Constructing a Transient Ischemia Attack Model Utilizing Flexible Spatial Targeting Photothrombosis with Real-Time Blood Flow Imaging Feedback
Abstract
:1. Introduction
2. Results
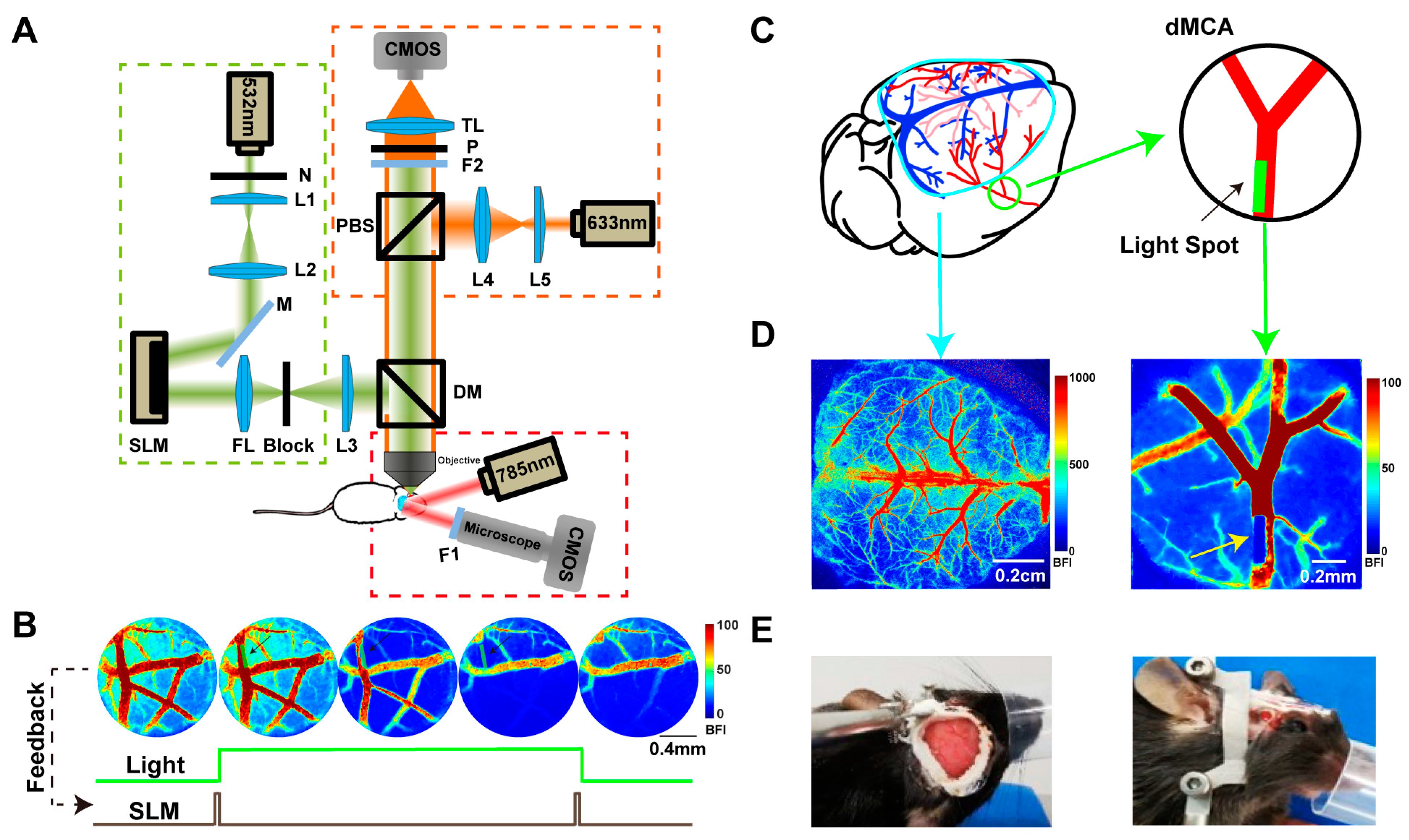
2.1. Establishment of a Photochemical TIA Model
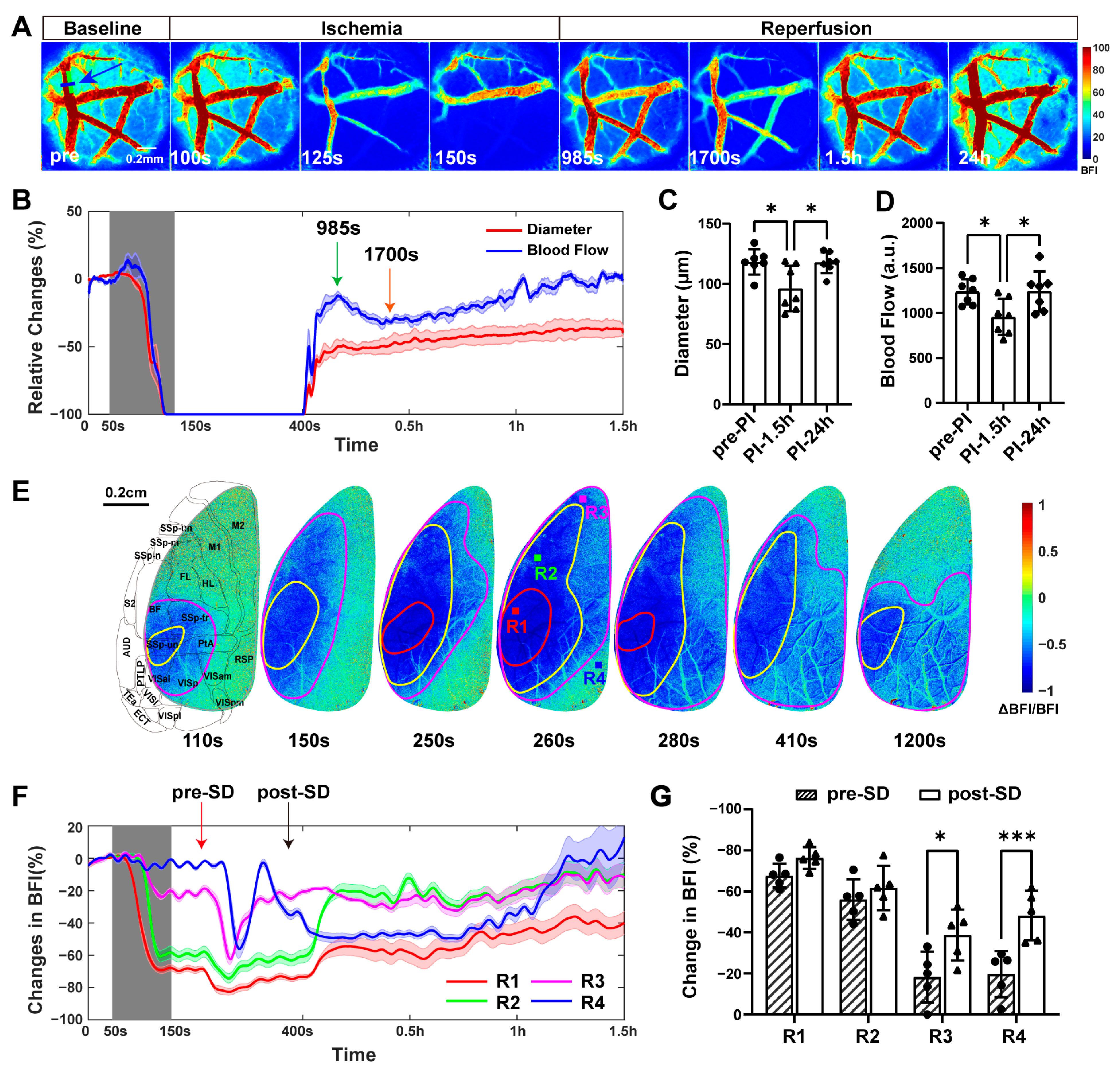
2.1.1. Condition 1: Occlusion and Reperfusion of the Distal Middle Cerebral Artery
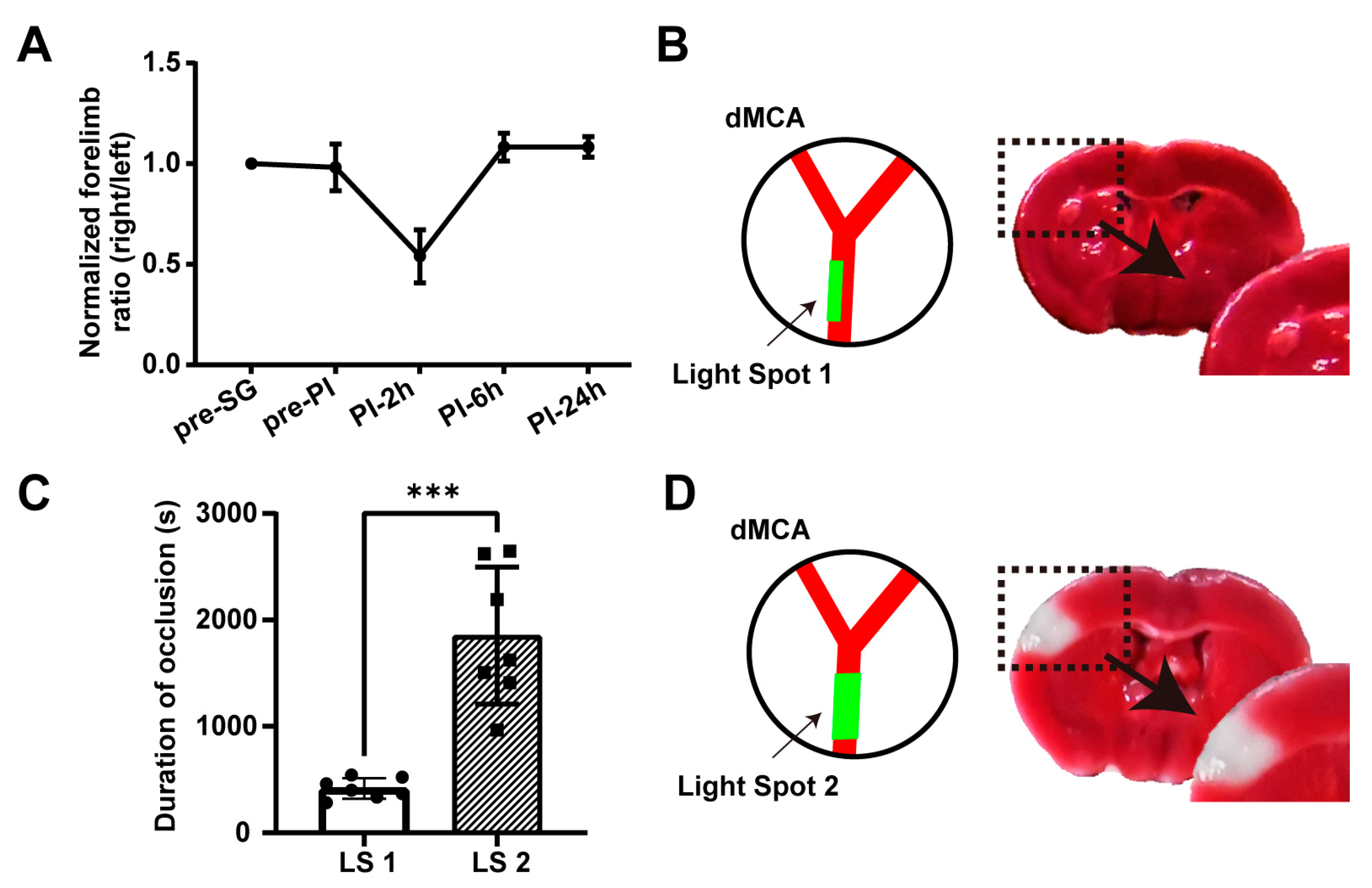
2.1.2. Conditions 2 and 3: No Permanent Neurological Deficits and No Acute Cerebral Infarction
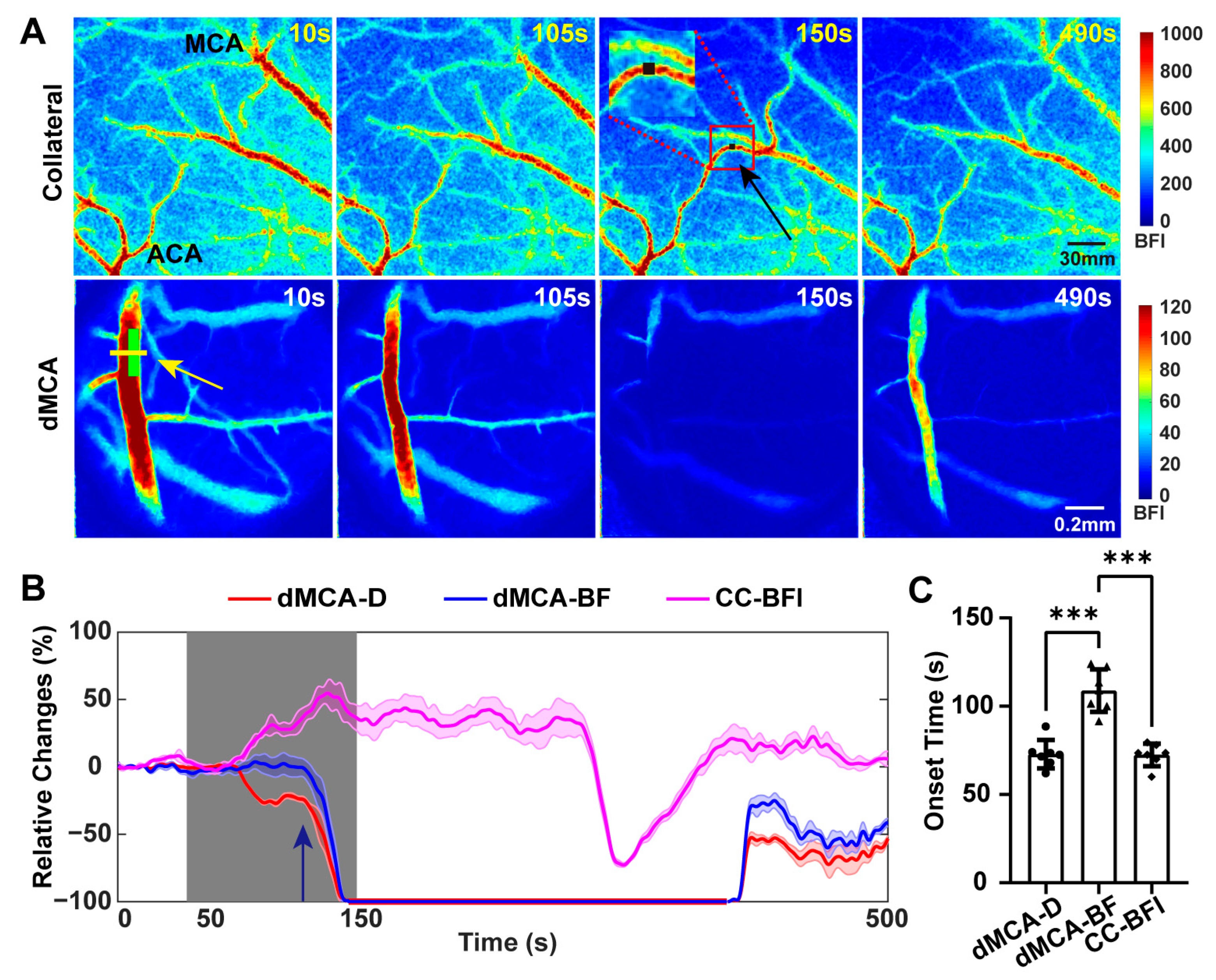
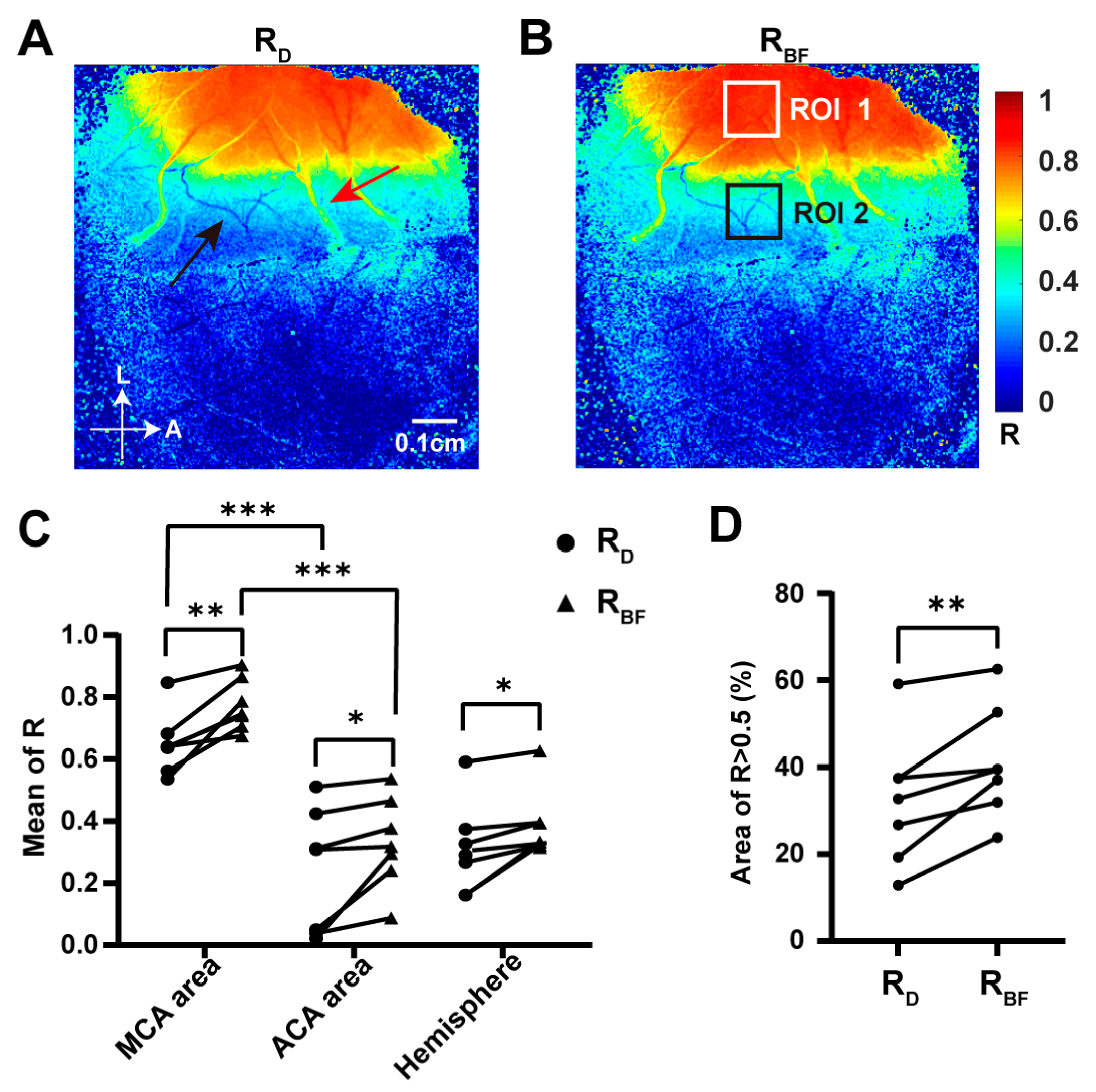
2.2. Establishment of Collateral Circulation Observed in the Dorsal Cortex during TIA
2.3. Thrombus Could Be Observed Downstream from the dMCA after TIA
2.4. Blood Flow in the dMCA Acted as a More Reliable Indicator of Ischemic Changes in the Cortex Than the Vascular Diameter Did
2.5. Inducing Secondary Transient Ischemia at the Same Location in the dMCA
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
4.2. Imaging System
4.3. Photochemical Ischemia
4.4. TTC Staining
4.5. Antibodies and Fluorescent Tracers
4.6. Cylinder Test
4.7. Data Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and Evaluation of Transient Ischemic Attack: A Scientific Statement for Healthcare Professionals from the American Heart association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardio. Stroke 2009, 40, 2276–2293. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Warlow, C.P. Timing of TIAs Preceding Stroke: Time Window for Prevention Is Very Short. Neurology 2005, 64, 817–820. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, E364–E467. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnaya, Y.N.; Khaitin, A.M.; Demyanenko, S.V. Modeling Transient Ischemic Attack via Photothrombosis. Biophys. Rev. 2023, 15, 1279–1286. [Google Scholar] [CrossRef]
- Durukan Tolvanen, A.; Tatlisumak, E.; Pedrono, E.; Abo-Ramadan, U.; Tatlisumak, T. TIA Model Is Attainable in Wistar Rats by Intraluminal Occlusion of the MCA for 10 Min or Shorter. Brain Res. 2017, 1663, 166–173. [Google Scholar] [CrossRef]
