Placental Protein 13: Vasomodulatory Effects on Human Uterine Arteries and Potential Implications for Preeclampsia
Abstract
:1. Introduction
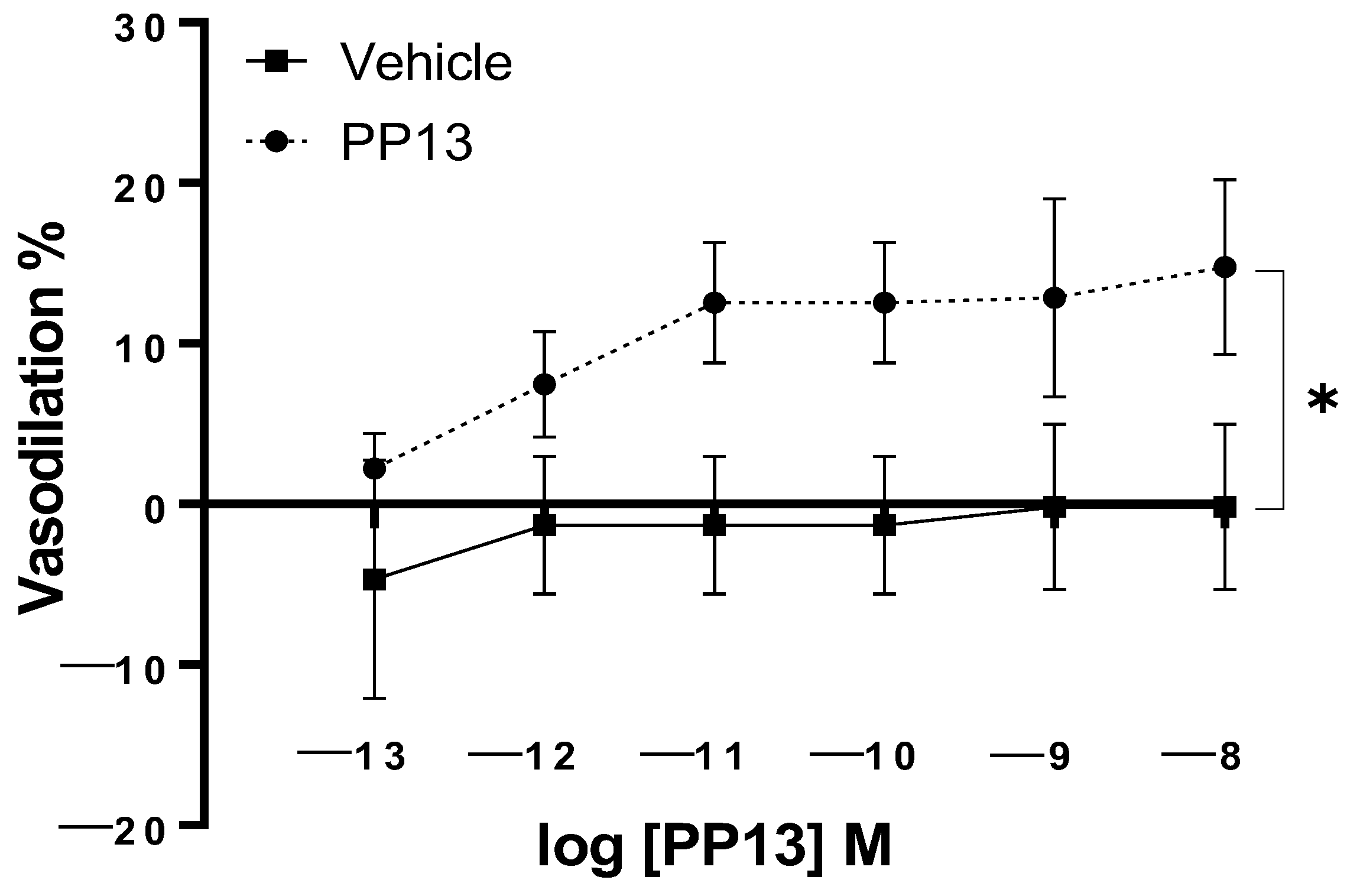
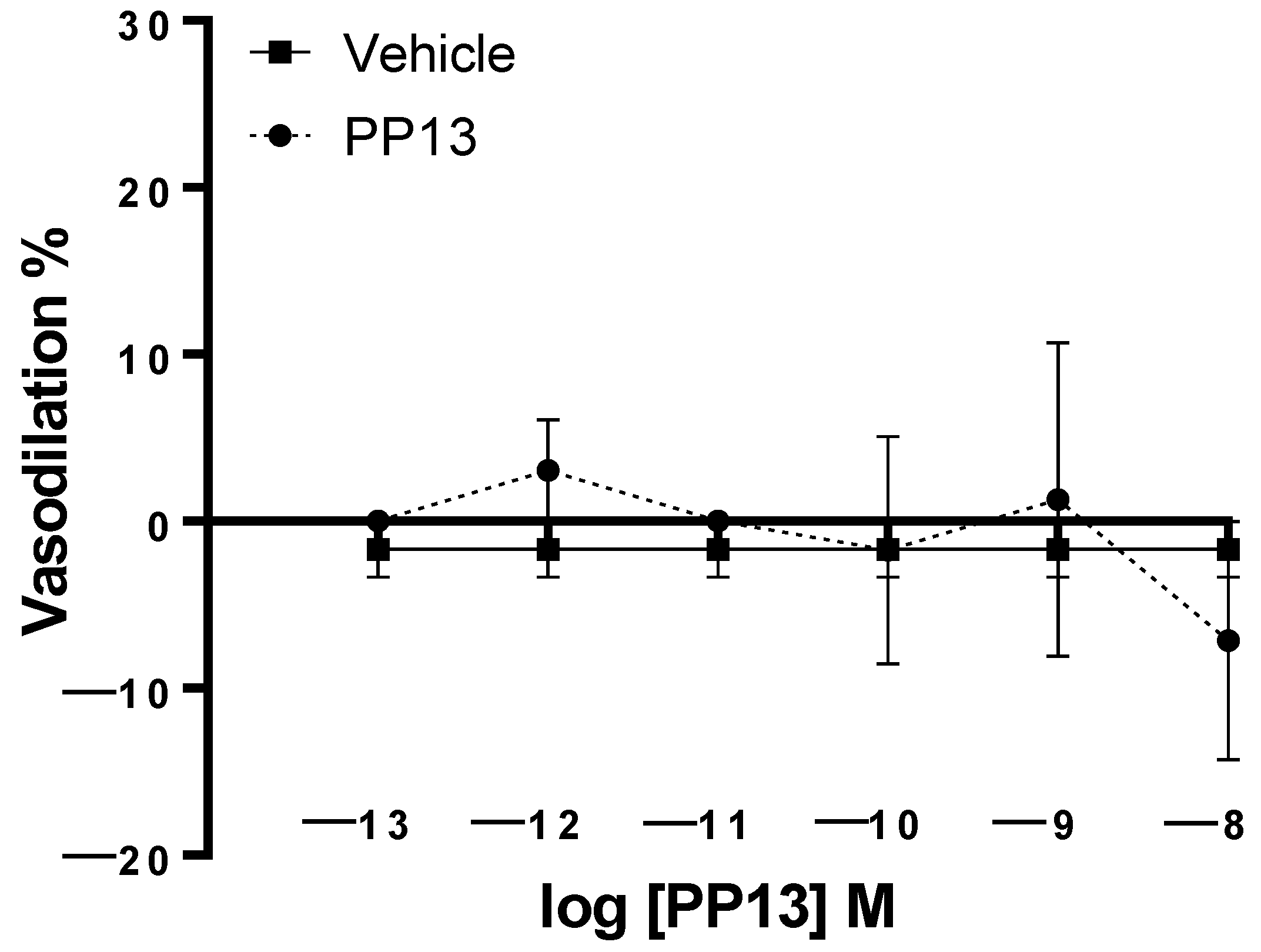
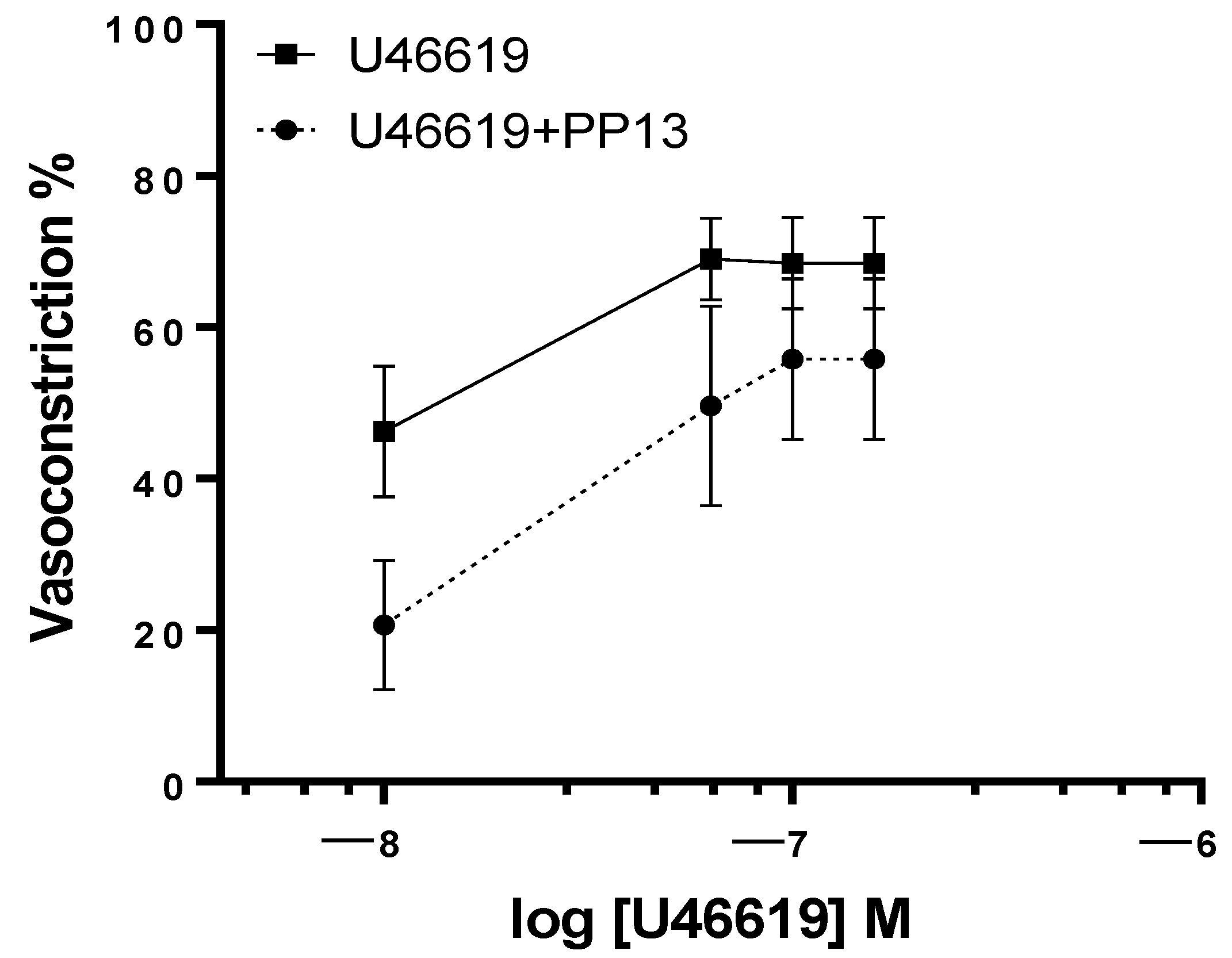
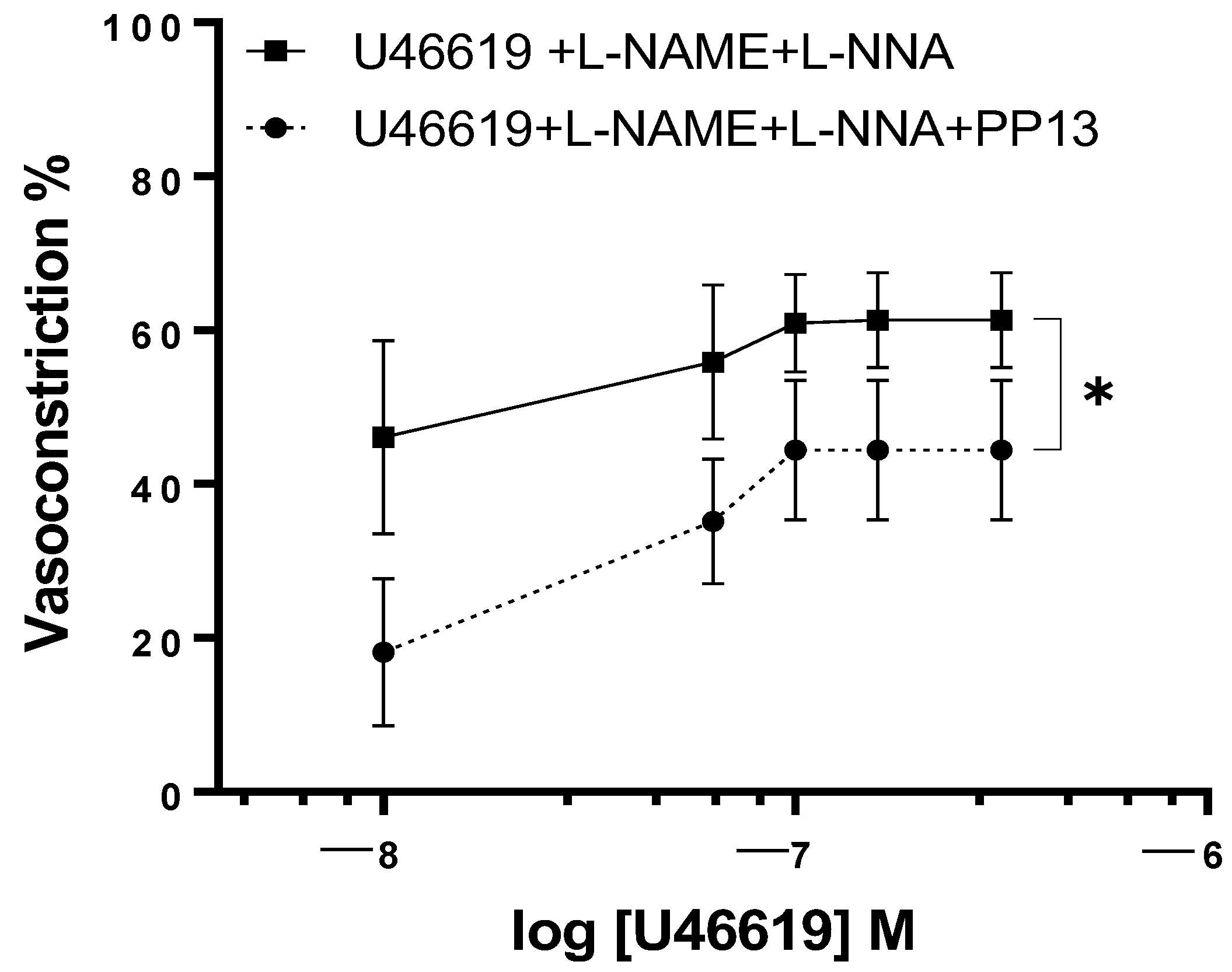
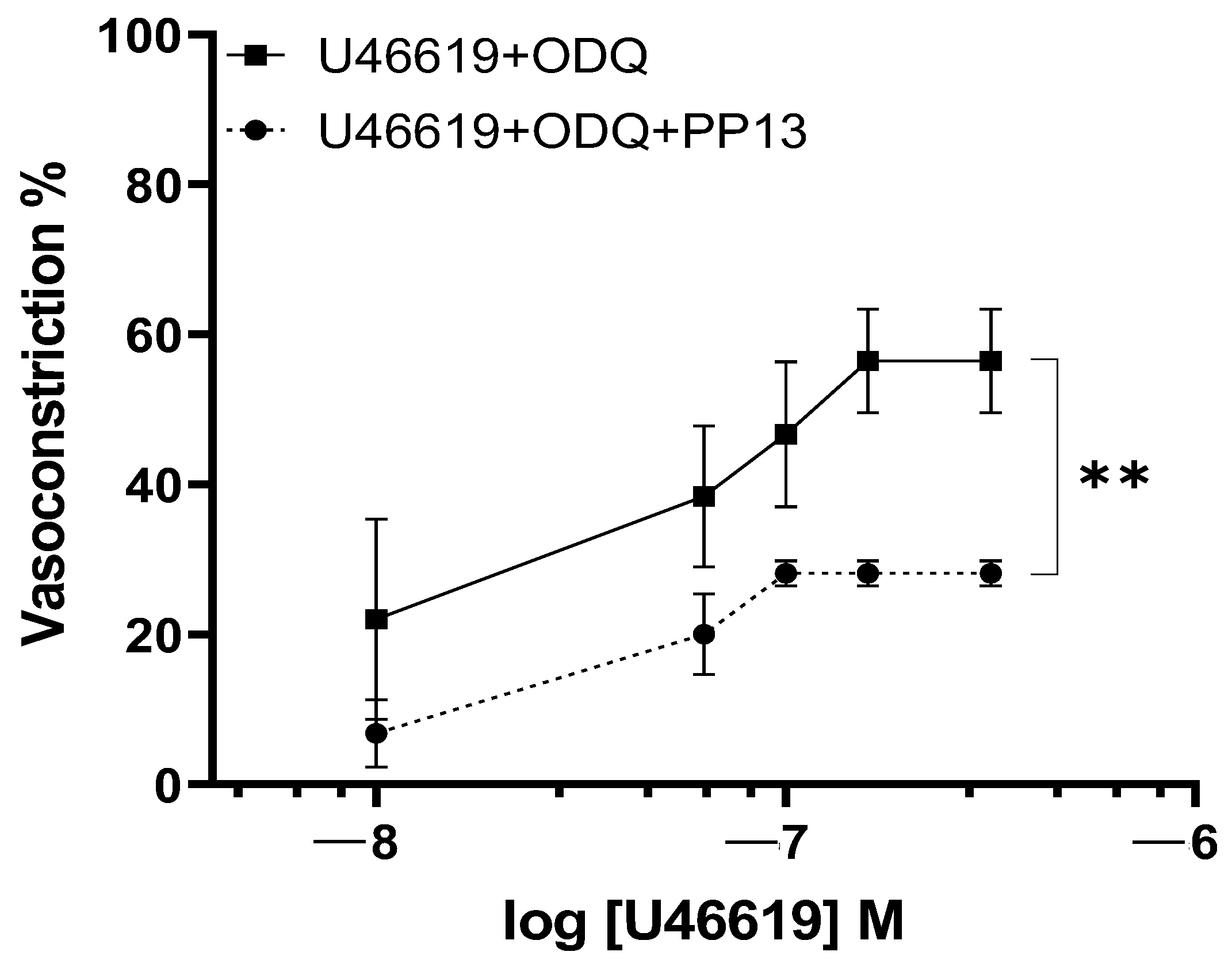
2. Results
3. Discussion
4. Material and Methods
4.1. Patient Recruitment and Selection
4.2. Samples Collection
4.3. Vessel Preparation
4.4. Pressure Myography
4.5. Experimental Protocol
4.6. Chemicals and Solutions
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Davis, E.P.; Narayan, A.J. Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Dev. Psychopathol. 2020, 32, 1625–1639. [Google Scholar] [CrossRef]
- McNestry, C.; Killeen, S.L.; Crowley, R.K.; McAuliffe, F.M. Pregnancy complications and later life women’s health. Acta Obstet. Gynecol. Scand. 2023, 102, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Drobnjak, T.; Mandala, M.; Gizurarson, S.; Huppertz, B.; Meiri, H. Galectin 13 (PP13) Facilitates Remodeling and Structural Stabilization of Maternal Vessels during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3192. [Google Scholar] [CrossRef]
