The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases
Abstract
1. Introduction
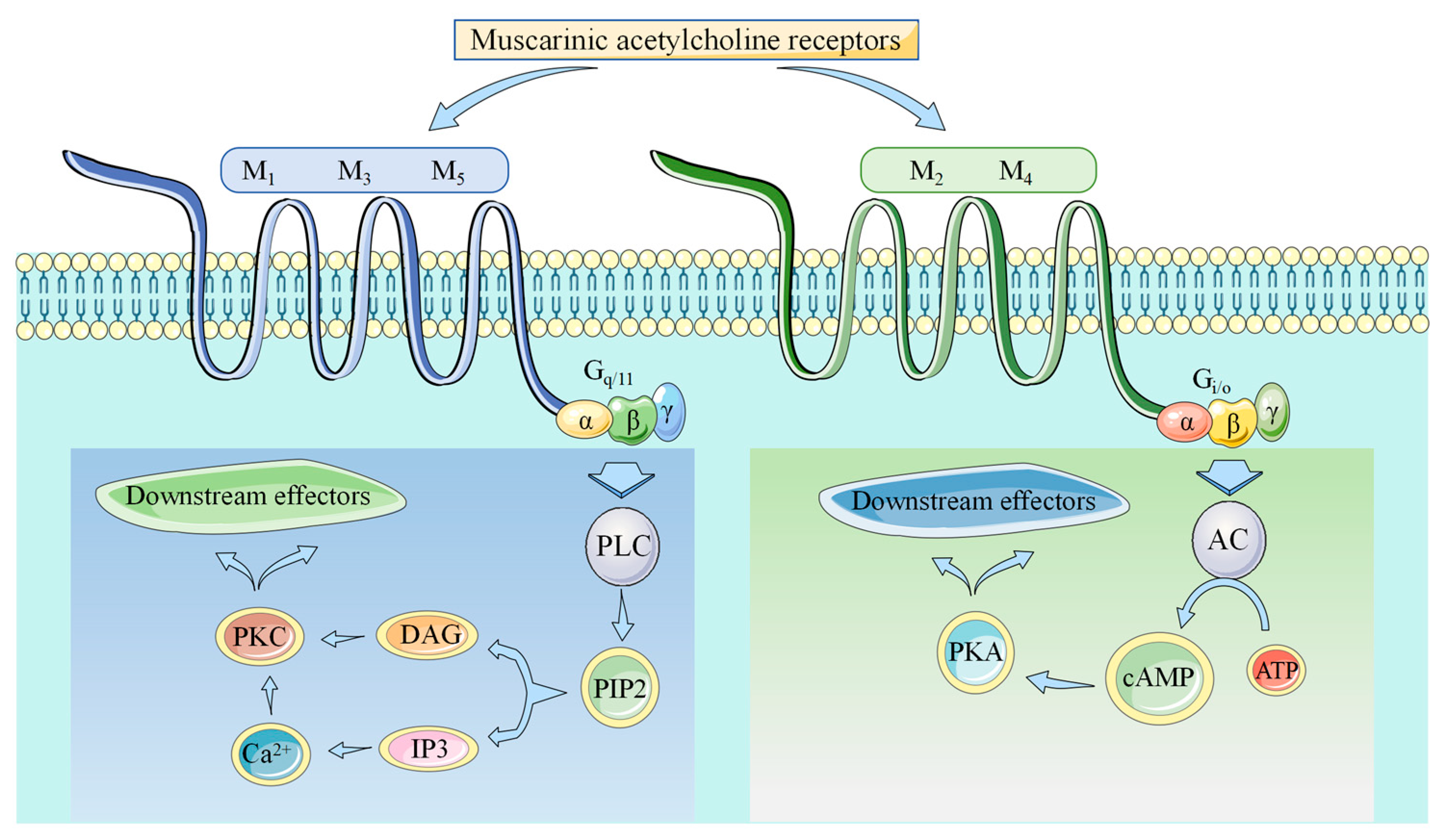
2. Muscarinic Acetylcholine Receptors
3. Choline and M3-mAChR
4. Acetylcholine and M2/M3-mAChR
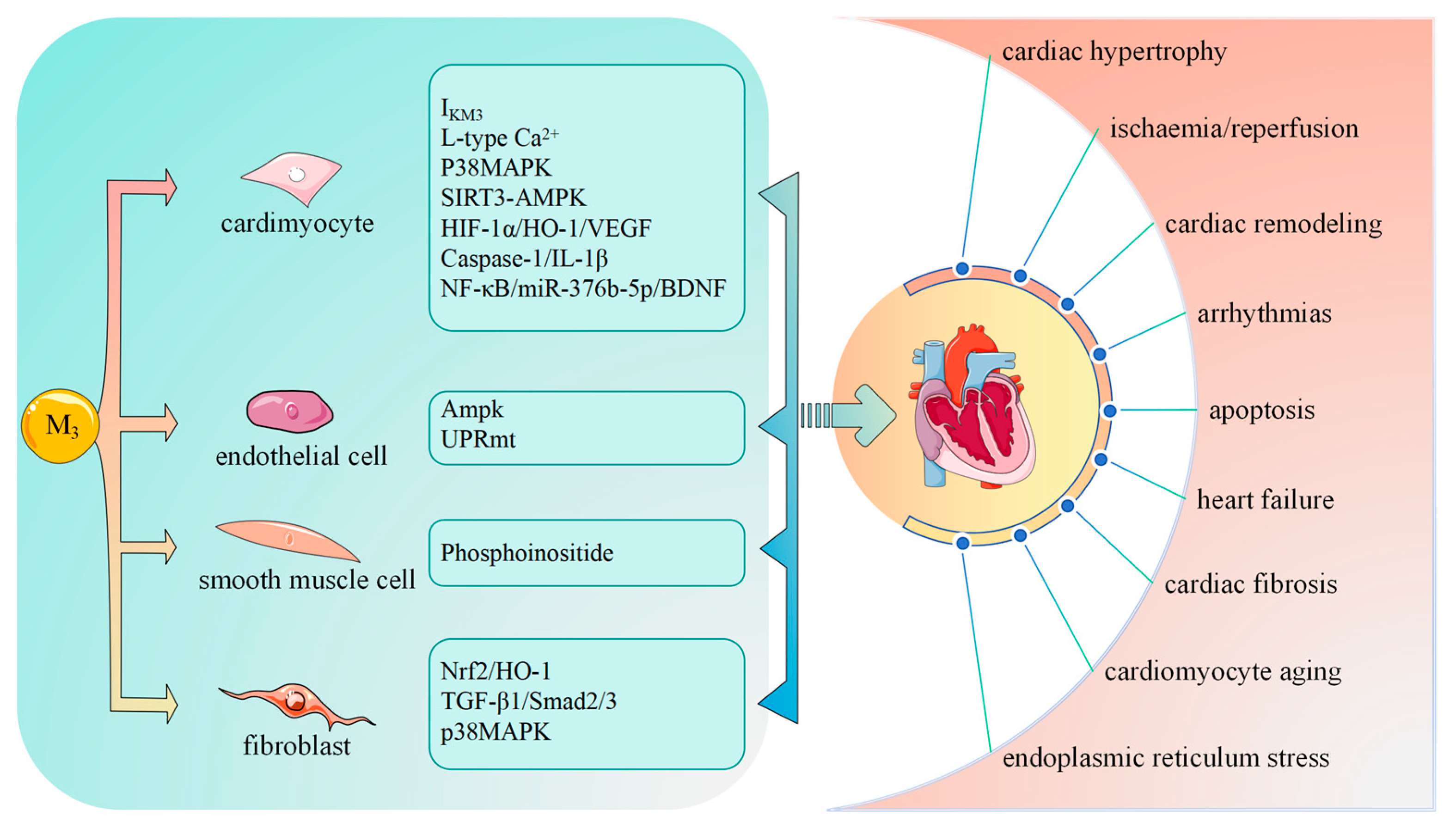
5. M3-mAChR in Different Cardiac Cell Types
5.1. M3-mAChR in Cardiomyocytes
M3-mAChR Signal in Cardiomyocytes
5.2. M3-mAChR in Endothelial Cells
M3-mAChR Signal in Endothelial Cells
5.3. M3-mAChR in Smooth Muscle Cells
M3-mAChR Signal in Smooth Muscle Cells
5.4. M3-mAChR in Fibroblasts
M3-mAChR Signal in Fibroblasts
6. M3-mAChR and Cardiovascular Diseases
6.1. M3-mAChR in Ischemia/Reperfusion
6.2. M3-mAChR in Cardiac Hypertrophy
6.3. M3-mAChR in Heart Failure
7. Future Directions
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulme, E.C.; Birdsall, N.J.; Buckley, N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 633–673. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Chen, J.; Dai, C.; Liu, D.; Pan, W.; Wang, L.; Fasae, M.B.; Sun, L.; Wang, L.; et al. Activation of M3 Muscarinic Acetylcholine Receptors Delayed Cardiac Aging by Inhibiting the Caspase-1/IL-1beta Signaling Pathway. Cell Physiol. Biochem. 2018, 49, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, Y.; Qi, H.; Fan, K.; Wang, S.; Zhao, H.; Fan, Y.; Xie, J.; Guo, F.; Hou, Y.; et al. M3 subtype of muscarinic acetylcholine receptor promotes cardioprotection via the suppression of miR-376b-5p. PLoS ONE 2012, 7, e32571. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Zhang, Y.; Du, Z.; Xiao, J.; Pan, Z.; Wang, N.; Yu, H.; Ma, W.; Qin, H.; Wang, W.H.; et al. Ischemia impairs the association between connexin 43 and M3 subtype of acetylcholine muscarinic receptor (M3-mAChR) in ventricular myocytes. Cell Physiol. Biochem. 2006, 17, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Shiraishi, A.; Murata, J.; Matsubara, S.; Nakaoka, S.; Kirimoto, S.; Osawa, M. Muscarinic receptor M3 contributes to intestinal stem cell maintenance via EphB/ephrin-B signaling. Life Sci. Alliance 2021, 4, e202000962. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.; Zimliki, C.L.; Gautam, D.; Cui, Y.; Mears, D.; Wess, J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes 2004, 53, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Han, S.J.; Hamdan, F.F.; Jeon, J.; Li, B.; Li, J.H.; Cui, Y.; Mears, D.; Lu, H.; Deng, C.; et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Kanda, G.N.; Yamada, R.G.; Shi, S.; Sunagawa, G.A.; Ukai-Tadenuma, M.; Fujishima, H.; Matsumoto, N.; Masumoto, K.H.; Nagano, M.; et al. Muscarinic Acetylcholine Receptors Chrm1 and Chrm3 Are Essential for REM Sleep. Cell Rep. 2018, 24, 2231–2247.e7. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.R.; Pan, H.L. Muscarinic receptor subtypes differentially control synaptic input and excitability of cerebellum-projecting medial vestibular nucleus neurons. J. Neurochem. 2016, 137, 226–239. [Google Scholar] [CrossRef]
- He, X.; Deng, J.; Yu, X.J.; Yang, S.; Yang, Y.; Zang, W.J. Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) Signaling by Choline Alleviates Vascular Smooth Muscle Cell Phenotypic Switching and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2649–2664. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of Muscarinic Receptor Subtypes in Gastrointestinal Smooth Muscle: A Review of Studies with Receptor-Knockout Mice. Int. J. Mol. Sci. 2021, 22, 926. [Google Scholar] [CrossRef]