- Pedrono, E.; Durukan, A.; Strbian, D.; Marinkovic, I.; Shekhar, S.; Pitkonen, M.; Abo-Ramadan, U.; Tatlisumak, T. An Optimized Mouse Model for Transient Ischemic Attack. J. Neuropathol. Exp. Neurol. 2010, 69, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Yu, H.; Li, G.; Bai, S.; Chen, S.; Zhang, P.; Tang, Z. Dl-3-N-Butylphthalide Promotes Angiogenesis in an Optimized Model of Transient Ischemic Attack in C57BL/6 Mice. Front. Pharmacol. 2021, 12, 751397. [Google Scholar] [CrossRef] [PubMed]
- Berthet, C.; Lei, H.; Gruetter, R.; Hirt, L. Early Predictive Biomarkers for Lesion after Transient Cerebral Ischemia. Stroke 2011, 42, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Emmrich, J.V.; Sawiak, S.J.; Williamson, D.J.; Baron, J.C. Cortical Selective Neuronal Loss, Impaired Behavior, and Normal Magnetic Resonance Imaging in a New Rat Model of True Transient Ischemic Attacks. Stroke 2015, 46, 1084–1092. [Google Scholar] [CrossRef]
- Knapp, L.; Gellért, L.; Herédi, J.; Kocsis, K.; Oláh, G.; Fuzik, J.; Kis, Z.; Vécsei, L.; Toldi, J.; Farkas, T. A Simple Novel Technique to Induce Short-Lasting Local Brain Ischaemia in the Rat. Neuropathol. Appl. Neurobiol. 2014, 40, 603–609. [Google Scholar] [CrossRef]
- Barthels, D.; Das, H. Current Advances in Ischemic Stroke Research and Therapies. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Schroeter, M.; Jander, S.; Stoll, G. Non-Invasive Induction of Focal Cerebral Ischemia in Mice by Photothrombosis of Cortical Microvessels: Characterization of Inflammatory Responses. J. Neurosci. Methods 2002, 117, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Kuo, Y.-M.; Chen, H.-R.; Short-Miller, J.C.; Smucker, M.R.; Kuan, C.-Y. A Murine Photothrombotic Stroke Model with an Increased Fibrin Content and Improved Responses to tPA-Lytic Treatment. Blood Adv. 2020, 4, 1222–1231. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Tuor, U.I.; Deng, Q.; Rushforth, D.; Foniok, T.; Qiao, M. Model of Minor Stroke with Mild Peri-Infarct Ischemic Injury. J. Neurosci. Methods 2016, 268, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics-2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e39. [Google Scholar] [CrossRef]
