Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities
Abstract
1. Introduction
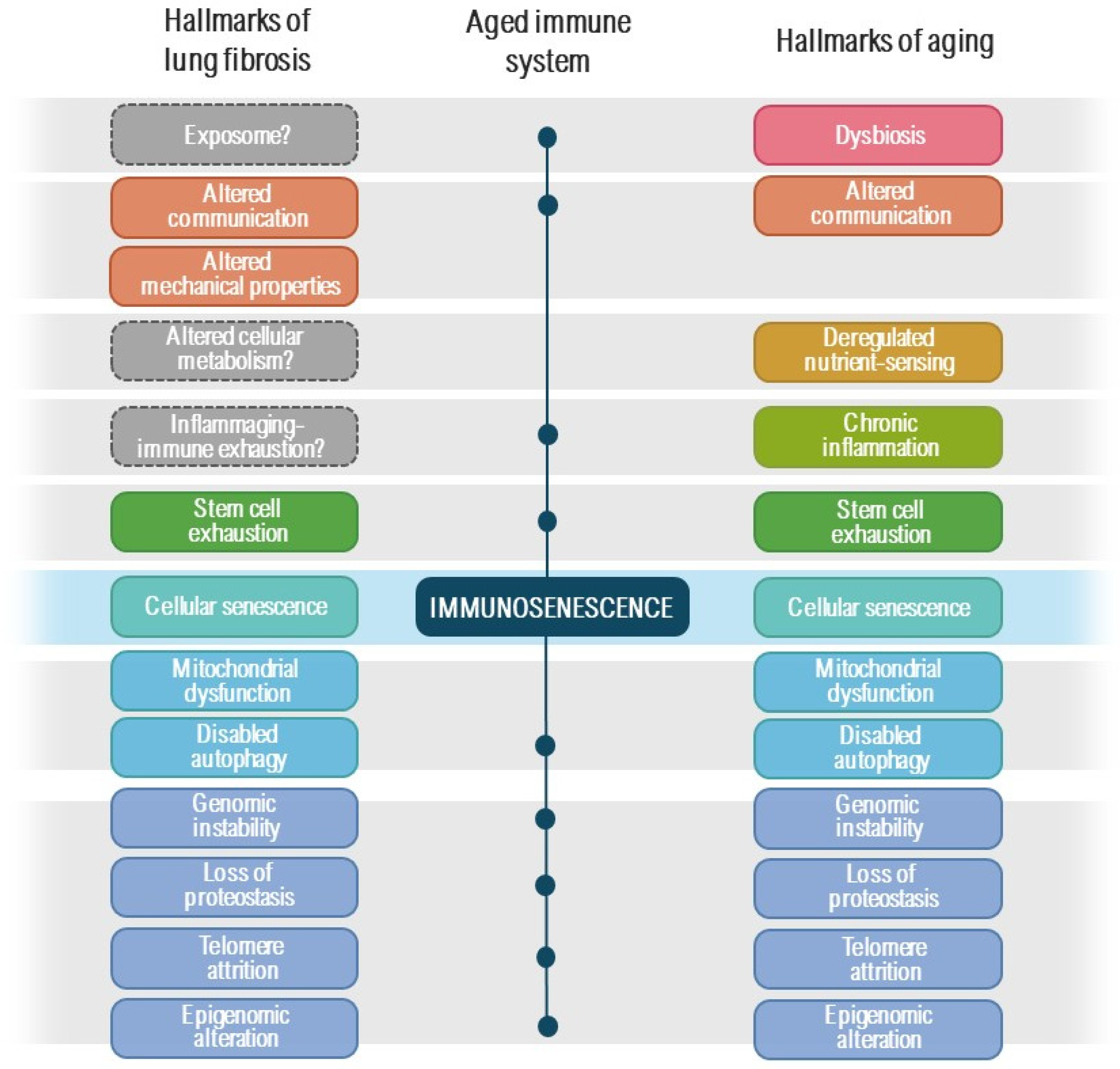
2. Brief Overview of Lung Fibrosis Pathogenesis
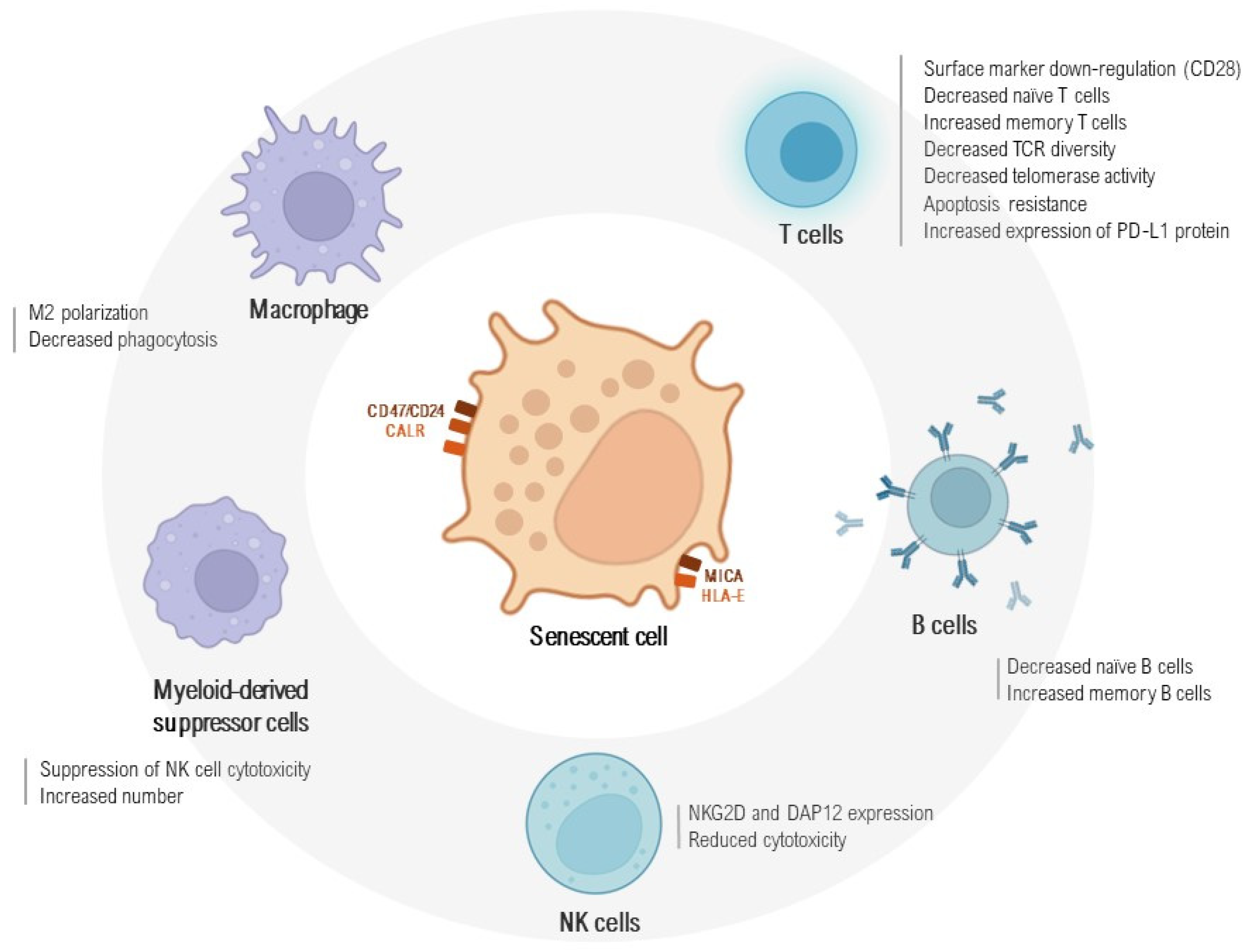
3. Immunosenescence and Senescent Cell Immunosurveillance
4. Senescence-Related Immune Cells Subsets in Fibrosing Interstitial Lung Diseases
4.1. Stem Cell Exhaustion
4.2. Macrophages and Their Interaction with Senescent Cells
4.3. B and T Cell Immune Response in a State of Exhaustion
4.4. NK Cells and NKT-like Cell Contribution to an Aged Immunophenotype
4.5. Myeloid-Derived Suppressor Cells and the Immune System–Senescence–Fibrosis Axis
5. The Potential Link between an Aged Immune System, Autoimmune Disease and Fibrosis
5.1. Pulmonary Fibrosis and Autoimmune Features
5.2. Inflammaging and Immunosenescence in Autoimmune Diseases
5.3. A Tripartite Relationship: Senescence, Autoimmunity, and Lung Fibrosis
6. Potential Therapeutic Strategies to Improve Responses among Fibrosing Interstitial Lung Diseases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The economic value of targeting aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Kapetanaki, M.G.; Mora, A.L.; Rojas, M. Influence of age on wound healing and fibrosis. J. Pathol. 2013, 229, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Noble, P.W. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am. J. Physiol. Cell Physiol. 2014, 306, C987–C996. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. Idiopathic Pulmonary Fibrosis: From Common Microscopy to Single-Cell Biology and Precision Medicine. Am. J. Respir. Crit. Care Med. 2024, 209, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, F.; Prats, N.; Ramponi, V.; Lopez-Dominguez, J.A.; Meyer, K.; Aguilera, M.; Munoz Martin, M.I.; Martinez, D.; Agusti, A.; Faner, R.; et al. Human senescent fibroblasts trigger progressive lung fibrosis in mice. Aging 2023, 15, 6641–6657. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Le Page, A.; Fortin, C.; Witkowski, J.M.; Dupuis, G.; Larbi, A. Cellular signaling in the aging immune system. Curr. Opin. Immunol. 2014, 29, 105–111. [Google Scholar] [CrossRef]
- Murray, M.A.; Chotirmall, S.H. The Impact of Immunosenescence on Pulmonary Disease. Mediat. Inflamm. 2015, 2015, 692546. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Akbar, A.; Beverley, P.; Caruso, C.; Derhovanessian, E.; Fulop, T.; Griffiths, P.; Grubeck-Loebenstein, B.; Hamprecht, K.; Jahn, G.; et al. Immunosenescence and Cytomegalovirus: Where do we stand after a decade? Immun. Ageing 2010, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Manoharan, M.S.; Lee, G.C.; McKinnon, L.R.; Meunier, J.A.; Steri, M.; Harper, N.; Fiorillo, E.; Smith, A.M.; Restrepo, M.I.; et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 2023, 14, 3286. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Gladyshev, V.N. The meaning of adaptation in aging: Insights from cellular senescence, epigenetic clocks and stem cell alterations. Nat. Aging 2023, 3, 766–775. [Google Scholar] [CrossRef]
