2.6. Alterations in sws2 Expression Levels and Genes Related to Skin Color following sws2 Knockdown
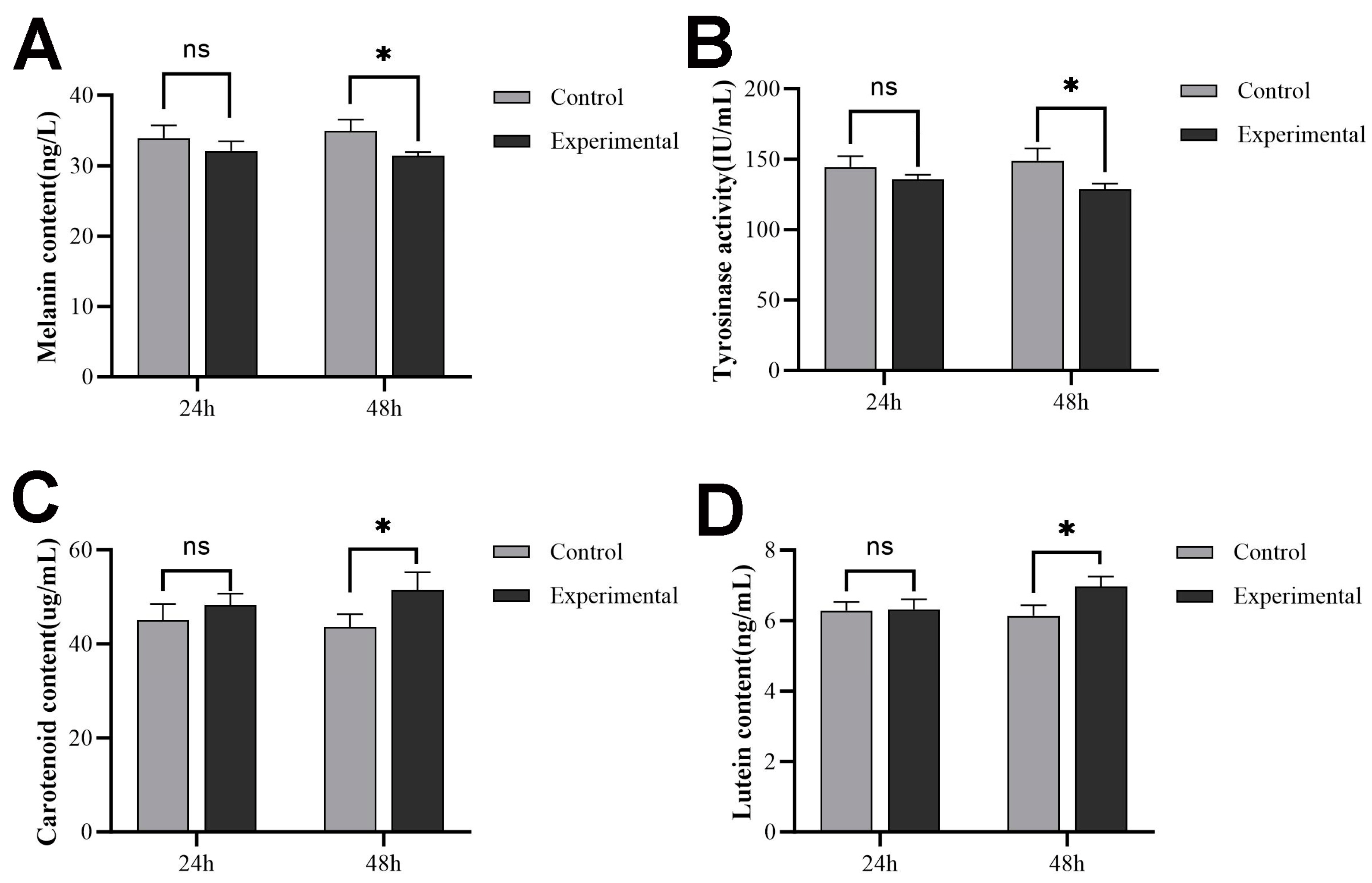
2.7. Pigment Content and Tyrosinase Activity after sws2 Knockdown
2.8. Expression Levels of Skin-Color-Related Genes after Retinoic Acid Treatment
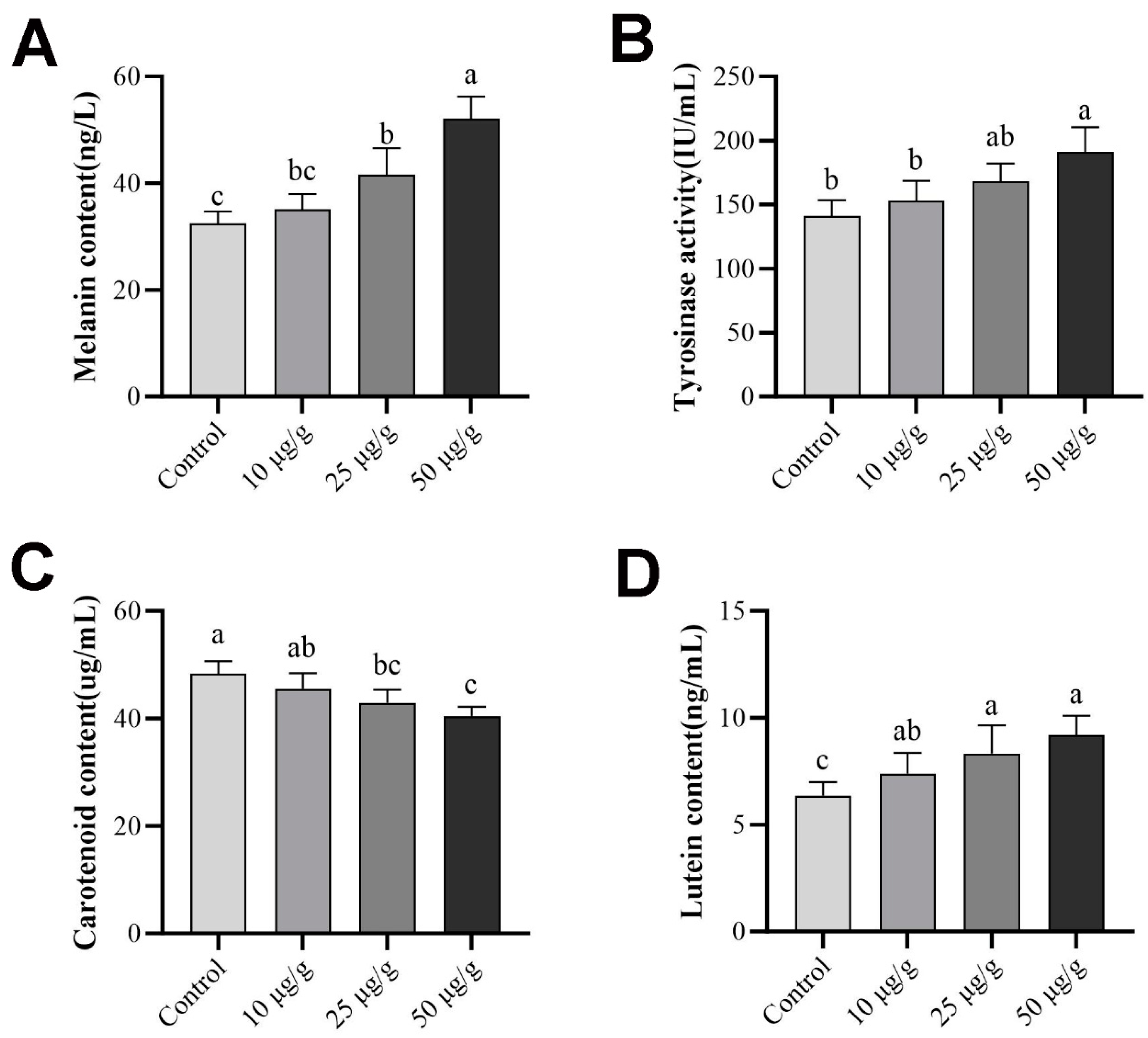
2.9. Pigment Content and Tyrosinase Activity after Retinoic Acid Treatment
3. Discussion
4. Materials and Methods
4.1. Fish
4.2. Fish Sampling, RNA Extraction, and First-Strand cDNA Synthesis
4.3. Molecular Cloning and Bioinformatics Analysis
4.4. qRT-PCR (Quantitative Real-Time PCR) Analysis
4.5. Immunohistochemistry
4.6. Fluorescence In Situ Hybridization
4.7. Effect of Sws2 Knockdown on Skin Color
4.8. Retinoic Acid Treatment
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, M.K.; Lu, G.Q.; Yin, H.R.; Wang, L.M.; Atuganile, M.; Dong, Z.J. Fish pigmentation and coloration: Molecular mechanisms and aquaculture perspectives. Rev. Aquacult. 2021, 13, 2395–2412. [Google Scholar] [CrossRef]
- Duarte, R.C.; Stevens, M.; Flores, A.A.V. Shape, colour plasticity, and habitat use indicate morph-specific camouflage strategies in a marine shrimp. BMC. Evol. Biol. 2016, 16, 218. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Mastin, A.; Dufaut, C.; Mondal, D.; Benvenuto, C. Background matching in the brown shrimp Crangon crangon: Adaptive camouflage and behavioural-plasticity. Sci. Rep. 2018, 8, 3292. [Google Scholar] [CrossRef]
- McLean, E. Background color and cultured invertebrates-A review. Aquaculture 2021, 537, 736523. [Google Scholar] [CrossRef]
- McLean, E. Fish tank color: An overview. Aquaculture 2021, 530, 35750. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Slominski, A.T.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominsk, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Rennison, D.J.; Owens, G.L.; Taylor, J.S. Opsin gene duplication and divergence in ray-finned fish. Mol. Phylogenet. Evol. 2012, 62, 986–1008. [Google Scholar] [CrossRef]
- Liang, Q.L.; Afriyie, G.; Chen, Z.Z.; Xu, Z.M.; Dong, Z.D.; Guo, Y.S.; Wang, Z.D. Analysis of opsin gene family of Crimson snapper (Lutjanus erythropterus). Gene 2021, 807, 145960. [Google Scholar] [CrossRef]