- Taylor, Z.; Shih, A. Targeted Occlusion of Individual Pial Vessels of Mouse Cortex. Bio-Protocol 2013, 3, e897. [Google Scholar] [CrossRef]
- Delafontaine-Martel, P.; Zhang, C.; Lu, X.; Damseh, R.; Lesage, F.; Marchand, P.J. Targeted Capillary Photothrombosis via Multiphoton Excitation of Rose Bengal. J. Cereb. Blood Flow Metab. 2023, 43, 1713–1725. [Google Scholar] [CrossRef]
- Liu, Z.; He, B.; Wang, X.; Peng, J.; Sun, Q.; Luo, C. Deep Cortical Microinfarction Induced by Femtosecond Laser in Mice: Long-Term Secondary Pathological Changes in Corresponding Superficial Cortex. Neurosci. Lett. 2023, 802, 137170. [Google Scholar] [CrossRef]
- Yao, H.; Nabika, T. Characterizing Photothrombotic Distal Middle Cerebral Artery Occlusion and YAG Laser-Induced Reperfusion Model in the Izumo Strain of Spontaneously Hypertensive Rats. Cell. Mol. Neurobiol. 2011, 31, 57–63. [Google Scholar] [CrossRef]
- Qian, C.; Li, P.-C.; Jiao, Y.; Yao, H.-H.; Chen, Y.-C.; Yang, J.; Ding, J.; Yang, X.-Y.; Teng, G.-J. Precise Characterization of the Penumbra Revealed by MRI: A Modified Photothrombotic Stroke Model Study. PLoS ONE 2016, 11, e0153756. [Google Scholar] [CrossRef] [PubMed]
- Vaz, P.G.; Humeau-Heurtier, A.; Figueiras, E.; Correia, C.; Cardoso, J. Laser Speckle Imaging to Monitor Microvascular Blood Flow: A Review. IEEE Rev. Biomed. Eng. 2016, 9, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, W.; Zhou, F.; Li, P.; Luo, Q. Dynamic Change of Collateral Flow Varying with Distribution of Regional Blood Flow in Acute Ischemic Rat Cortex. J. Biomed. Opt. 2012, 17, 125001. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Li, Y.; Zhu, X.; Chen, M.; Lu, J.; Li, P. Selective Photoactivation of Neural Activity Combined with Laser Speckle Imaging of Cerebral Blood Flow. Opt. Lett. 2018, 43, 3798. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Knutsson, L.; Zhou, J.; van Zijl, P.C.M. Imaging the Physiological Evolution of the Ischemic Penumbra in Acute Ischemic Stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1500–1516. [Google Scholar] [CrossRef] [PubMed]
