Trigonometric Bundling Disulfide Unit Starship Synergizes More Effectively to Promote Cellular Uptake
Abstract
1. Introduction
2. Results and Discussion
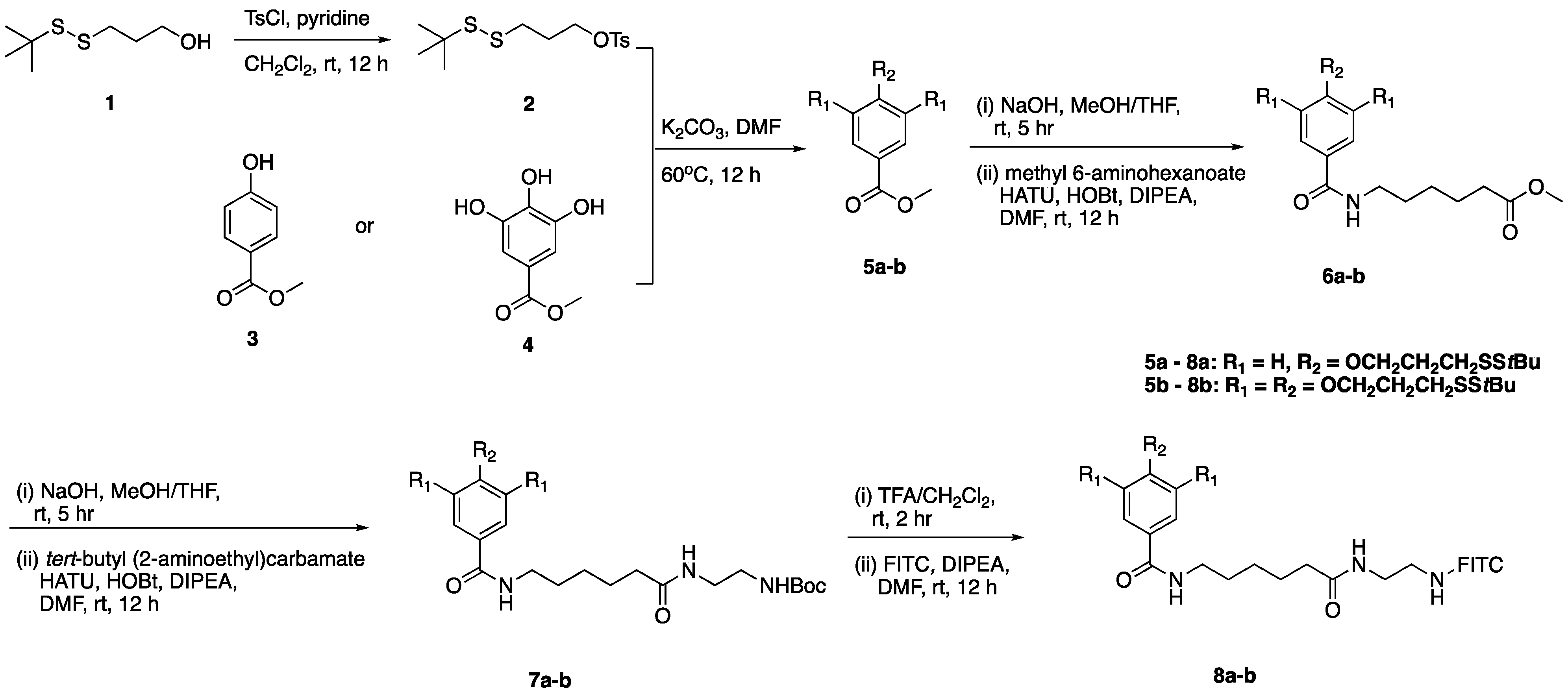
2.1. Chemistry
2.2. Biological Activity
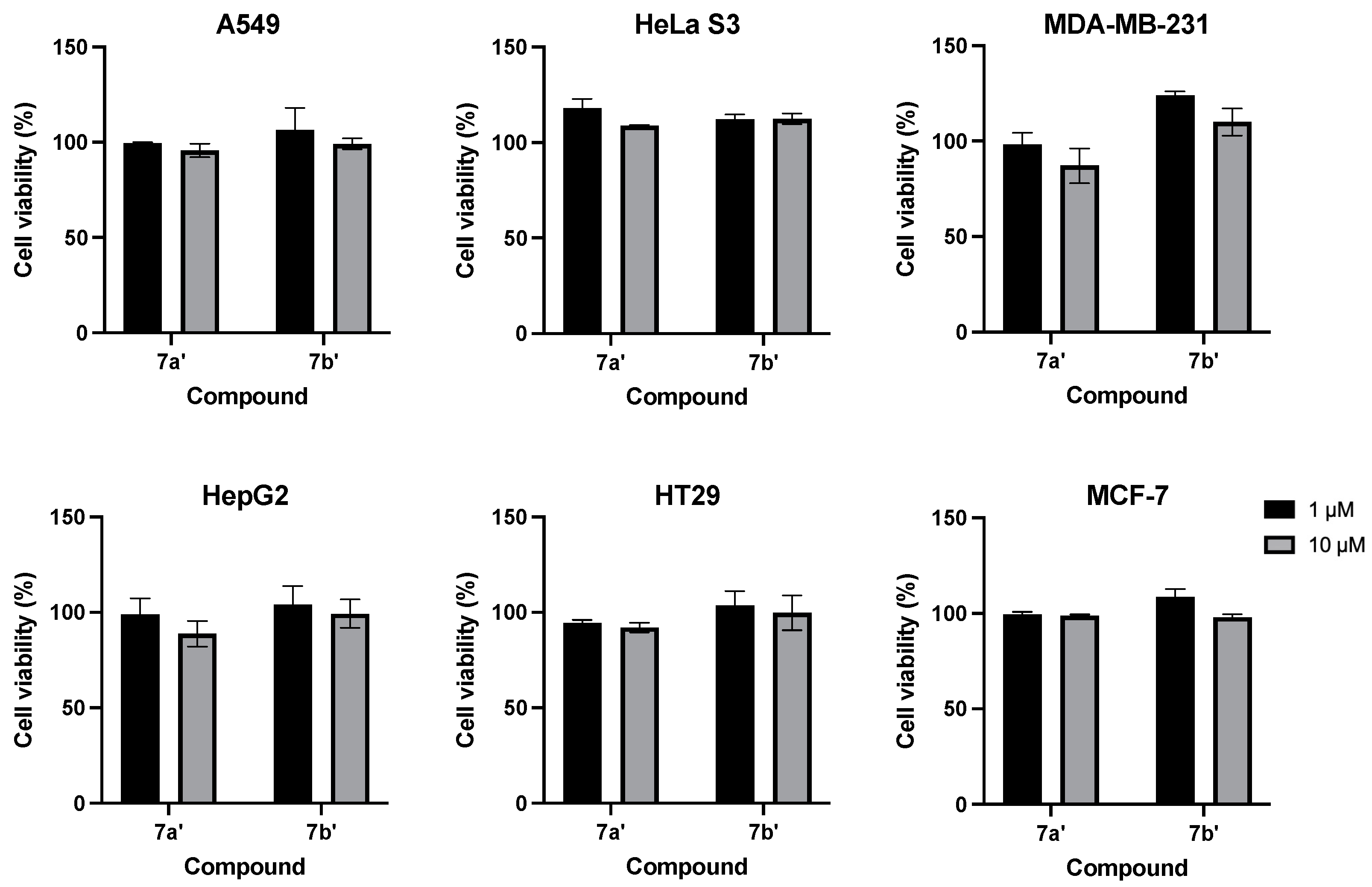
2.2.1. CCK-8 Assay
2.2.2. Time-Dependent Cellular Uptake of SS1/3-FITC Probe
2.2.3. Concentration-Dependent Cellular Uptake of SS1/3-FITC Probe
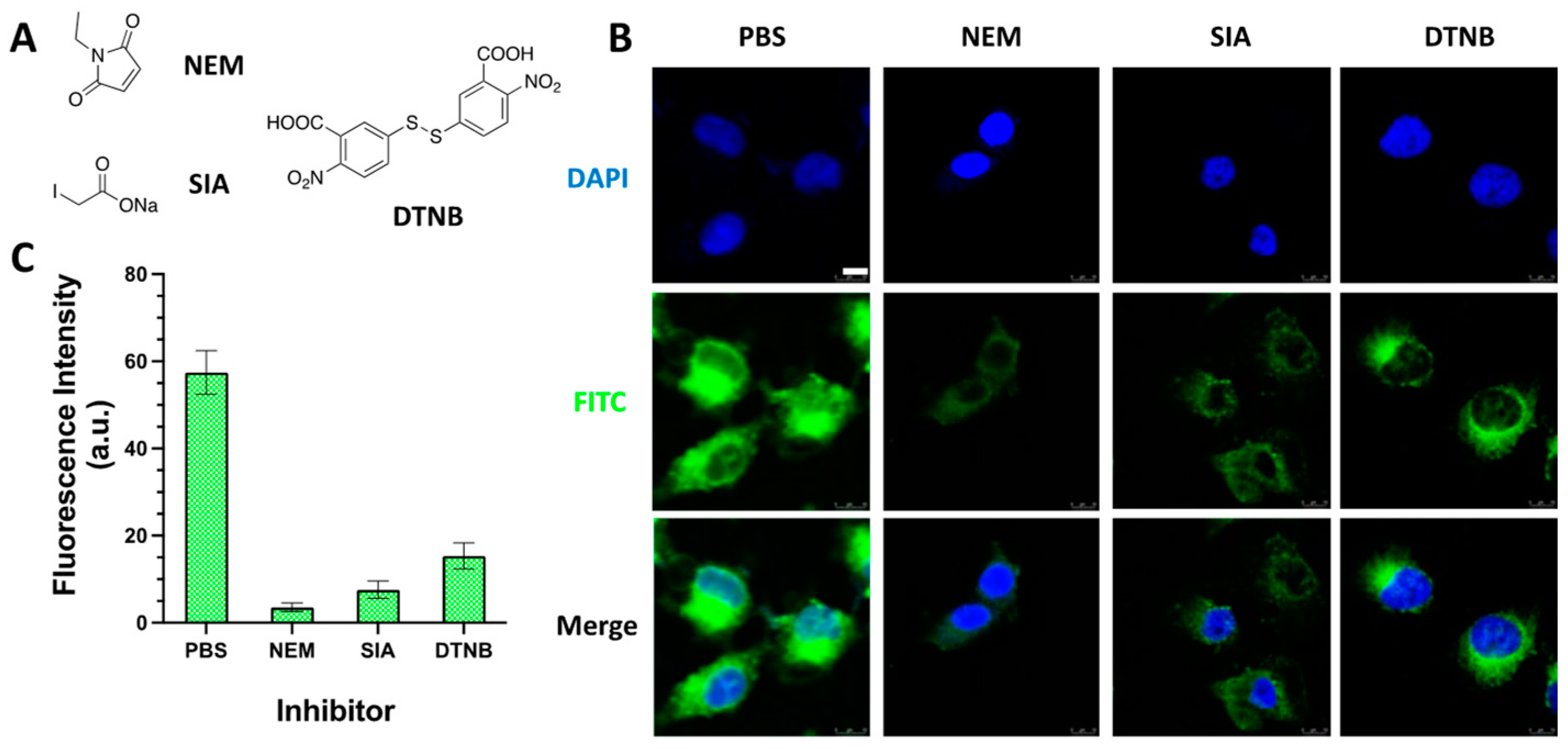
2.2.4. Cellular Uptake of SS3-FITC Probe with Thiols Inhibitors
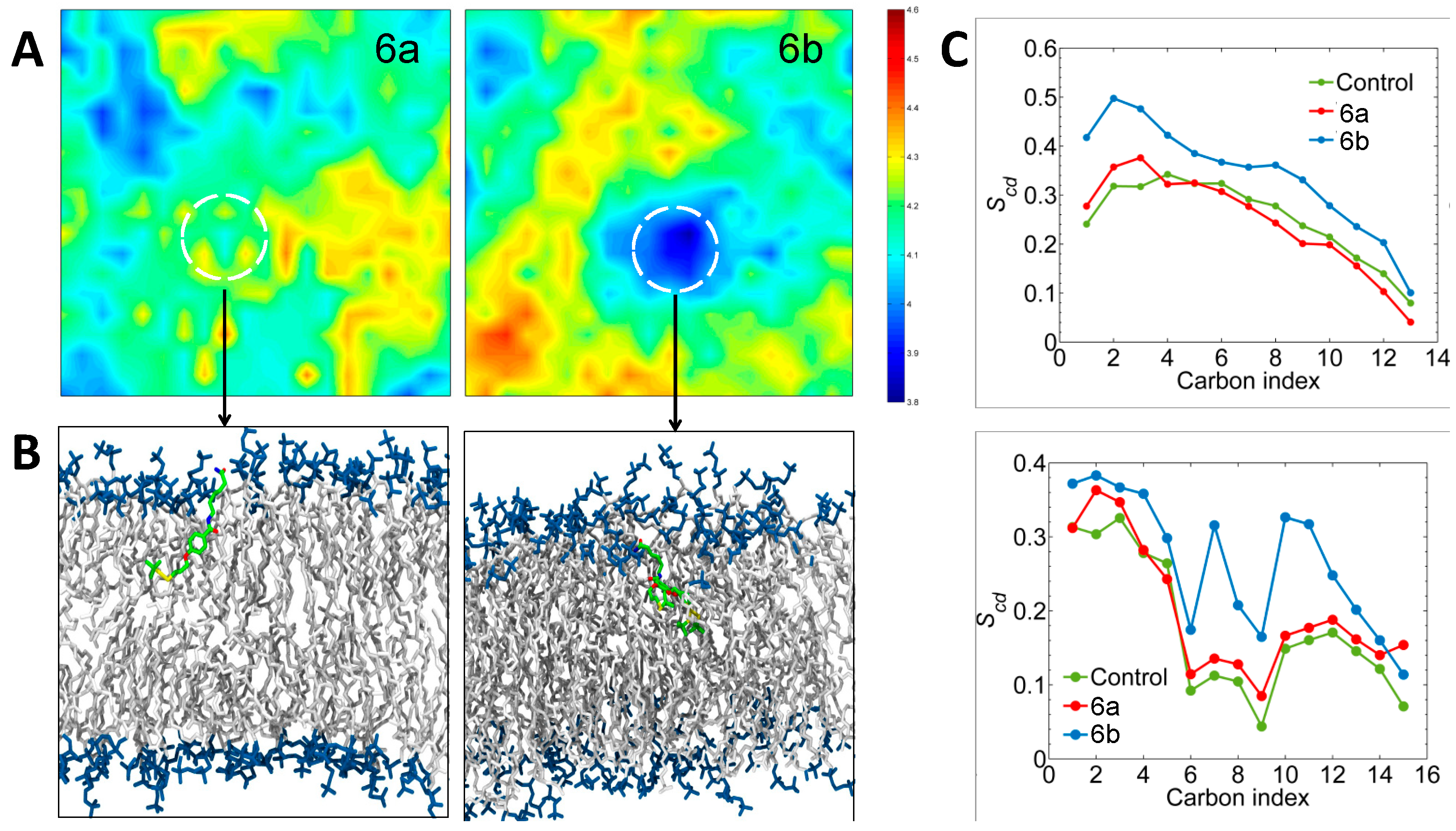
2.2.5. Molecular Dynamics Simulations Analysis
3. Materials and Methods
3.1. General Method
3.2. General Method for Preparation of Probe SS1/3-FITC
3.2.1. Synthesis of 3-(Tert-butyldisulfaneyl)propyl-4-methylbenzenesulfonate (2)
3.2.2. Synthesis of Ethyl 4-(3-(Tert-butyldisulfaneyl)propoxy)benzoate (5a)
3.2.3. Synthesis of Methyl 3,4,5-tris(3-(Tert-butyldisulfaneyl)propoxy)benzoate (5b)
3.2.4. Synthesis of Methyl 6-(4-(3-(Tert-butyldisulfaneyl)propoxy)benzamido) Hexanoate (6a)
3.2.5. Synthesis of Methyl 6-(3,4,5-tris(3-(Tert-butyldisulfaneyl)propoxy)benzamido) Hexanoate (6b)
3.2.6. Synthesis of Tert-butyl (2-(6-(4-(3-(tert-butyldisulfaneyl)propoxy)benzamido)hexan-amido)ethyl)carbamate (7a)
3.2.7. Synthesis of Tert-butyl (2-(6-(3,4,5-tris(3-(tert-butyldisulfaneyl)propoxy)benzamido)hexan-amido)ethyl)carbamate (7b)
3.2.8. Synthesis of SS1-FITC (8a)
3.2.9. Synthesis of SS3-FITC (8b)
3.3. Cell Culture
3.4. Time-Dependent Fluorescence Microscopy Measurement of the Cancer Cells Treated with the SS1-FITC and SS3-FITC Probe
3.5. Concentration-Dependent Fluorescence Microscopy Measurement of the Cancer Cells Treated with the SS1-FITC and SS3-FITC Probe