- Lee, E.; Carreras-Gallo, N.; Lopez, L.; Turner, L.; Lin, A.; Mendez, T.L.; Went, H.; Tomusiak, A.; Verdin, E.; Corley, M.; et al. Exploring the effects of Dasatinib, Quercetin, and Fisetin on DNA methylation clocks: A longitudinal study on senolytic interventions. Aging 2024, 16, 3088–3106. [Google Scholar] [CrossRef] [PubMed]
- Giannoula, Y.; Kroemer, G.; Pietrocola, F. Cellular senescence and the host immune system in aging and age-related disorders. Biomed. J. 2023, 46, 100581. [Google Scholar] [CrossRef] [PubMed]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Thompson, P.J.; Wang, Y.; Bhattacharyya, A.; Apostolopoulou, H.; Hatano, R.; Naikawadi, R.P.; Shah, A.; Wolters, P.J.; Koliwad, S.; et al. Invariant Natural Killer T cells coordinate removal of senescent cells. Med 2021, 2, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cellular Senescence and Lung Function during Aging. Yin and Yang. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S402–S406. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Serrano, M.; Pietrocola, F. Cellular senescence enhances adaptive anticancer immunosurveillance. Oncoimmunology 2023, 12, 2154115. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Serrano, M.; Pietrocola, F. Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. NPJ Aging 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, D.; Lindenthal, L.; Sauer, J.; Chung, K.J.; Chavakis, T.; Griesser, E.; Baskaran, P.; Maier-Habelsberger, U.; Fundel-Clemens, K.; Schlotthauer, I.; et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis. J. Cell Biol. 2023, 222, e202207097. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; He, M.; Li, X.; Wang, R. Role of Circular RNAs in Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 10493. [Google Scholar] [CrossRef] [PubMed]
- Lagnado, A.; Leslie, J.; Ruchaud-Sparagano, M.H.; Victorelli, S.; Hirsova, P.; Ogrodnik, M.; Collins, A.L.; Vizioli, M.G.; Habiballa, L.; Saretzki, G.; et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021, 40, e106048. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Vartholomatos, G.; Tzavaras, T.; Hatziapostolou, M.; Fackelmayer, F.O.; Sandaltzopoulos, R.; Polytarchou, C.; Kolettas, E. Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp. Gerontol. 2017, 96, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. The Roles of MicroRNAs and Extracellular Vesicles in the Pathogeneses of Idiopathic Pulmonary Fibrosis and Acute Respiratory Distress Syndrome. Tohoku J. Exp. Med. 2020, 251, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Roberts, W.N.; Roman, J.; Gangemi, S. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 160125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Z.; Gao, L.; Chen, M.; Duan, Y.; Zhou, P.; Liu, Z.; Wu, C.; Zhang, J.; Zhu, Q. C5a/C5aR1 axis as a key driver promotes epithelial-to-mesenchymal transition in airway epithelial cells in silica nanoparticles-induced pulmonary fibrosis. Int. Immunopharmacol. 2023, 125 Pt B, 111112. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am. J. Respir. Crit. Care Med. 2014, 189, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Rao, S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front. Microbiol. 