- Tannetta, D.; Collett, G.; Vatish, M.; Redman, C.; Sargent, I. Syncytiotrophoblast extracellular vesicles—Circulating biopsies reflecting placental health. Placenta 2017, 52, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Gadde, R.; CD, D.; Sheela, S.R. Placental protein 13 An important biological protein in preeclampsia. J. Circ. Biomark. 2018, 7, 1849454418786159. [Google Scholar] [CrossRef]
- Huppertz, B.; Meiri, H.; Gizurarson, S.; Osol, G.; Sammar, M. Placental protein 13 (PP13): A new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum. Reprod. Update 2013, 19, 391–405. [Google Scholar] [CrossRef]
- Soongsatitanon, A.; Phupong, V. Prediction of preeclampsia using first trimester placental protein 13 and uterine artery Doppler. J. Matern.-Fetal Neonatal Med. 2022, 35, 4412–4417. [Google Scholar] [CrossRef]
- Sekizawa, A.; Purwosunu, Y.; Yoshimura, S.; Nakamura, M.; Shimizu, H.; Okai, T.; Rizzo, N.; Farina, A. PP13 mRNA Expression in Trophoblasts from Preeclamptic Placentas. Reprod. Sci. 2008, 16, 408–413. [Google Scholar] [CrossRef]
- Sammar, M.; Nisemblat, S.; Fleischfarb, Z.; Golan, A.; Sadan, O.; Meiri, H.; Huppertz, B.; Gonen, R. Placenta-bound and Body Fluid PP13 and its mRNA in Normal Pregnancy Compared to Preeclampsia, HELLP and Preterm Delivery. Placenta 2011, 32, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Ko, N.L.; Mandalà, M. Plasticity of the Maternal Vasculature During Pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Balogh, A.; Romero, R.; Kárpáti, É.; Erez, O.; Szilágyi, A.; Kovalszky, I.; Sammar, M.; Gizurarson, S.; Matkó, J.; et al. Placental Protein 13 (PP13)—A Placental Immunoregulatory Galectin Protecting Pregnancy. Front. Immunol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Asiltas, B.; Surmen-Gur, E.; Uncu, G. Prediction of first-trimester preeclampsia: Relevance of the oxidative stress marker MDA in a combination model with PP-13, PAPP-A and beta-HCG. Pathophysiology 2018, 25, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Guardia, C.M.A.; Gauto, D.F.; Di Lella, S.; Rabinovich, G.A.; Martí, M.A.; Estrin, D.A. An Integrated Computational Analysis of the Structure, Dynamics, and Ligand Binding Interactions of the Human Galectin Network. J. Chem. Inf. Model. 2011, 51, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Toth, E.; Romero, R.; Parej, K.; Csala, D.; Szenasi, N.L.; Hajdu, I.; Juhasz, K.; Kovacs, A.F.; Meiri, H.; et al. Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response. Front. Immunol. 2019, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Vokalova, L.; Balogh, A.; Toth, E.; Van Breda, S.V.; Schäfer, G.; Hoesli, I.; Lapaire, O.; Hahn, S.; Than, N.G.; Rossi, S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Drobnjak, T.; Gizurarson, S.; Gokina, N.I.; Meiri, H.; Mandalá, M.; Huppertz, B.; Osol, G. Placental protein 13 (PP13)-induced vasodilation of resistance arteries from pregnant and nonpregnant rats occurs via endothelial-signaling pathways. Hypertens. Pregnancy 2017, 36, 186–195. [Google Scholar] [CrossRef]
- Mariacarmela, G.; Milena, E.; Sveinbjorn, G.; Daniel, H.; Maurizio, M. Placental protein 13 dilation of pregnant rat uterine vein is endothelium dependent and involves nitric oxide/calcium activated potassium channels signals. Placenta 2022, 126, 233–238. [Google Scholar] [CrossRef]
- Gizurarson, S.; Sigurdardottir, E.R.; Meiri, H.; Huppertz, B.; Sammar, M.; Sharabi-Nov, A.; Mandalá, M.; Osol, G. Placental Protein 13 Administration to Pregnant Rats Lowers Blood Pressure and Augments Fetal Growth and Venous Remodeling. Fetal Diagn. Ther. 2015, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.K.; Zamudio, S.; Coffin, C.; Parker, S.; Stamm, E.; Moore, L.G. Quantitative Estimation of Human Uterine Artery Blood Flow and Pelvic Blood Flow Redistribution in Pregnancy. Obstet. Gynecol. 1992, 80, 1000–1006. [Google Scholar] [PubMed]