3.6. CCK-8 Assay
3.7. Fluorescence Microscopy Measurement of the Cancer Cells Pretreated with Thiols Inhibitors
3.8. Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, G.; Yin, X.; Luo, J.; Gu, R.; Wang, S.; Feng, J.; Chen, B. Non-small cell lung cancer-targeted, redox-sensitive lipid-polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor-Afatinib: In vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 120, 109493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Gan, Y.; Qiu, N.; Gu, Y.; Zhu, H. Conjugates of TAT and folate with DOX-loaded chitosan micelles offer effective intracellular delivery ability. Pharm. Dev. Technol. 2019, 24, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, W.; Gan, Y.; Li, Q.; Li, X.; Shao, L.; Zhu, D.; Guo, H. Combined Modality Therapy Based on Hybrid Gold Nanostars Coated with Temperature Sensitive Liposomes to Overcome Paclitaxel-Resistance in Hepatic Carcinoma. Pharmaceutics 2019, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Yu, Y.; Chen, Y.; Liu, Y.; Su, G. Facile Construction of i-Motif DNA-Conjugated Gold Nanostars as Near-Infrared and pH Dual-Responsive Targeted Drug Delivery Systems for Combined Cancer Therapy. Mol. Pharm. 2020, 17, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Qian, Y.; Fang, G. Development of Lipid-Polymer Hybrid Nanoparticles for Improving Oral Absorption of Enoxaparin. Pharmaceutics 2020, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Long, M.; Liu, X.; Huang, X.; Lu, M.; Wu, X.; Weng, L.; Chen, Q.; Wang, X.; Zhu, L.; Chen, Z. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J. Control. Release 2021, 334, 303–317. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, E.; Wang, Y.; Miao, S.; Liu, Y.; Hu, Y., 3rd; Liu, J.; Xu, B.; Chen, D.; Shen, Y. Emerging Antibacterial Strategies with Application of Targeting Drug Delivery System and Combined Treatment. Int. J. Nanomed. 2021, 16, 6141–6156. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shao, L.; Chen, Y.; Han, W.; Zhou, Y.; Liu, T.; Gu, J.; Zhu, H. Sequential Dual Delivery System Based on siCOX-2-Loaded Gold Nanostar and Thermal-Sensitive Liposomes Overcome Hypoxia-Mediated Multidrug Resistance in Tumors. Mol. Pharm. 2022, 19, 2390–2405. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Jin, Y.; Zhao, S.; Yuan, H.; Shi, J.; Zhao, H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed. Pharmacother. 2022, 153, 113463. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ma, J.; Chen, M.; Zhang, W.; Xu, C.; Nan, Y.; Wu, W.; Mao, X.; Cheng, X.; Cai, H.; et al. 4-Iodo-6-phenylpyrimidine (4-IPP) suppresses fibroblast-like synoviocyte-mediated inflammation and joint destruction associated with rheumatoid arthritis. Int. Immunopharmacol. 2023, 115, 109714. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, L.; Lu, Q.; Li, G.; Zhou, Y.; Yang, Y.; Zhang, L. Recent progress and applications of poly(beta amino esters)-based biomaterials. J. Control. Release 2023, 354, 337–353. [Google Scholar] [CrossRef]
- Xu, L.; Bai, E.; Zhu, Y.; Qin, J.; Du, X.; Huang, H. pH-Responsive Hydrogel as a Potential Oral Delivery System of Baicalin for Prolonging Gastroprotective Activity. Pharmaceutics 2023, 15, 257. [Google Scholar] [CrossRef]
- Banushi, B.; Joseph, S.R.; Lum, B.; Lee, J.J.; Simpson, F. Endocytosis in cancer and cancer therapy. Nat. Rev. Cancer 2023, 23, 450–473. [Google Scholar] [CrossRef]