2016, 7, 2111. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, F.; Mendoza, N.; Casas-Recasens, S.; Cruz, T.; Albacar, N.; Lopez-Saiz, G.; Alsina-Restoy, X.; Rojas, M.; Agusti, A.; Sellares, J.; et al. Peripheral Immune Cell Profiling Reveals Distinct Immune Hallmarks in Progressive Pulmonary Fibrosis. Arch. Bronconeumol. 2023, 59, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.; Mendoza, N.; Casas-Recasens, S.; Noell, G.; Hernandez-Gonzalez, F.; Frino-Garcia, A.; Alsina-Restoy, X.; Molina, M.; Rojas, M.; Agusti, A.; et al. Lung immune signatures define two groups of end-stage IPF patients. Respir. Res. 2023, 24, 236. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Alder, J.K.; Sellares, J.; Rojas, M.; Gurkar, A.U.; Mora, A.L. Cellular Senescence: The Trojan Horse in Chronic Lung Diseases. Am. J. Respir. Cell Mol. Biol. 2019, 61, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Lai, Y.C.; Romero, Y.; Brands, J.; St Croix, C.M.; Kamga, C.; Corey, C.; Herazo-Maya, J.D.; Sembrat, J.; Lee, J.S.; et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Investig. 2015, 125, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Mannem, H.; Winters, S.A.; Hoji, A.; Silveira, F.; McNally, E.; Pipeling, M.R.; Lendermon, E.A.; Morrell, M.R.; Pilewski, J.M.; et al. Impaired Cytomegalovirus Immunity in Idiopathic Pulmonary Fibrosis Lung Transplant Recipients with Short Telomeres. Am. J. Respir. Crit. Care Med. 2019, 199, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Behmoaras, J.; Gil, J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021, 220, e202010162. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Stolzing, A. Cellular senescence: Immunosurveillance and future immunotherapy. Ageing Res. Rev. 2018, 43, 17–25. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, F.; Liao, T.; Qian, H.; Liu, Y. Immunosenescence and macrophages: From basics to therapeutics. Int. J. Biochem. Cell Biol. 2023, 165, 106479. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Docherty, M.H.; Ferenbach, D.A.; Mylonas, K.J. The Role of Ageing and Parenchymal Senescence on Macrophage Function and Fibrosis. Front. Immunol. 2021, 12, 700790. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Nie, L.; Zhang, P.; Chen, Q.; Lv, Q.; Wang, Q. Tissue-resident macrophage inflammaging aggravates homeostasis dysregulation in age-related diseases. Cell Immunol. 2021, 361, 104278. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, Y.; Kitakaze, K.; Tsuboi, K.; Okamoto, Y. Growth differentiation factor 15 facilitates lung fibrosis by activating macrophages and fibroblasts. Exp. Cell Res. 2020, 391, 112010. [Google Scholar] [CrossRef]
- McQuattie-Pimentel, A.C.; Ren, Z.; Joshi, N.; Watanabe, S.; Stoeger, T.; Chi, M.; Lu, Z.; Sichizya, L.; Aillon, R.P.; Chen, C.I.; et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Investig. 2021, 131, e140299. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.O.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018, 10, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Habiel, D.M.; Espindola, M.S.; Kitson, C.; Azzara, A.V.; Coelho, A.L.; Stripp, B.; Hogaboam, C.M. Characterization of CD28(null) T cells in idiopathic pulmonary fibrosis. Mucosal Immunol. 2019, 12, 212–222. [Google Scholar] [CrossRef]