- Liu, C.W.; Yu, J.; Wang, Z.D.; Guo, Y.S. Progress on fish opsin research. Oceanol. Et Limnol. Sin. 2015, 46, 1564–1570. [Google Scholar]
- Moore, H.A.; Whitmore, D. Circadian rhythmicity and light sensitivity of the zebrafish brain. PLoS ONE 2014, 9, e86176. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zhao, W.; Li, Z. Progress in the studies on opsins. Chem. Life 2009, 29, 440–443. [Google Scholar]
- Sakmar, T.P.; Franke, R.R.; Khorana, H.G. Glutamic acid-113serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc. Natl. Acad. Sci. USA 1989, 86, 8309–8313. [Google Scholar] [CrossRef]
- Ward, M.N.; Churcher, A.M.; Dick, K.J.; Laver, C.R.; Owens, G.L.; Polack, M.D.; Ward, P.R.; Breden, F.; Taylor, J.S. The molecular basis of color vision in colorful fish: Four long wave-sensitive (LWS) opsins in guppies (Poecilia reticulata) are defined by amino acid substitutions at key functional sites. BMC. Evol. Biol. 2008, 8, 210. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zhang, W.; Du, X.X.; Liu, Y.X.; Qu, J.B.; Liu, X.B.; Liu, J.X.; Zhang, Q.Q. Genome-wide identification of nonvisual opsin family reveals amplification of RPE-retinal G protein receptor gene (RGR) and offers novel insights into functions of RGR(s) in Paralichthys olivaceus (Paralichthyidae, Teleostei). J. Exp. Zool. Part B Mol. Dev. Evol. 2020, 334, 25–36. [Google Scholar] [CrossRef]
- Chen, S.C.; Robertson, R.M.; Hawryshyn, C.W. Possible involvement of cone opsins in distinct photoresponses of intrinsically photosensitive dermal chromatophores in tilapia Oreochromis niloticus. PLoS ONE 2013, 8, e70342. [Google Scholar] [CrossRef]
- Chen, S.C.; Xiao, C.; Troje, N.F.; Robertson, R.M.; Hawryshyn, C.W. Functional characterisation of the chromatically antagonistic photosensitive mechanism of erythrophores in the tilapia Oreochromis niloticus. J. Exp. Biol. 2015, 218, 748–756. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of vertebrate visual pigments. Vis. Res. 2008, 48, 2022–2041. [Google Scholar] [CrossRef]
- Cheng, C.H.; Flamarique, I.N. Opsin expression: New approach to understanding visual sensitivity and spectral diversity in deep-sea fishes. BioEssays 2004, 26, 930–941. [Google Scholar]