- Campbell, S.; Griffin, D.R.; Pearce, J.M.; Diaz-Recasens, J.; Cohen-Overbeek, T.E.; Willson, K.; Teague, M.J. New doppler technique for assessing uteroplacental blood flow. Lancet 1983, 321, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Hwuang, E.; Vidorreta, M.; Schwartz, N.; Moon, B.F.; Kochar, K.; Tisdall, M.D.; Detre, J.A.; Witschey, W.R.T. Assessment of uterine artery geometry and hemodynamics in human pregnancy with 4d flow mri and its correlation with doppler ultrasound. J. Magn. Reson. 2019, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Grigoriadis, C.; Zygouris, D.; Vlachadis, N.; Antonakopoulos, N.; Iliodromiti, Z. The endocrine and paracrine role of placental cytokines, growth factors and peptides. HJOG 2015, 14, 33–38. [Google Scholar]
- Drobnjak, T.; Jónsdóttir, A.M.; Helgadóttir, H.; Runólfsdóttir, M.S.; Meiri, H.; Sammar, M.; Osol, G.J.; Mandalá, M.; Huppertz, B.; Gizurarson, S. Placental protein 13 (PP13) stimulates rat uterine vessels after slow subcutaneous administration. Int. J. Womens Health 2019, 11, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.H.; Steinsland, O.S.; Wang, Y.; Yallampalli, C.; Dong, Y.-L.; Sanchez, J.M. Increased Nitric Oxide Synthase Activity and Expression in the Human Uterine Artery During Pregnancy. Circ. Res. 2000, 87, 406–411. [Google Scholar] [CrossRef]