- Hornsby, P.J. Cellular senescence and tissue aging in vivo. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B251–B256. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Jersmann, H.; Tran, H.B.; Holmes, M.; Reynolds, P.N.; Hodge, S. Lymphocyte senescence in COPD is associated with loss of glucocorticoid receptor expression by pro-inflammatory/cytotoxic lymphocytes. Respir. Res. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.R.; Vuga, L.J.; Lindell, K.O.; Gibson, K.F.; Xue, J.; Kaminski, N.; Valentine, V.G.; Lindsay, E.K.; George, M.P.; Steele, C.; et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE 2010, 5, e8959. [Google Scholar] [CrossRef] [PubMed]
- Feghali-Bostwick, C.A.; Tsai, C.G.; Valentine, V.G.; Kantrow, S.; Stoner, M.W.; Pilewski, J.M.; Gadgil, A.; George, M.P.; Gibson, K.F.; Choi, A.M.; et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J. Immunol. 2007, 179, 2592–2599. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tanabe, N.; Vasilescu, D.M.; McDonough, J.E.; Coxson, H.O.; Ikezoe, K.; Kinose, D.; Ng, K.W.; Verleden, S.E.; Wuyts, W.A.; et al. The transition from normal lung anatomy to minimal and established fibrosis in idiopathic pulmonary fibrosis (IPF). EBioMedicine 2021, 66, 103325. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A.; Wells, A.U. Usual interstitial pneumonia as a stand-alone diagnostic entity: The case for a paradigm shift? Lancet Respir. Med. 2023, 11, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Homolka, J.; Ziegenhagen, M.W.; Gaede, K.I.; Entzian, P.; Zissel, G.; Muller-Quernheim, J. Systemic immune cell activation in a subgroup of patients with idiopathic pulmonary fibrosis. Respiration 2003, 70, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.A.; Hrusch, C.L.; Blaine, K.M.; Manns, S.T.; Vij, R.; Oldham, J.M.; Churpek, M.M.; Strek, M.E.; Noth, I.; Sperling, A.I. T cell Co-Stimulatory molecules ICOS and CD28 stratify idiopathic pulmonary fibrosis survival. Respir. Med. X 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.; Agudelo Garcia, P.A.; Chamucero-Millares, J.A.; Bondonese, A.; Mitash, N.; Sembrat, J.; Tabib, T.; Zhang, W.; Seyed, N.; Peters, V.; et al. End-Stage Idiopathic Pulmonary Fibrosis Lung Microenvironment Promotes Impaired NK Activity. J. Immunol. 2023, 211, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.N.; Brandes, J.C.; Weyand, C.M.; Goronzy, J.J. Modulation of CD28 expression: Distinct regulatory pathways during activation and replicative senescence. J. Immunol. 1999, 162, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Hacker, S.; Posch, M.; Hoetzenecker, K.; Pollreisz, A.; Lichtenauer, M.; Klepetko, W.; Ankersmit, H.J. T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin. Exp. Immunol. 