- Calizo, R.C.; Bell, M.K.; Ron, A.; Hu, M.; Bhattacharya, S.; Wong, N.J.; Janssen, W.G.M.; Perumal, G.; Pederson, P.; Scarlata, S.; et al. Cell shape regulates subcellular organelle location to control early Ca2+ signal dynamics in vascular smooth muscle cells. Sci. Rep. 2020, 10, 17866. [Google Scholar] [CrossRef]
- Kato, M.; Kolotuev, I.; Cunha, A.; Gharib, S.; Sternberg, P.W. Extrasynaptic acetylcholine signaling through a muscarinic receptor regulates cell migration. Proc. Natl. Acad. Sci. USA 2021, 118, e1904338118. [Google Scholar] [CrossRef] [PubMed]
- Chernyavsky, A.I.; Arredondo, J.; Wess, J.; Karlsson, E.; Grando, S.A. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J. Cell Biol. 2004, 166, 261–272. [Google Scholar] [CrossRef]
- Rhoden, A.; Speiser, J.; Geertz, B.; Uebeler, J.; Schmidt, K.; de Wit, C.; Eschenhagen, T. Preserved cardiovascular homeostasis despite blunted acetylcholine-induced dilation in mice with endothelial muscarinic M3 receptor deletion. Acta Physiol. 2019, 226, e13262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Su, Y.; Zhang, Y.; Pan, Z.; Yang, L.; Chen, X.; Liu, Y.; Lu, Y.; Du, Z.; Yang, B. Activation of cardiac muscarinic M3 receptors induces delayed cardioprotection by preserving phosphorylated connexin43 and up-regulating cyclooxygenase-2 expression. Br. J. Pharmacol. 2010, 159, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L.; Pan, Z.; Bai, Y.; Wang, N.; Zhao, J.; Xu, C.; Li, Z.; Li, B.; Du, Z.; et al. Overexpression of M3 muscarinic receptor is a novel strategy for preventing sudden cardiac death in transgenic mice. Mol. Med. 2011, 17, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Narayanan, N. Age related alteration in cholinergic but not alpha adrenergic response of rat coronary vasculature. Cardiovasc. Res. 1993, 27, 284–290. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, T.; Hang, P.; Li, W.; Guo, J.; Pan, Y.; Du, J.; Zheng, Y.; Du, Z. Choline Attenuates Cardiac Fibrosis by Inhibiting p38MAPK Signaling Possibly by Acting on M3 Muscarinic Acetylcholine Receptor. Front. Pharmacol. 2019, 10, 1386. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, C.; Song, H.; Han, H.; Hang, P.; Jiang, Y.; Wei, L.; Huo, R.; Sun, L.; et al. Upregulation of M3 muscarinic receptor inhibits cardiac hypertrophy induced by angiotensin II. J. Transl. Med. 2013, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, H.M.; Pan, Z.W.; Hang, P.Z.; Sun, L.H.; Jiang, Y.N.; Song, H.X.; Du, Z.M.; Liu, Y. Choline inhibits angiotensin II-induced cardiac hypertrophy by intracellular calcium signal and p38 MAPK pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 823–831. [Google Scholar] [CrossRef]
- Darby, M.; Schnoeller, C.; Vira, A.; Culley, F.J.; Bobat, S.; Logan, E.; Kirstein, F.; Wess, J.; Cunningham, A.F.; Brombacher, F.; et al. The M3 muscarinic receptor is required for optimal adaptive immunity to helminth and bacterial infection. PLoS Pathog. 2015, 11, e1004636. [Google Scholar] [CrossRef] [PubMed]