- Valen, R.; Edvardsen, R.B.; Søviknes, A.M.; Drivenes, Ø.; Helvik, J.V. Molecular evidence that only two opsin subfamilies, the blue light-(SWS2) and green light-sensitive (RH2), drive color vision in Atlantic cod (Gadus morhua). PLoS ONE 2014, 9, e115436. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, H.; Luo, D.; Chen, S. Evolution of opsins and visual function of ultraviolet sensitivity in vertebrates. Hereditas 2017, 155, 24. [Google Scholar]
- Kanazawa, N.; Goto, M.; Harada, Y.; Takimoto, C.; Sasaki, Y.; Uchikawa, T.; Kamei, Y.; Matsuo, M.; Fukamachi, S. Changes in a cone opsin repertoire affect color-dependent social behavior in medaka but not behavioral photosensitivity. Front. Genet. 2020, 11, 00801. [Google Scholar] [CrossRef]
- Shao, C.; Bao, B.; Xie, Z.; Chen, X.; Li, B.; Jia, X.; Yao, Q.; Orti, G.; Li, W.; Li, X.; et al. The genome and transcriptome of Japanese flounder provide insights into flatfish asymmetry. Nat. Genet. 2017, 49, 119–124. [Google Scholar] [CrossRef]
- Axel, D. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Osanai, M. Expression of the retinoic acid-metabolizing enzyme cyp26a1 limits programmed cell death. Mol. Pharmacol. 2005, 67, 1808–1817. [Google Scholar] [CrossRef]
- He, C. The Study of Retinoic Acid Regulating Tyrosinase Expression in Crassostrea gigas; Ludong University: Yantai, China, 2019. [Google Scholar]
- Song, F.B.; Shi, L.P.; Yao, F.C.; Gu, Y.; Zheng, D.; Zhang, W.W.; Liang, Y.S.; Zhang, K.X.; Yang, M.; Wang, L.; et al. The Effect of Background Color on Skin Color Variation of Juvenile Plectropomus leopardus. Animals 2022, 12, 3349. [Google Scholar] [CrossRef]
- Song, F.; Zheng, D.; Yang, Z.; Shi, L.; Lu, X.; Yao, F.; Liang, H.; Wang, L.; Wang, X.; Chen, H.; et al. Weighted correlation network analysis of the genes in the eyes of juvenile Plectropomus leopardus provide novel insights into the molecular mechanisms of the adaptation to the background color. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101123. [Google Scholar] [CrossRef]
- Härer, A.; Torres-Dowdall, J.; Meyer, A. Rapid adaptation to a novel light environment: The importance of ontogeny and phenotypic plasticity in shaping the visual system of Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.). Mol. Ecol. 2017, 26, 5582–5593. [Google Scholar] [CrossRef]