2009, 155, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Fasth, A.E.; Dastmalchi, M.; Rahbar, A.; Salomonsson, S.; Pandya, J.M.; Lindroos, E.; Nennesmo, I.; Malmberg, K.J.; Soderberg-Naucler, C.; Trollmo, C.; et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J. Immunol. 2009, 183, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Kempuraj, D.; Kandere, K.; Di Gioacchino, M.; Barbacane, R.C.; Castellani, M.L.; Felaco, M.; Boucher, W.; Letourneau, R.; Theoharides, T.C. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 2003, 86, 123–129. [Google Scholar] [CrossRef]
- Nakagome, K.; Dohi, M.; Okunishi, K.; Tanaka, R.; Miyazaki, J.; Yamamoto, K. In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-beta in the lung. Thorax 2006, 61, 886–894. [Google Scholar] [CrossRef]
- Tseng, C.C.; Sung, Y.W.; Chen, K.Y.; Wang, P.Y.; Yen, C.Y.; Sung, W.Y.; Wu, C.C.; Ou, T.T.; Tsai, W.C.; Liao, W.T.; et al. The Role of Macrophages in Connective Tissue Disease-Associated Interstitial Lung Disease: Focusing on Molecular Mechanisms and Potential Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11995. [Google Scholar] [CrossRef] [PubMed]
- Desdin-Mico, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabande-Rodriguez, E.; Blanco, E.M.; Alfranca, A.; Cusso, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Guy, T.; Pillai, S. "Age could not wither her..." because of her cytotoxic CD4(+) T cells? Sci. Immunol. 2023, 8, eadi4345. [Google Scholar] [CrossRef] [PubMed]
- Fagnoni, F.F.; Vescovini, R.; Mazzola, M.; Bologna, G.; Nigro, E.; Lavagetto, G.; Franceschi, C.; Passeri, M.; Sansoni, P. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology 1996, 88, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28− T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, H.F.; Effros, R.B. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin. Immunol. 2002, 105, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Boix, O.; Garcia-Garijo, A.; Sirois, I.; Caballe, A.; Zarzuela, E.; Ruano, I.; Attolini, C.S.; Prats, N.; Lopez-Dominguez, J.A.; et al. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023, 13, 410–431. [Google Scholar] [CrossRef] [PubMed]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci. Transl. Med. 2018, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.E.; Scholz, J.L.; Quinn, W.J., 3rd; Stadanlick, J.E.; Treml, J.F.; Treml, L.S.; Hao, Y.; Goenka, R.; O’Neill, P.J.; Matthews, A.H.; et al. Homeostatic control of B lymphocyte subsets. Immunol. Res. 2008, 42, 75–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donahoe, M.; Valentine, V.G.; Chien, N.; Gibson, K.F.; Raval, J.S.; Saul, M.; Xue, J.; Zhang, Y.; Duncan, S.R. Autoantibody-Targeted Treatments for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. PLoS ONE 2015, 10, e0127771. [Google Scholar] [CrossRef]
- Hoyne, G.F.; Elliott, H.; Mutsaers, S.E.; Prele, C.M. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol. Cell Biol. 2017, 95, 577–583. [Google Scholar] [CrossRef]