- Sakurai, F.; Inoue, R.; Nishino, Y.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J. Control. Release 2000, 66, 255–269. [Google Scholar] [CrossRef]
- Radler, J.O.; Koltover, I.; Salditt, T.; Safinya, C.R. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997, 275, 810–814. [Google Scholar] [CrossRef]
- Chen, N.; He, Y.; Zang, M.; Zhang, Y.; Lu, H.; Zhao, Q.; Wang, S.; Gao, Y. Approaches and materials for endocytosis-independent intracellular delivery of proteins. Biomaterials 2022, 286, 121567. [Google Scholar] [CrossRef]
- Laurent, Q.; Martinent, R.; Lim, B.; Pham, A.T.; Kato, T.; Lopez-Andarias, J.; Sakai, N.; Matile, S. Thiol-Mediated Uptake. JACS Au 2021, 1, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Goerdeler, F.; Reuber, E.E.; Luhle, J.; Leichnitz, S.; Freitag, A.; Nedielkov, R.; Groza, R.; Ewers, H.; Moller, H.M.; Seeberger, P.H.; et al. Thiol-Mediated Uptake of a Cysteine-Containing Nanobody for Anticancer Drug Delivery. ACS Cent. Sci. 2023, 9, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Shybeka, I.; Maynard, J.R.J.; Saidjalolov, S.; Moreau, D.; Sakai, N.; Matile, S. Dynamic Covalent Michael Acceptors to Penetrate Cells: Thiol-Mediated Uptake with Tetrel-Centered Exchange Cascades, Assisted by Halogen-Bonding Switches. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, M.; Hu, W.; Wang, J.; Zhang, Z.G.; Zhang, K.; Yu, B.; Xu, F.J. Controllable Disulfide Exchange Polymerization of Polyguanidine for Effective Biomedical Applications by Thiol-Mediated Uptake. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200535. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liew, S.S.; Li, L.; Yao, S.Q. Bypassing Endocytosis: Direct Cytosolic Delivery of Proteins. J. Am. Chem. Soc. 2018, 140, 15986–15996. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Fu, J.; Yuan, P.; Du, S.; Huang, W.; Li, L.; Yao, S.Q. Intracellular Delivery of Native Proteins Facilitated by Cell-Penetrating Poly(disulfide)s. Angew. Chem. Int. Ed. Engl. 2018, 57, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, L.; Wang, L.; Liu, Y.; Li, J.; Li, J.; Li, J.; Yang, H. Self-Assembled and Size-Controllable Oligonucleotide Nanospheres for Effective Antisense Gene Delivery through an Endocytosis-Independent Pathway. Angew. Chem. Int. Ed. Engl. 2019, 58, 5236–5240. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Bang, E.K.; Molinard, G.; Tulumello, D.V.; Ward, S.; Kelley, S.O.; Roux, A.; Sakai, N.; Matile, S. Cellular uptake of substrate-initiated cell-penetrating poly(disulfide)s. J. Am. Chem. Soc. 2014, 136, 6069–6074. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Sargsyan, G.; Bang, E.K.; Sakai, N.; Matile, S. Ring Tension Applied to Thiol-Mediated Cellular Uptake. Angew. Chem. Int. Ed. Engl. 2015, 54, 7328–7331. [Google Scholar] [CrossRef]
- Chuard, N.; Gasparini, G.; Moreau, D.; Lorcher, S.; Palivan, C.; Meier, W.; Sakai, N.; Matile, S. Strain-Promoted Thiol-Mediated Cellular Uptake of Giant Substrates: Liposomes and Polymersomes. Angew. Chem. Int. Ed. Engl. 2017, 56, 2947–2950. [Google Scholar]
- Zong, L.; Bartolami, E.; Abegg, D.; Adibekian, A.; Sakai, N.; Matile, S. Epidithiodiketopiperazines: Strain-Promoted Thiol-Mediated Cellular Uptake at the Highest Tension. ACS Cent. Sci. 2017, 3, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qu, D.H.; Feringa, B.L.; Tian, H. Disulfide-Mediated