- Marques, D.A.; Taylor, J.S.; Jones, F.C.; Palma, F.D.; Kingsley, D.M.; Reimchen, T.E. Convergent evolution of SWS2 opsin facilitates adaptive radiation of three spine stickleback into different light environments. PLoS. Biol. 2017, 15, e2001627. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-L.; Liang, X.-F.; Li, L.; Wu, J.; Lu, K. Genome-wide identification and expression patterns of opsin genes during larval development in Chinese perch (Siniperca chuatsi). Gene 2022, 825, 146434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Zhang, W.; Du, X.; Liu, J. Benthic visual adaptation by fine-tuning light sensitivity in Japanese flounder (Paralichthys olivaceus). Front. Mar. Sci. 2022, 9, 1019660. [Google Scholar] [CrossRef]
- Lu, K.; Wu, J.; Tang, S.; Jia, X.; Liang, X.-F. Knockout of sws2a and sws2b in Medaka (Oryzias latipes) reveals their roles in regulating vision-guided behavior and eye development. Int. J. Mol. Sci. 2023, 24, 8786. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, H.; Bao, B. Cloning of SWS1 gene and expression of five opsin genes in larval flounder Paralichthys olivaceus. J. Shanghai Ocean. Univ. 2015, 24, 641–649. [Google Scholar]
- Chang, C.H.; Catchen, J.; Moran, R.L.; Rivera-Colón, A.G.; Wang, Y.C.; Fuller, R.C. Sequence Analysis and Ontogenetic Expression Patterns of Cone Opsin Genes in the Bluefin Killifish (Lucania goodei). J. Hered. 2021, 112, 357–366. [Google Scholar]
- Horth, L. Sensory genes and mate choice: Evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors. Genomics 2007, 90, 159–175. [Google Scholar] [PubMed]
- Carleton, K.L.; Escobar-Camacho, D.; Stieb, S.M.; Cortesi, F.; Marshall, N.J. Seeing the rainbow: Mechanisms underlying spectral sensitivity in teleost fishes. J. Exp. Biol. 2020, 223, 193334. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.N. Light and photoreception: Visual pigments and photoreception. In Adaptive Mechanisms in the Ecology of Vision; Springer: Torregrande, Oristano, Italy, 1999; pp. 25–42. [Google Scholar]
- Yokoyama, S. Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 2000, 19, 385–419. [Google Scholar] [PubMed]