- Hayakawa, H.; Hirata, Y.; Kakoki, M.; Suzuki, Y.; Nishimatsu, H.; Nagata, D.; Suzuki, E.; Kikuchi, K.; Nagano, T.; Kangawa, K.; et al. Role of Nitric Oxide–cGMP Pathway in Adrenomedullin-Induced Vasodilation in the Rat. Hypertension 1999, 33, 689–693. [Google Scholar] [CrossRef]
- Giachini, F.R.; Lima, V.V.; Carneiro, F.S.; Tostes, R.C.; Webb, R.C. Decreased cGMP Level Contributes to Increased Contraction in Arteries from Hypertensive Rats. Hypertension 2011, 57, 655–663. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Salmeron, A.G.; Bai, J.; Chen, D. Estrogen-Induced Uterine Vasodilation in Pregnancy and Preeclampsia. J. Matern.-Fetal Med. 2022, 4, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.; Chesler, N.C.; Magness, R.R. Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: Experimental studies and hemodynamic models of shear stresses on endothelial cells. Int. J. Dev. Biol. 2010, 54, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, S. Nitric Oxide-Induced Calcium Release: Activation of Type 1 Ryanodine Receptor, a Calcium Release Channel, through Non-Enzymatic Post-Translational Modification by Nitric Oxide. Front. Endocrinol. 2013, 4, 142. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, M.; Esposito, M.; Morelli, M.; De Rose, S.; Gizurarson, S.; Meiri, H.; Mandalà, M. Placental Protein 13: Vasomodulatory Effects on Human Uterine Arteries and Potential Implications for Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7522. https://doi.org/10.3390/ijms25147522
Gatto M, Esposito M, Morelli M, De Rose S, Gizurarson S, Meiri H, Mandalà M. Placental Protein 13: Vasomodulatory Effects on Human Uterine Arteries and Potential Implications for Preeclampsia. International Journal of Molecular Sciences. 2024; 25(14):7522. https://doi.org/10.3390/ijms25147522
Chicago/Turabian StyleGatto, Mariacarmela, Milena Esposito, Michele Morelli, Silvia De Rose, Sveinbjorn Gizurarson, Hamutal Meiri, and Maurizio Mandalà. 2024. "Placental Protein 13: Vasomodulatory Effects on Human Uterine Arteries and Potential Implications for Preeclampsia" International Journal of Molecular Sciences 25, no. 14: 7522. https://doi.org/10.3390/ijms25147522
APA StyleGatto, M., Esposito, M., Morelli, M., De Rose, S., Gizurarson, S., Meiri, H., & Mandalà, M. (2024). Placental Protein 13: Vasomodulatory Effects on Human Uterine Arteries and Potential Implications for Preeclampsia. International Journal of Molecular Sciences, 25(14), 7522. https://doi.org/10.3390/ijms25147522