- Ermine, C.M.; Bivard, A.; Parsons, M.W.; Baron, J.C. The Ischemic Penumbra: From Concept to Reality. Int. J. Stroke 2021, 16, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.T.; Tuohy, M.C.; Chow, D.; Kozberg, M.G.; Kim, S.H.; Shaik, M.A.; Hillman, E.M.C. Neurovascular Dynamics of Repeated Cortical Spreading Depolarizations after Acute Brain Injury. Cell Rep. 2021, 37, 109794. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Burdett, N.G.; Carpenter, T.A.; Hall, L.D.; Pambakian, P.S.; Patel, S.; Wood, N.I.; James, M.F. Evolution of Photochemically Induced Focal Cerebral Ischemia in the Rat: Magnetic Resonance Imaging and Histology. Stroke 1996, 27, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.; Tatlisumak, T. Chapter 3 Animal Models of Ischemic Stroke. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 92, pp. 43–66. [Google Scholar]
- Purroy, F.; Jimenez Caballero, P.E.; Gorospe, A.; Torres, M.J.; Alvarez-Sabin, J.; Santamarina, E.; Martinez-Sanchez, P.; Canovas, D.; Freijo, M.J.; Egido, J.A.; et al. Recurrent Transient Ischaemic Attack and Early Risk of Stroke: Data from the PROMAPA Study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 596–603. [Google Scholar] [CrossRef]
- Coutts, S.B.; Modi, J.; Patel, S.K.; Demchuk, A.M.; Goyal, M.; Hill, M.D. CT/CT Angiography and MRI Findings Predict Recurrent Stroke After Transient Ischemic Attack and Minor Stroke. Stroke 2012, 43, 1013–1017. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Tang, Z. Animal Models of Transient Ischemic Attack: A Review. Acta Neurol. Belg. 2020, 120, 267–275. [Google Scholar] [CrossRef]
- Conti, E.; Carlini, N.; Piccardi, B.; Allegra Mascaro, A.L.; Pavone, F.S. Photothrombotic Middle Cerebral Artery Occlusion in Mice: A Novel Model of Ischemic Stroke. eNeuro 2023, 10, ENEURO.0244-22.2022. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Ham, J.; Duc, N.T.; Lee, B. Quantification of Stroke Lesion Volume Using Epidural EEG in a Cerebral Ischaemic Rat Model. Sci. Rep. 2021, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, M.; Fu, P.; Liu, Y.; Zhang, H.; Roe, A.W.; Xi, W. Precision 1070 Nm Ultrafast Laser-Induced Photothrombosis of Depth-Targeted Vessels In Vivo. Small Methods 2023, 7, e2200917. [Google Scholar] [CrossRef]