- Asashima, H.; Tsuboi, H.; Takahashi, H.; Hirota, T.; Iizuka, M.; Kondo, Y.; Matsui, M.; Matsumoto, I.; Sumida, T. The anergy induction of M3 muscarinic acetylcholine receptor-reactive CD4+ T cells suppresses experimental sialadenitis-like Sjogren’s syndrome. Arthritis Rheumatol. 2015, 67, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, C.; Chen, X.; Zhao, F.; Liu, A.M.; Cho, D.B.; Chong, J.; Yang, P.C. Role of muscarinic receptor activation in regulating immune cell activity in nasal mucosa. Allergy 2010, 65, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Azua, I.; Gautam, D.; Jain, S.; Guettier, J.M.; Wess, J. Critical metabolic roles of beta-cell M3 muscarinic acetylcholine receptors. Life Sci. 2012, 91, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Henquin, J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Wang, S.; Han, H.M.; Jiang, Y.N.; Wang, C.; Song, H.X.; Pan, Z.Y.; Fan, K.; Du, J.; Fan, Y.H.; Du, Z.M.; et al. Activation of cardiac M3 muscarinic acetylcholine receptors has cardioprotective effects against ischaemia-induced arrhythmias. Clin. Exp. Pharmacol. Physiol. 2012, 39, 343–349. [Google Scholar] [CrossRef]
- Tansey, E.M. Henry Dale and the discovery of acetylcholine. Comptes Rendus Biol. 2006, 329, 419–425. [Google Scholar] [CrossRef]
- Moran, S.P.; Maksymetz, J.; Conn, P.J. Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders. Trends Pharmacol. Sci. 2019, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar] [PubMed]
- Hamilton, S.E.; Loose, M.D.; Qi, M.; Levey, A.I.; Hille, B.; McKnight, G.S.; Idzerda, R.L.; Nathanson, N.M. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 13311–13316. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Yohn, S.E.; Popiolek, M.; Miller, A.C.; Felder, C.C. Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia. Am. J. Psychiatry 2022, 179, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Colecraft, H.M.; Egamino, J.P.; Sharma, V.K.; Sheu, S.S. Signaling mechanisms underlying muscarinic receptor-mediated increase in contraction rate in cultured heart cells. J. Biol. Chem. 1998, 273, 32158–32166. [Google Scholar] [CrossRef][Green Version]
- Islam, M.A.; Nojima, H.; Kimura, I. Muscarinic M1 receptor activation reduces maximum upstroke velocity of action potential in mouse right atria. Eur. J. Pharmacol. 1998, 346, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.P.; Alloatti, G.; Eva, C.; Oberto, A.; Levi, R.C. M1 muscarinic receptors increase calcium current and phosphoinositide turnover in guinea-pig ventricular cardiocytes. J. Physiol. 1993, 471, 41–60. [Google Scholar] [CrossRef]
- Chiba, S.; Tsukada, M. Possible involvement of muscarinic M1 and M3 receptor subtypes mediating vasodilation in isolated, perfused canine lingual arteries. Clin. Exp. Pharmacol. Physiol. 1996, 23, 839–843. [Google Scholar] [CrossRef]
- Tsai, B.M.; Wang, M.; Turrentine, M.W.; Mahomed, Y.; Brown, J.W.; Meldrum, D.R. Hypoxic pulmonary vasoconstriction in cardiothoracic surgery: Basic mechanisms to potential therapies. Ann. Thorac. Surg. 2004, 78, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Gomeza, J.; Klebroff, W.; Zhou, H.; Wess, J. Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: A study with M2- and M4-receptor-deficient mice. Br. J. Pharmacol. 2003, 138, 469–480. [Google Scholar] [CrossRef]
- Dhein, S.; van Koppen, C.J.; Brodde, O.E. Muscarinic receptors in the mammalian heart. Pharmacol. Res. 2001, 44, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Lamping, K.G.; Wess, J.; Cui, Y.; Nuno, D.W.; Faraci, F.M. Muscarinic (M) receptors in coronary circulation: Gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, H.; Zhang, L.; Shi, H.; Schram, G.; Nattel, S.; Wang, Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol. Pharmacol. 2001, 59, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E.; Michel, M.C. Adrenergic and muscarinic receptors in the human heart. Pharmacol. Rev. 1999, 51, 651–690. [Google Scholar]