- Storch, H.; Zimmermann, B.; Resch, B.; Tykocinski, L.O.; Moradi, B.; Horn, P.; Kaya, Z.; Blank, N.; Rehart, S.; Thomsen, M.; et al. Activated human B cells induce inflammatory fibroblasts with cartilage-destructive properties and become functionally suppressed in return. Ann. Rheum. Dis. 2016, 75, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, A.; Burton, D.G.; Moshayev, Z.; Vadai, E.; Wensveen, F.; Ben-Dor, S.; Golani, O.; Polic, B.; Krizhanovsky, V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 2016, 8, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8(+) T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, N.; Casas-Recasens, S.; Olvera, N.; Hernandez-Gonzalez, F.; Cruz, T.; Albacar, N.; Alsina-Restoy, X.; Frino-Garcia, A.; Lopez-Saiz, G.; Robres, L.; et al. Blood Immunophenotypes of Idiopathic Pulmonary Fibrosis: Relationship with Disease Severity and Progression. Int. J. Mol. Sci. 2023, 24, 13832. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; Cameli, P.; d’Alessandro, M.; Vagaggini, C.; Refini, R.M.; Landi, C.; Pieroni, M.G.; Spalletti, M.; Sestini, P.; Bargagli, E. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin. Exp. Med. 2019, 19, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.; Jia, M.; Sembrat, J.; Tabib, T.; Agostino, N.; Bruno, T.C.; Vignali, D.; Sanchez, P.; Lafyatis, R.; Mora, A.L.; et al. Reduced Proportion and Activity of Natural Killer Cells in the Lung of Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Greiffo, F.R.; Frankenberger, M.; Bandres, J.; Heinzelmann, K.; Neurohr, C.; Hatz, R.; Hartl, D.; Behr, J.; Eickelberg, O. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur. Respir. J. 2016, 48, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Menard, C.; Martin, F.; Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: Relevance during tumor progression. Immunol. Rev. 2006, 214, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, A.N.R.; Suo, S.; Badrinath, S.; Kumar, S.; Melms, J.; Luoma, A.; Bagati, A.; Saadatpour, A.; Izar, B.; Yuan, G.C.; et al. Immunosuppressive Myeloid Cells Induce Nitric Oxide-Dependent DNA Damage and p53 Pathway Activation in CD8(+) T Cells. Cancer Immunol. Res. 2021, 9, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Koether, K.; Besnard, V.; Sandig, H.; Carruthers, A.; Miranda, E.; Grootenboer-Mignot, S.; Taille, C.; Chevret, S.; Valeyre, D.; Nunes, H.; et al. Autoantibodies are associated with disease progression in idiopathic pulmonary fibrosis. Eur. Respir. J. 2023, 61, 2102381. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Kolb, M. A novel take on idiopathic pulmonary fibrosis disease progression: Localised autoimmunity. Eur. Respir. J. 2023, 61, 2300653. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.M.; Lozada-Martinez, I.D.; Torres, I.; Shoenfeld, Y. Autoimmunity in centenarians. A paradox. J. Transl. Autoimmun. 2024, 8, 100237. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.M.; Chen, D.Y. Biological ageing: A promising target for prevention and management of rheumatoid arthritis. Lancet Healthy Longev. 2024, 5, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Han, M.; Rui, K.; Ai, X.; Tian, J.; Zhang, W.; Zhao, F.; Zhao, Y.; Jiang, Q.; Lu, L. New insights into follicular helper T cell response and regulation in autoimmune pathogenesis. Cell Mol. Immunol. 