- Fukuda, M.; Matsumura, T.; Suda, T.; Hirase, H. Depth-Targeted Intracortical Microstroke by Two-Photon Photothrombosis in Rodent Brain. Neurophotonics 2022, 9, 021910. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-S.; Hsieh, F.-I.; Yeh, H.-L.; Chen, W.-H.; Chiu, H.-C.; Tang, S.-C.; Liu, C.-H.; Lin, H.-J.; Hsu, S.-P.; Lo, Y.-K.; et al. Impact of MCA Stenosis on the Early Outcome in Acute Ischemic Stroke Patients. PLoS ONE 2017, 12, e0175434. [Google Scholar] [CrossRef]
- Dreier, J.P. The Role of Spreading Depression, Spreading Depolarization and Spreading Ischemia in Neurological Disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M.; Dreier, J.P.; Fabricius, M.; Hartings, J.A.; Graf, R.; Strong, A.J. Clinical Relevance of Cortical Spreading Depression in Neurological Disorders: Migraine, Malignant Stroke, Subarachnoid and Intracranial Hemorrhage, and Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2011, 31, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Arsava, E.M.; Gurer, G.; Gursoy-Ozdemir, Y.; Karatas, H.; Dalkara, T. A New Model of Transient Focal Cerebral Ischemia for Inducing Selective Neuronal Necrosis. Brain Res. Bull. 2009, 78, 226–231. [Google Scholar] [CrossRef]
- Walz, E.T.; Brink, T.; Slivka, A. Pattern and Frequency of Recurrent Transient Ischemic Attacks. J. Stroke Cerebrovasc. Dis. 1997, 6, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chaudhari, K.; Winters, A.; Sun, Y.; Berry, R.; Tang, C.; Yang, S.-H.; Liu, R. Recurrent Transient Ischemic Attack Induces Neural Cytoskeleton Modification and Gliosis in an Experimental Model. Transl. Stroke Res. 2023, 14, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Tuor, U.I.; Zhao, Z.; Barber, P.A.; Qiao, M. Recurrent Mild Cerebral Ischemia: Enhanced Brain Injury Following Acute Compared to Subacute Recurrence in the Rat. BMC Neurosci. 