- Yang, B.; Lin, H.; Xu, C.; Liu, Y.; Wang, H.; Han, H.; Wang, Z. Choline produces cytoprotective effects against ischemic myocardial injuries: Evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell Physiol. Biochem. 2005, 16, 163–174. [Google Scholar] [CrossRef]
- Dauphin, F.; Hamel, E. Muscarinic receptor subtype mediating vasodilation feline middle cerebral artery exhibits M3 pharmacology. Eur. J. Pharmacol. 1990, 178, 203–213. [Google Scholar] [CrossRef]
- Gericke, A.; Steege, A.; Manicam, C.; Bohmer, T.; Wess, J.; Pfeiffer, N. Role of the M3 muscarinic acetylcholine receptor subtype in murine ophthalmic arteries after endothelial removal. Investig. Ophthalmol. Vis. Sci. 2014, 55, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Stengel, P.W.; Gomeza, J.; Wess, J.; Cohen, M.L. M(2) and M(4) receptor knockout mice: Muscarinic receptor function in cardiac and smooth muscle in vitro. J. Pharmacol. Exp. Ther. 2000, 292, 877–885. [Google Scholar]
- Xu, M.; Xue, R.Q.; Lu, Y.; Yong, S.Y.; Wu, Q.; Cui, Y.L.; Zuo, X.T.; Yu, X.J.; Zhao, M.; Zang, W.J. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc. Res. 2019, 115, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Z.; Bi, X.Y.; He, X.; Zhao, M.; Xu, M.; Yu, X.J.; Zhao, Z.H.; Zang, W.J. Activation of M3 cholinoceptors attenuates vascular injury after ischaemia/reperfusion by inhibiting the Ca2+/calmodulin-dependent protein kinase II pathway. Br. J. Pharmacol. 2015, 172, 5619–5633. [Google Scholar] [CrossRef]
- Guo, J.; Hang, P.; Yu, J.; Li, W.; Zhao, X.; Sun, Y.; Fan, Z.; Du, Z. The association between RGS4 and choline in cardiac fibrosis. Cell Commun. Signal 2021, 19, 46. [Google Scholar] [CrossRef]
- Wang, Y.P.; Hang, P.Z.; Sun, L.H.; Zhang, Y.; Zhao, J.L.; Pan, Z.W.; Ji, H.R.; Wang, L.A.; Bi, H.; Du, Z.M. M3 muscarinic acetylcholine receptor is associated with beta-catenin in ventricular myocytes during myocardial infarction in the rat. Clin. Exp. Pharmacol. Physiol. 2009, 36, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, H.; Lu, Y.; Yang, B.; Wang, Z. Choline modulates cardiac membrane repolarization by activating an M3 muscarinic receptor and its coupled K+ channel. J. Membr. Biol. 1999, 169, 55–64. [Google Scholar] [CrossRef]
- Grogan, A.; Lucero, E.Y.; Jiang, H.; Rockman, H.A. Pathophysiology and pharmacology of G protein-coupled receptors in the heart. Cardiovasc. Res. 2023, 119, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y.; He, X.; Xu, M.; Zhao, M.; Yu, X.J.; Lu, X.Z.; Zang, W.J. Acetylcholine ameliorates endoplasmic reticulum stress in endothelial cells after hypoxia/reoxygenation via M3 AChR-AMPK signaling. Cell Cycle 2015, 14, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bi, X.Y.; Xue, X.R.; Lu, X.Z.; Li, Q.G.; Jian, Q.; Sun, J.Y. Activation of the M3AChR and Notch1/HSF1 Signaling Pathway by Choline Alleviates Angiotensin II-Induced Cardiomyocyte Apoptosis. Oxid. Med. Cell Longev. 2021, 2021, 9979706. [Google Scholar] [CrossRef]
- Hui, Y.; Zhao, Y.; Ma, N.; Peng, Y.; Pan, Z.; Zou, C.; Zhang, P.; Du, Z. M3-mAChR stimulation exerts anti-apoptotic effect via activating the HIF-1alpha/HO-1/VEGF signaling pathway in H9c2 rat ventricular cells. J. Cardiovasc. Pharmacol. 2012, 60, 474–482. [Google Scholar] [CrossRef]