- Palczewski, K.; Kiser, P.D. Shedding new light on the generation of the visual chromophore. Proc. Natl. Acad. Sci. USA 2020, 117, 19629–19638. [Google Scholar] [CrossRef]
- Moldstad, A.J. Development of Vision and the Effect of Spectral Environment on the Cone Photoreceptor Mosaic of the Bluefin Killifish, Lucania goodei; Florida State University: Tallahassee, FL, USA, 2008. [Google Scholar]
- Carleton, K. Cichlid fish visual systems: Mechanisms of spectral tuning. Integr. Zool. 2009, 4, 75–86. [Google Scholar]
- Padhi, N.; Jena, S.K.; Ail, S.K.S.; Ferosekhan, S.; Sahoo, S.N.; Udit, U.K.; Bairwa, M.K.; Swain, S.K. Does tank background colour influence the growth, survival, and carotenoid content in fishes? An illustration in filament barb, Dawkinsia filamentosa (Va-lenciennes, 1844). Aquaculture 2022, 560, 738536. [Google Scholar] [CrossRef]
- Sundvold, H.; Helgeland, H.; Baranski, M.; Omholt, S.W.; Våge, D.I. Characterisation of a novel paralog of scavenger re-ceptor class B member I (SCARB1) in Atlantic salmon (Salmo salar). BMC Genet. 2011, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, H.P.; Zhang, H.K.; Deng, L.H.; Liu, W.H.; Wang, S.Q.; Meng, F.; Wang, Y.J.; Guo, Z.C.; Li, S.K.; et al. A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration. BMC Genom. 2015, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, T.L.; Xu, G.L.; Xing, W.; Ma, Z.H.; Jiang, N.; Luo, L. Mechanisms underlying carotenoid-based coloration in fishes. J. Shanghai Ocean. Univ. 2018, 27, 206–212. [Google Scholar]
- Qiu, C.; Bao, B. Effects of UV illumination on number of melanocytes and expression of related genes in sws1 mutant zebrafish. J. Shanghai Ocean. Univ. 2022, 31, 1–10. [Google Scholar]
- Patel, D.; Barnes, J.E.; Davies, W.I.L.; Stenkamp, D.L.; Patel, J.S. Short-wavelength-sensitive 2 (Sws2) visual photopigment models combined with atomistic molecular simulations to predict spectral peaks of absorbance. PLoS Comput. Biol. 2020, 16, e1008212. [Google Scholar] [CrossRef]




| Primer | Sequence (5′-3′) | Application | Gene Name | Accession Number |
|---|---|---|---|---|
| sws2 RACE F1 (first) | TCTTTGGGTCGTCAACAACCG | sws2 cloning | - | - |
| sws2 RACE R1 (first) | GAGGTGCCCGCAGTGAATAA | - | - | |
| sws2 RACE F2 (nested) | GCGGGCAGCCTTTTGACCT | - | - | |