2016, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stalker, T.J.; Traxler, E.A.; Wu, J.; Wannemacher, K.M.; Cermignano, S.L.; Voronov, R.; Diamond, S.L.; Brass, L.F. Hierarchical Organization in the Hemostatic Response and Its Relationship to the Platelet-Signaling Network. Blood 2013, 121, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fan, Y.; Zhang, F.; Lin, Y. Cerebral Microinfarct Is Emergency Consequence of Alzheimer’s Disease: A New Insight into Development of Neurodegenerative Diseases. Int. J. Biol. Sci. 2022, 18, 1569–1579. [Google Scholar] [CrossRef]
- Wang, M.; Iliff, J.J.; Liao, Y.; Chen, M.J.; Shinseki, M.S.; Venkataraman, A.; Cheung, J.; Wang, W.; Nedergaard, M. Cognitive Deficits and Delayed Neuronal Loss in a Mouse Model of Multiple Microinfarcts. J. Neurosci. 2012, 32, 17948–17960. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Kuo, Y.M.; Tsai, Y.H. Transient Ischemic Attack Induced by Melted Solid Lipid Microparticles Protects Rat Brains from Permanent Focal Ischemia. Neuroscience 2014, 275, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.C.; Woods, S.D.; Brown, A.T.; Lowery, J.D.; Hennings, L.J.; Skinner, R.D.; Borrelli, M.J.; Roberson, P.K. Three Variations in Rabbit Angiographic Stroke Models. J. Neurosci. Methods 2013, 212, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Li, A.; Lou, Y.; Chen, S.; Long, B.; Peng, J.; Yang, Z.; Xu, T.; Yang, X.; Li, X.; et al. Precise Cerebral Vascular Atlas in Stereotaxic Coordinates of Whole Mouse Brain. Front. Neuroanat. 2017, 11, 128. [Google Scholar] [CrossRef]
- Dickinson, R.; Peterson, B.K.; Banks, P.; Simillis, C.; Martin, J.C.S.; Valenzuela, C.A.; Maze, M.; Franks, N.P. Competitive Inhibition at the Glycine Site of the N-Methyl-D-Aspartate Receptor by the Anesthetics Xenon and Isoflurane: Evidence from Molecular Modeling and Electrophysiology. Anesthesiology 2007, 107, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective Strategies for Ischemic Stroke—Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4334. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Zhu, L.; Guo, J.; Zeng, L.; Gong, Q.; He, L.; Chen, H. Aberrant Functional Connectivity of Resting State Networks in Transient Ischemic Attack. PLoS ONE 2013, 8, e71009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, L.; Song, Y.; Han, Y.; Zhou, C.; Zhou, D.; Zhang, F.; Xue, Q.; Liu, J.; Zhao, L.; et al. Altered Functional Connectivity within Default Mode Network in Patients with Transient Ischemic Attack: A Resting-State Functional Magnetic Resonance Imaging Study. Cerebrovasc. Dis. 2019, 48, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Etgen, A.M. Neuroprotective Action of Acute Estrogens: Animal Models of Brain Ischemia and Clinical Implications. Steroids 2013, 78, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.J.; Shih, A.Y.; Driscoll, J.D.; Knutsen, P.M.; Blinder, P.; Davalos, D.; Akassoglou, K.; Tsai, P.S.; Kleinfeld, D. Chronic Optical Access through a Polished and Reinforced Thinned Skull. Nat. Methods 2010, 7, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Silasi, G.; Xiao, D.; Vanni, M.P.; Chen, A.C.N.; Murphy, T.H. Intact Skull Chronic Windows for Mesoscopic Wide-Field Imaging in Awake Mice. J. Neurosci. Methods 2016, 267, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Llovera, G.; Roth, S.; Plesnila, N.; Veltkamp, R.; Liesz, A. Modeling Stroke in Mice: Permanent Coagulation of the Distal Middle Cerebral Artery. J. Vis. Exp. 2014, 89, e51729. [Google Scholar] [CrossRef]
- Choi, W.J.; Li, Y.; Wang, R.K. Monitoring Acute Stroke Progression: Multi-Parametric OCT Imaging of Cortical Perfusion, Flow, and Tissue Scattering in a Mouse Model of Permanent Focal Ischemia. IEEE Trans. Med. Imaging 2019, 38, 1427–1437. [Google Scholar] [CrossRef]
- Liao, L.-D.; Bandla, A.; Ling, J.M.; Liu, Y.-H.; Kuo, L.-W.; Chen, Y.-Y.; King, N.K.; Lai, H.-Y.; Lin, Y.-R.; Thakor, N.V. Improving Neurovascular Outcomes with Bilateral Forepaw Stimulation in a Rat Photothrombotic Ischemic Stroke Model. Neurophotonics 2014, 1, 11007. [Google Scholar] [CrossRef]