- Roy, A.; Guatimosim, S.; Prado, V.F.; Gros, R.; Prado, M.A. Cholinergic activity as a new target in diseases of the heart. Mol. Med. 2015, 20, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Gavioli, M.; Lara, A.; Almeida, P.W.; Lima, A.M.; Damasceno, D.D.; Rocha-Resende, C.; Ladeira, M.; Resende, R.R.; Martinelli, P.M.; Melo, M.B.; et al. Cholinergic signaling exerts protective effects in models of sympathetic hyperactivity-induced cardiac dysfunction. PLoS ONE 2014, 9, e100179. [Google Scholar] [CrossRef]
- Stiegler, A.; Li, J.H.; Shah, V.; Tsaava, T.; Tynan, A.; Yang, H.; Tamari, Y.; Brines, M.; Tracey, K.J.; Chavan, S.S. Systemic administration of choline acetyltransferase decreases blood pressure in murine hypertension. Mol. Med. 2021, 27, 133. [Google Scholar] [CrossRef]
- Bandoni, R.L.; Bricher Choque, P.N.; Delle, H.; de Moraes, T.L.; Porter, M.H.M.; da Silva, B.D.; Neves, G.A.; Irigoyen, M.C.; De Angelis, K.; Pavlov, V.A.; et al. Cholinergic stimulation with pyridostigmine modulates a heart-spleen axis after acute myocardial infarction in spontaneous hypertensive rats. Sci. Rep. 2021, 11, 9563. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Dakroub, M.; Tezini, G.C.; Liu, Y.; Guatimosim, S.; Feng, Q.; Salgado, H.C.; Prado, V.F.; Prado, M.A.; Gros, R. Cardiac acetylcholine inhibits ventricular remodeling and dysfunction under pathologic conditions. FASEB J. 2016, 30, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Gergs, U.; Wackerhagen, S.; Fuhrmann, T.; Schafer, I.; Neumann, J. Further investigations on the influence of protein phosphatases on the signaling of muscarinic receptors in the atria of mouse hearts. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Saternos, H.C.; Almarghalani, D.A.; Gibson, H.M.; Meqdad, M.A.; Antypas, R.B.; Lingireddy, A.; AbouAlaiwi, W.A. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol. Genom. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, V.; Schwertfeger, E.; Rutz, T.; Beyersdorf, F.; Rump, L.C. Acetylcholine release in human heart atrium: Influence of muscarinic autoreceptors, diabetes, and age. Circulation 2001, 103, 1638–1643. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Miao, Y.; Zhou, J.; Zhao, M.; Liu, J.; Sun, L.; Yu, X.; He, X.; Pan, X.; Zang, W. Acetylcholine attenuates hypoxia/reoxygenation-induced mitochondrial and cytosolic ROS formation in H9c2 cells via M2 acetylcholine receptor. Cell Physiol. Biochem. 2013, 31, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, M.; Yang, Y.; Xue, R.Q.; Yu, X.J.; Liu, J.K.; Zang, W.J. Acetylcholine Attenuates Hypoxia/Reoxygenation Injury by Inducing Mitophagy Through PINK1/Parkin Signal Pathway in H9c2 Cells. J. Cell Physiol. 2016, 231, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Rampa, L.; Santangelo, R.; Gaspardone, C.; Cerutti, A.; Magnani, G.; Piscazzi, F.; Sgherzi, G.; Fiore, G.; Filippi, M.; Agosta, F.; et al. Potential Cardiologic Protective Effects of Acetylcholinesterase Inhibitors in Patients With Mild to Moderate Dementia. Am. J. Cardiol. 2023, 200, 162–170. [Google Scholar] [CrossRef]
- Cui, Y.L.; Xue, R.Q.; Xi, H.; Ming, Z.; Yu, X.J.; Liu, L.Z.; Wu, Q.; Si, Y.; Li, D.L.; Zang, W.J. Cholinergic drugs ameliorate endothelial dysfunction by decreasing O-GlcNAcylation via M3 AChR-AMPK-ER stress signaling. Life Sci. 2019, 222, 1–12. [Google Scholar] [CrossRef]
- Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Tapilina, S.V.; Ivanova, A.D.; Filatova, T.S.; Galenko-Yaroshevsky, P.A.; Abramochkin, D.V. The role of M3 receptors in regulation of electrical activity deteriorates in the rat heart during ageing. Curr. Res. Physiol. 2022, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rusiecka, O.M.; Montgomery, J.; Morel, S.; Batista-Almeida, D.; Van Campenhout, R.; Vinken, M.; Girao, H.; Kwak, B.R. Canonical and Non-Canonical Roles of Connexin43 in Cardioprotection. Biomolecules 2020, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, H.; Wang, Z. Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol. Pharmacol. 1999, 55, 497–507. [Google Scholar] [PubMed]
- Shi, H.; Wang, H.; Wang, Z. M3 muscarinic receptor activation of a delayed rectifier potassium current in canine atrial myocytes. Life Sci. 1999, 64, PL251–PL257. [Google Scholar] [CrossRef]
- Chen, X.; Bai, Y.; Sun, H.; Su, Z.; Guo, J.; Sun, C.; Du, Z. Overexpression of M3 Muscarinic Receptor Suppressed Adverse Electrical Remodeling in Hypertrophic Myocardium Via Increasing Repolarizing K+ Currents. Cell Physiol. Biochem. 2017, 43, 915–925. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Deng, J.; Wu, Q.; Zang, W.J. Amelioration of circadian disruption and calcium-handling protein defects by choline alleviates cardiac remodeling in abdominal aorta coarctation rats. Lab. Investig. 2021, 101, 878–896. [Google Scholar] [CrossRef]
- Filatova, T.S.; Naumenko, N.; Galenko-Yaroshevsky, P.A.; Abramochkin, D.V. M3 cholinoreceptors alter electrical activity of rat left atrium via suppression of L-type Ca2+ current without affecting K+ conductance. J. Physiol. Biochem. 2017, 73, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial cell-cardiomyocyte crosstalk in heart development and disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef]
- He, X.; Bi, X.Y.; Lu, X.Z.; Zhao, M.; Yu, X.J.; Sun, L.; Xu, M.; Wier, W.G.; Zang, W.J. Reduction of Mitochondria-Endoplasmic Reticulum Interactions by Acetylcholine Protects Human Umbilical Vein Endothelial Cells From Hypoxia/Reoxygenation Injury. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1623–1634. [Google Scholar] [CrossRef]
- Xu, M.; Bi, X.; He, X.; Yu, X.; Zhao, M.; Zang, W. Inhibition of the mitochondrial unfolded protein response by acetylcholine alleviated hypoxia/reoxygenation-induced apoptosis of endothelial cells. Cell Cycle 2016, 15, 1331–1343. [Google Scholar] [CrossRef]
- Jiao, Z.Y.; Wu, J.; Liu, C.; Wen, B.; Zhao, W.Z.; Du, X.L. Type 3 muscarinic acetylcholine receptor stimulation is a determinant of endothelial barrier function and adherens junctions integrity: Role of protein-tyrosine phosphatase 1B. BMB Rep. 2014, 47, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995, 75, 487–517. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, T.; Wright, A.C.; Yang, J.; Zhou, S.; Li, L.; Yang, J.; Small, A.; Parmacek, M.S. Myocardin is required for maintenance of vascular and visceral smooth muscle homeostasis during postnatal development. Proc. Natl. Acad. Sci. USA 2015, 112, 4447–4452. [Google Scholar] [CrossRef]
- Liu, L.; Rippe, C.; Hansson, O.; Kryvokhyzha, D.; Fisher, S.; Ekman, M.; Sward, K. Regulation of the Muscarinic M3 Receptor by Myocardin-Related Transcription Factors. Front. Physiol. 2021, 12, 710968. [Google Scholar] [CrossRef] [PubMed]