| sws2 RACE R2 (nested) | AGGAAGGGGCTGAAAGCTGT | - | - | |
| mc1r F | TGCCAGAACCACCAGGAT | Quantitative RT-PCR | melanocortin 1 receptor | XM042485859.1 |
| mc1r R | ACGGAAACGACCAACAGG | |||
| sws2 F | GTTGTCGGTGGTGTACCAGT | - | - | |
| sws2 R | GGGTGTTCGCACTAATTGCC | - | - | |
| tyr F | GTCTTCAACATCCTCAGCGGT | tyrosinase | XM042486951.1 | |
| tyr R | GGTCGCATAGACAGTGCTTCC | |||
| tyrp1 F | CGTAAGAGTAGCCAAGATTTTCA | tyrosinase related protein 1 | Transcriptome data | |
| tyrp1 R | GTTGAGGAGACACAGTCCAGAT | |||
| pomc F | GTCGAGATCTGACGGAGGAG | proopiomelanocortin | Transcriptome data | |
| pomc R | AGTCAGTGCTGGGAACATCC | |||
| Scarb1 F | ACCAGTCCGCTGTCATAACC | scavenger receptor class B, member 1 | XM042509253.1 | |
| Scarb1 R | CACCGTGTCCTACAGGGAGT | |||
| β-actin F | TCTGGGCAACGGAACCTCT | beta-actin mRNA | OK483033.1 | |
| β-actin R | CACCACAGCCGAGAGGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Chen, H.; Wang, X.; Zhang, Y.; Tan, Y.; Wang, L.; Sun, J.; Luo, J.; Song, F. Sws2 Gene Positively Regulates Melanin Production in Plectropomus leopardus Skin via Direct Regulation of the Synthesis of Retinoic Acid. Int. J. Mol. Sci. 2024, 25, 7513. https://doi.org/10.3390/ijms25147513
Yu H, Chen H, Wang X, Zhang Y, Tan Y, Wang L, Sun J, Luo J, Song F. Sws2 Gene Positively Regulates Melanin Production in Plectropomus leopardus Skin via Direct Regulation of the Synthesis of Retinoic Acid. International Journal of Molecular Sciences. 2024; 25(14):7513. https://doi.org/10.3390/ijms25147513
Chicago/Turabian StyleYu, Haoran, Huapeng Chen, Xinxin Wang, Yichun Zhang, Yafang Tan, Lei Wang, Junlong Sun, Jian Luo, and Feibiao Song. 2024. "Sws2 Gene Positively Regulates Melanin Production in Plectropomus leopardus Skin via Direct Regulation of the Synthesis of Retinoic Acid" International Journal of Molecular Sciences 25, no. 14: 7513. https://doi.org/10.3390/ijms25147513
APA StyleYu, H., Chen, H., Wang, X., Zhang, Y., Tan, Y., Wang, L., Sun, J., Luo, J., & Song, F. (2024). Sws2 Gene Positively Regulates Melanin Production in Plectropomus leopardus Skin via Direct Regulation of the Synthesis of Retinoic Acid. International Journal of Molecular Sciences, 25(14), 7513. https://doi.org/10.3390/ijms25147513