Reversible Polymerization toward Intrinsically Dynamic Smart Materials. J. Am. Chem. Soc. 2022, 144, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Pięta, M.; Purohit, V.B.; Pietrasik, J.; Plummer, C.M. Disulfide-containing monomers in chain-growth polymerization. Polym. Chem. 2023, 14, 7–31. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Wu, Q.; Moore, J.S. Architecture-Controlled Ring-Opening Polymerization for Dynamic Covalent Poly(disulfide)s. J. Am. Chem. Soc. 2019, 141, 17075–17080. [Google Scholar] [CrossRef] [PubMed]
- Levkovskyi, I.O.; Mochizuki, S.; Zheng, A.; Zhang, X.; Zhang, F. Lipoic acid-based poly(disulfide)s: Synthesis and biomedical applications. Nano TransMed 2023, 2, 100006. [Google Scholar] [CrossRef]
- Tirla, A.; Hansen, M.E.; Rivera-Fuentes, P. Synthesis of Asparagusic Acid Modified Lysine and its Application in Solid-Phase Synthesis of Peptides with Enhanced Cellular Uptake. Synlett 2017, 29, 1289–1292. [Google Scholar]
- Shu, Z.; Tanaka, I.; Ota, A.; Fushihara, D.; Abe, N.; Kawaguchi, S.; Nakamoto, K.; Tomoike, F.; Tada, S.; Ito, Y.; et al. Disulfide-Unit Conjugation Enables Ultrafast Cytosolic Internalization of Antisense DNA and siRNA. Angew. Chem. Int. Ed. Engl. 2019, 58, 6611–6615. [Google Scholar] [CrossRef]
- Li, T.; Takeoka, S. Enhanced cellular uptake of maleimide-modified liposomes via thiol-mediated transport. Int. J. Nanomed. 2014, 9, 2849–2861. [Google Scholar]
- Liu, Y.; Song, M.; Wu, J.; Xie, S.; Zhou, Y.; Liu, L.; Huang, M.; Jiang, L.; Xu, P.; Li, J. Exploring the mechanism of photosensitizer conjugation on membrane perturbation of antimicrobial peptide: A multiscale molecular simulation study. Int. J. Biol. Macromol. 2023, 247, 125698. [Google Scholar] [CrossRef]