- Roffel, A.F.; Meurs, H.; Elzinga, C.R.; Zaagsma, J. Characterization of the muscarinic receptor subtype involved in phosphoinositide metabolism in bovine tracheal smooth muscle. Br. J. Pharmacol. 1990, 99, 293–296. [Google Scholar] [CrossRef]
- Ehlert, F.J.; Sawyer, G.W.; Esqueda, E.E. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999, 64, 387–394. [Google Scholar] [CrossRef]
- Lin, S.; Kajimura, M.; Takeuchi, K.; Kodaira, M.; Hanai, H.; Kaneko, E. Expression of muscarinic receptor subtypes in rat gastric smooth muscle: Effect of M3 selective antagonist on gastric motility and emptying. Dig. Dis. Sci. 1997, 42, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, R.S.; Ehlert, F.J. Comparison of functional antagonism between isoproterenol and M2 muscarinic receptors in guinea pig ileum and trachea. J. Pharmacol. Exp. Ther. 1999, 288, 969–976. [Google Scholar]
- Blackstone, N.W. The impact of mitochondrial endosymbiosis on the evolution of calcium signaling. Cell Calcium 2015, 57, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Akazawa, H.; Komuro, I. Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ. Res. 2015, 117, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Aspects Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2023, 8, e169756. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, Y.; Wang, J.; Liu, Z.; Lai, Y.; Zhou, Z.; Liu, Z.; Zhou, Y.; Xu, X.; Li, Z.; et al. Choline Protects the Heart from Doxorubicin-Induced Cardiotoxicity through Vagal Activation and Nrf2/HO-1 Pathway. Oxid. Med. Cell Longev. 2022, 2022, 4740931. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell Cardiol. 2019, 134, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Wang, J.; Hong, L.; Qiao, S.; Wang, C.; An, J. Cholinergic receptors play a role in the cardioprotective effects of anesthetic preconditioning: Roles of nitric oxide and the CaMKKbeta/AMPK pathway. Exp. Ther. Med. 2021, 21, 137. [Google Scholar] [CrossRef]
- Hang, P.; Zhao, J.; Su, Z.; Sun, H.; Chen, T.; Zhao, L.; Du, Z. Choline Inhibits Ischemia-Reperfusion-Induced Cardiomyocyte Autophagy in Rat Myocardium by Activating Akt/mTOR Signaling. Cell Physiol. Biochem. 2018, 45, 2136–2144. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Tenma, T.; Mitsuyama, H.; Watanabe, M.; Kakutani, N.; Otsuka, Y.; Mizukami, K.; Kamada, R.; Takahashi, M.; Takada, S.; Sabe, H.; et al. Small-conductance Ca2+-activated K+ channel activation deteriorates hypoxic ventricular arrhythmias via CaMKII in cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H262–H272. [Google Scholar] [CrossRef] [PubMed]
- Abi-Gerges, A.; Castro, L.; Leroy, J.; Domergue, V.; Fischmeister, R.; Vandecasteele, G. Selective changes in cytosolic beta-adrenergic cAMP signals and L-type Calcium Channel regulation by Phosphodiesterases during cardiac hypertrophy. J. Mol. Cell Cardiol. 2021, 150, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, L.; Zhou, K.; Zheng, N.; Yan, K. Effect of muscarinic receptors agonist in the rat model of coronary heart disease: A potential therapeutic target in cardiovascular diseases. Pak. J. Pharm. Sci. 2018, 31, 2769–2774. [Google Scholar] [PubMed]
- Zhao, Y.; Wang, C.; Wu, J.; Wang, Y.; Zhu, W.; Zhang, Y.; Du, Z. Choline protects against cardiac hypertrophy induced by increased after-load. Int. J. Biol. Sci. 2013, 9, 295–302. [Google Scholar] [CrossRef]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primers 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef]