- van Golen, R.F.; Stevens, K.M.; Colarusso, P.; Jaeschke, H.; Heger, M. Platelet Aggregation but Not Activation and Degranulation during the Acute Post-Ischemic Reperfusion Phase in Livers with No Underlying Disease. J. Clin. Transl. Res. 2015, 1, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, M.G.; Trueman, R.C.; Boltze, J.; Corbett, D.; Jolkkonen, J. Behavioral Outcome Measures to Improve Experimental Stroke Research. Behav. Brain Res. 2018, 352, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Schaar, K.L.; Brenneman, M.M.; Savitz, S.I. Functional Assessments in the Rodent Stroke Model. Exp. Transl. Stroke Med. 2010, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.D.; Kirkpatrick, S.J. Spatio-Temporal Algorithms for Processing Laser Speckle Imaging Data. In Proceedings of the Optics in Tissue Engineering and Regenerative Medicine II, San Jose, CA, USA, 20–21 January 2008; SPIE: St Bellingham, WA, USA, 2008; Volume 6858, p. 685802. [Google Scholar]
- Qiu, J. Spatiotemporal Laser Speckle Contrast Analysis for Blood Flow Imaging with Maximized Speckle Contrast. J. Biomed. Opt. 2010, 15, 16003. [Google Scholar] [CrossRef]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser Speckle Contrast Imaging: Theoretical and Practical Limitations. J. Biomed. Opt. 2013, 18, 66018. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yi, Z.; Li, R.; Wang, C.; Zhu, W.; Ma, M.; Lu, J.; Li, P. Constructing a Transient Ischemia Attack Model Utilizing Flexible Spatial Targeting Photothrombosis with Real-Time Blood Flow Imaging Feedback. Int. J. Mol. Sci. 2024, 25, 7557. https://doi.org/10.3390/ijms25147557
Zhu X, Yi Z, Li R, Wang C, Zhu W, Ma M, Lu J, Li P. Constructing a Transient Ischemia Attack Model Utilizing Flexible Spatial Targeting Photothrombosis with Real-Time Blood Flow Imaging Feedback. International Journal of Molecular Sciences. 2024; 25(14):7557. https://doi.org/10.3390/ijms25147557
Chicago/Turabian StyleZhu, Xuan, Zichao Yi, Ruolan Li, Chen Wang, Wenting Zhu, Minghui Ma, Jinling Lu, and Pengcheng Li. 2024. "Constructing a Transient Ischemia Attack Model Utilizing Flexible Spatial Targeting Photothrombosis with Real-Time Blood Flow Imaging Feedback" International Journal of Molecular Sciences 25, no. 14: 7557. https://doi.org/10.3390/ijms25147557