2021, 18, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Du, W.; Wang, X.; Yuan, S.; Cai, X.; Liu, D.; Li, J.; Lu, L. Multiple Functions of B Cells in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 6021. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Han, M.; Zhu, X.; Xiao, F.; Huang, E.; Che, N.; Tang, X.; Zou, H.; Jiang, Q.; Lu, L. The Multiple Roles of B Cells in the Pathogenesis of Sjogren’s Syndrome. Front. Immunol. 2021, 12, 684999. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fujii, H.; Weyand, C.M. Telomeres, immune aging and autoimmunity. Exp. Gerontol. 2006, 41, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hodes, R.J.; Hathcock, K.S.; Weng, N.P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated With Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, A.V.; Rubtsova, K.; Fischer, A.; Meehan, R.T.; Gillis, J.Z.; Kappler, J.W.; Marrack, P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 2011, 118, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Aouba, A.; Mouthon, L.; Londono-Vallejo, J.A.; Lepelletier, Y.; Gabet, A.S.; Hermine, O. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmun. Rev. 2010, 9, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.Y.; Chen, J.J.; Cogan, J.D.; Alder, J.K.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A., 3rd; et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Koetz, K.; Bryl, E.; Spickschen, K.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9203–9208. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Oates, J.C.; Markiewicz, M. Association Between the Anti-Aging Gene Klotho and Selected Rheumatologic Autoimmune Diseases. Am. J. Med. Sci. 2021, 361, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.W.; Duncan, D.; Helton, S.; Hutcheson, S.; Kurundkar, D.; Logsdon, N.J.; Locy, M.; Garth, J.; Denson, R.; Farver, C.; et al. Role of fibroblast growth factor 23 and klotho cross talk in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L141–L154. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Haque, S.; Rakieh, C.; Marriage, F.; Ho, P.; Gorodkin, R.; Teh, L.S.; Snowden, N.; Day, P.J.; Bruce, I.N. Shortened telomere length in patients with systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hsieh, S.C.; Li, K.J.; Lu, M.C.; Yu, C.L. Premature telomere shortening in polymorphonuclear neutrophils from patients with systemic lupus erythematosus is related to the lupus disease activity. Lupus 2007, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Haak, A.J.; Tschumperlin, D.J.; LeBrasseur, N.K. Targeting Senescent Cells in Fibrosis: Pathology, Paradox, and Practical Considerations. Curr. Rheumatol. Rep. 2018, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Miller, J.D.; LeBrasseur, N.K. Cellular senescence: Implications for metabolic disease. Mol. Cell Endocrinol. 2017, 455, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martinez, F.; Picallos-Rabina, P.; Da Silva-Alvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Pedrosa, P.; Ferreiros, A.; Barradas, M.; et al. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, Z.N.; Zubova, S.G.; Bukreeva, E.I.; Pospelov, V.A.; Pospelova, T.V.; Blagosklonny, M.V. Rapamycin decelerates cellular senescence. Cell Cycle 2009, 8, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Sun, B.; Gutierrez-Munoz, C.; Hernandez-Gonzalez, F.; Mellone, M.; Guiho, R.; Duran, I.; Pombo, J.; Pietrocola, F.; Birch, J.; et al. COPI vesicle formation and N-myristoylation are targetable vulnerabilities of senescent cells. Nat. Cell Biol. 2023, 25, 1804–1820. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Deschenes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Martin-Montalvo, A.; Dluzen, D.F.; Zhang, Y.; Bernier, M.; Zonderman, A.B.; Becker, K.G.; Gorospe, M.; de Cabo, R.; Evans, M.K. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 2016, 15, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Ozsvari, B.; Nuttall, J.R.; Sotgia, F.; Lisanti, M.P. Azithromycin and Roxithromycin define a new family of "senolytic" drugs that target senescent human fibroblasts. Aging 2018, 10, 3294–3307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Li, W.C.; Tang, B.X.; Li, J.; Zang, Y. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol. Sin. 2021, 42, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, G.; Luckhardt, T.; Antony, V.; Zhou, Y.; Carter, A.B.; Thannickal, V.J.; Liu, R.-M. Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease. Aging Cell 2017, 16, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hollstein, M.; Xu, Y. Ser46 phosphorylation regulates p53-dependent apoptosis and replicative senescence. Cell Cycle 2006, 5, 2812–2819. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Gonzalez, F.; Pietrocola, F.; Cameli, P.; Bargagli, E.; Prieto-González, S.; Cruz, T.; Mendoza, N.; Rojas, M.; Serrano, M.; Agustí, A.; et al. Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities. Int. J. Mol. Sci. 2024, 25, 7554. https://doi.org/10.3390/ijms25147554
Hernandez-Gonzalez F, Pietrocola F, Cameli P, Bargagli E, Prieto-González S, Cruz T, Mendoza N, Rojas M, Serrano M, Agustí A, et al. Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities. International Journal of Molecular Sciences. 2024; 25(14):7554. https://doi.org/10.3390/ijms25147554
Chicago/Turabian StyleHernandez-Gonzalez, Fernanda, Federico Pietrocola, Paolo Cameli, Elena Bargagli, Sergio Prieto-González, Tamara Cruz, Nuria Mendoza, Mauricio Rojas, Manuel Serrano, Alvar Agustí, and et al. 2024. "Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities" International Journal of Molecular Sciences 25, no. 14: 7554. https://doi.org/10.3390/ijms25147554
APA StyleHernandez-Gonzalez, F., Pietrocola, F., Cameli, P., Bargagli, E., Prieto-González, S., Cruz, T., Mendoza, N., Rojas, M., Serrano, M., Agustí, A., Faner, R., Gómez-Puerta, J. A., & Sellares, J. (2024). Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities. International Journal of Molecular Sciences, 25(14), 7554. https://doi.org/10.3390/ijms25147554