- de Souza, R.M.; Siani, P.; Schmidt, T.F.; Itri, R.; Dias, L.G. Methylene Blue Location in (Hydroperoxized) Cardiolipin Monolayer: Implication in Membrane Photodegradation. J. Phys. Chem. B 2017, 121, 8512–8522. [Google Scholar] [CrossRef]
- Menichetti, R.; Kanekal, K.H.; Bereau, T. Drug-Membrane Permeability across Chemical Space. ACS Cent. Sci. 2019, 5, 290–298. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, D.; Lei, W.; Sun, T.; Gu, B.; Dong, H.; Taniguchi, Y.; Liu, Y.; Ling, Y. Trigonometric Bundling Disulfide Unit Starship Synergizes More Effectively to Promote Cellular Uptake. Int. J. Mol. Sci. 2024, 25, 7518. https://doi.org/10.3390/ijms25147518
Wang L, Wang D, Lei W, Sun T, Gu B, Dong H, Taniguchi Y, Liu Y, Ling Y. Trigonometric Bundling Disulfide Unit Starship Synergizes More Effectively to Promote Cellular Uptake. International Journal of Molecular Sciences. 2024; 25(14):7518. https://doi.org/10.3390/ijms25147518
Chicago/Turabian StyleWang, Lei, Dezhi Wang, Wenzhuo Lei, Tiantian Sun, Bei Gu, Han Dong, Yosuke Taniguchi, Yichang Liu, and Yong Ling. 2024. "Trigonometric Bundling Disulfide Unit Starship Synergizes More Effectively to Promote Cellular Uptake" International Journal of Molecular Sciences 25, no. 14: 7518. https://doi.org/10.3390/ijms25147518
APA StyleWang, L., Wang, D., Lei, W., Sun, T., Gu, B., Dong, H., Taniguchi, Y., Liu, Y., & Ling, Y. (2024). Trigonometric Bundling Disulfide Unit Starship Synergizes More Effectively to Promote Cellular Uptake. International Journal of Molecular Sciences, 25(14), 7518. https://doi.org/10.3390/ijms25147518