- Ho, H.T.; Belevych, A.E.; Liu, B.; Bonilla, I.M.; Radwanski, P.B.; Kubasov, I.V.; Valdivia, H.H.; Schober, K.; Carnes, C.A.; Gyorke, S. Muscarinic Stimulation Facilitates Sarcoplasmic Reticulum Ca Release by Modulating Ryanodine Receptor 2 Phosphorylation Through Protein Kinase G and Ca/Calmodulin-Dependent Protein Kinase II. Hypertension 2016, 68, 1171–1178. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.; Li, D.; Nattel, S.; Wang, Z. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding K+ channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell Physiol. Biochem. 2004, 14, 31–40. [Google Scholar] [CrossRef]
- Kato, M.; Komamura, K.; Kitakaze, M. Tiotropium, a novel muscarinic M3 receptor antagonist, improved symptoms of chronic obstructive pulmonary disease complicated by chronic heart failure. Circ. J. 2006, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
| Cell Types | Main Mechanism | Functional Outcome | Cardiovascular Significance |
|---|---|---|---|
| cardiomyocytes | potassium currents and repolarization [76] | adverse electrical remodeling | cardiac hypertrophy |
| SIRT3-AMPK [49] | metabolic dysfunction in the heart | ||
| p38 MAPK [21] | attenuated the increment cell size | ||
| L-type Ca2+ currents [72] | AP shortening | arrhythmia | |
| Notch1/HSF1 [56] | impedes oxidative damage and cardiomyocyte apoptosis | early stage heart failure | |
| HIF-1α, HO-1, VEGF [57] | cytoprotection | apoptotic | |
| NF-κB, miR-376b-5p, BDNF [3] | cardioprotection | ischemia-induced cardiac injury | |
| IL-1β, Caspase-1 [2] | cardiomyocyte aging | age-related cardiac impairment | |
| endothelial cells | AMPK [55,70] | ER stress and apoptosis [55], endothelium damage O-glycosylation, and apoptosis [70] | ischemia/reperfusion injury [55] |
| VDAC1/glucose-regulated protein 75/inositol 1,4,5-trisphosphate receptor 1 complex and mitofusin 2 [80] | ER-mitochondria Ca2+ cross talk | reperfusion injury, endothelial protection | |
| mtROS, mitonuclear protein imbalance [81] | unfolded protein response | hypoxia/reoxygenation, endothelial cell damage | |
| maintaining PTP1B activity, keeping the adherens junction proteins dephosphorylation [82] | preserves the endothelial barrier function | ||
| smooth muscle cells | Phosphoinositide [87] | contraction in smooth muscle | |
| fibroblasts | Nrf2, HO-1 [95] | inflammation | DOX-induced cardiotoxicity |
| TGF-β1, Smad2/3, p38MAPK [19] | interstitial fibrosis, collagen I and III production | fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yu, Y.; Zhang, H.; Zhang, M.; Liu, Y. The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 7560. https://doi.org/10.3390/ijms25147560
Liu X, Yu Y, Zhang H, Zhang M, Liu Y. The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases. International Journal of Molecular Sciences. 2024; 25(14):7560. https://doi.org/10.3390/ijms25147560
Chicago/Turabian StyleLiu, Xinxing, Yi Yu, Haiying Zhang, Min Zhang, and Yan Liu. 2024. "The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases" International Journal of Molecular Sciences 25, no. 14: 7560. https://doi.org/10.3390/ijms25147560
APA StyleLiu, X., Yu, Y., Zhang, H., Zhang, M., & Liu, Y. (2024). The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases. International Journal of Molecular Sciences, 25(14), 7560. https://doi.org/10.3390/ijms25147560









