Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress
Abstract
:1. Introduction
2. Results
2.1. The Effect of Green Light Replacing Some Red and Blue Light on the Morphology of Melon Seedlings under Drought Stress
2.2. Effect of Green Light Replacing Some Red and Blue Light on Photosynthetic Characteristics of Melon Seedlings under Drought Stress
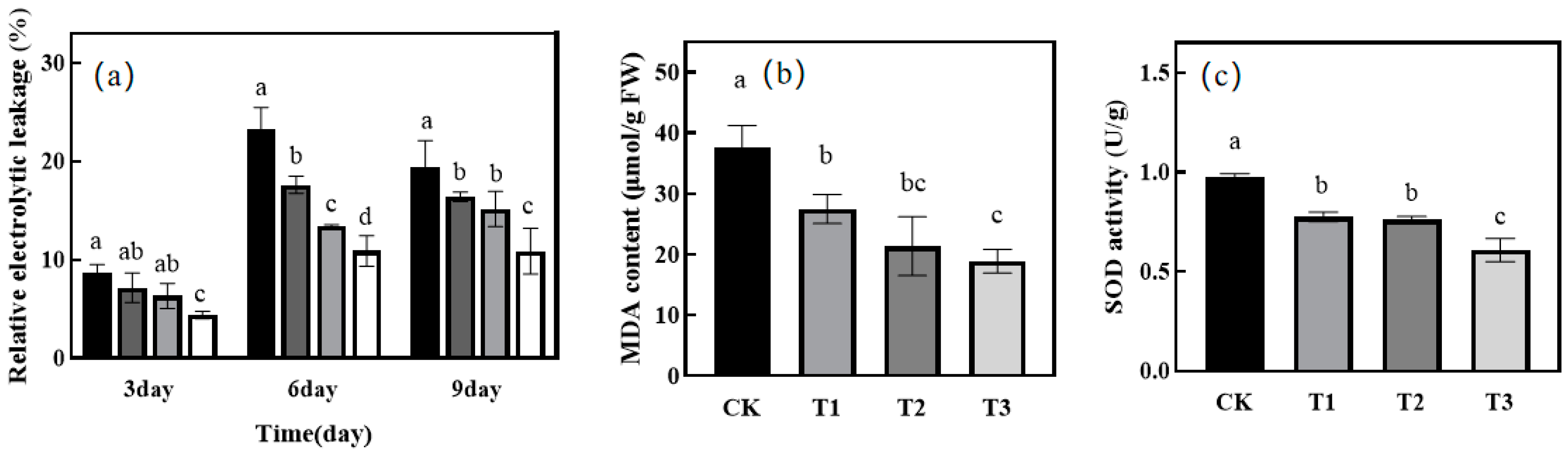
2.3. Effect of Green Light Replacing Some Red and Blue Light on Stomatal Aperture and REC of Melon Seedlings under Drought Stress
2.4. Effect of Green Light Replacing Some Red and Blue Light on MDA and SOD in Leaves of Melon Seedlings under Drought Stress
2.5. Effect of Green Light Replacing Some Red and Blue Light on H2O2 and O2∙− in Leaves of Melon Seedlings under Drought Stress
2.6. Transcriptomic Analysis of Green Light Instead of Partial Red and Blue Light on Leaves of Melon Seedlings under Drought Stress
2.6.1. RNA-Seq Analysis
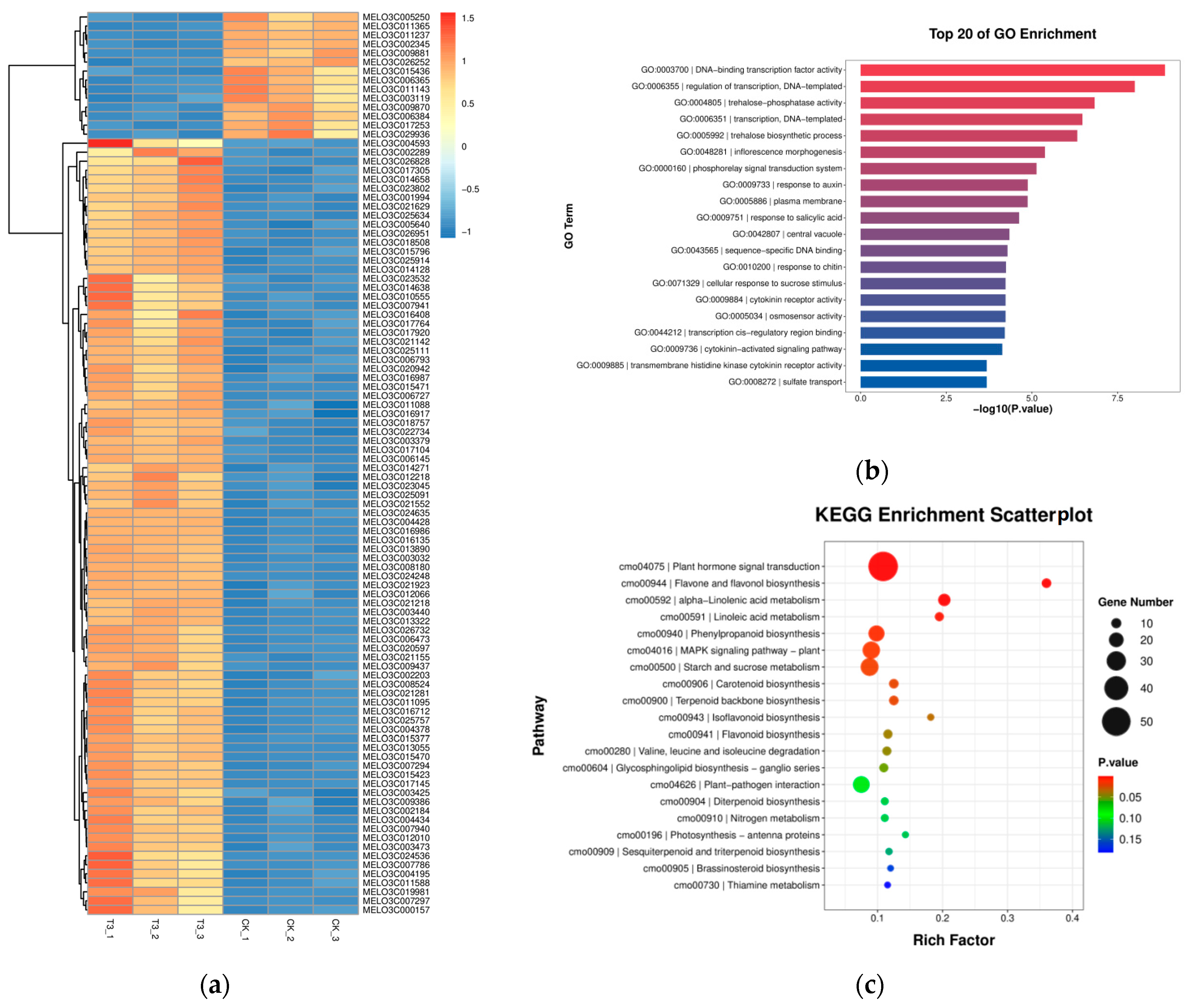
2.6.2. Identification of Differentially Expressed Genes (DEGs) between Groups
2.6.3. GO Pathway Annotation Analysis of Differential Genes
2.6.4. KEGG Pathway Annotation Analysis of Differential Genes
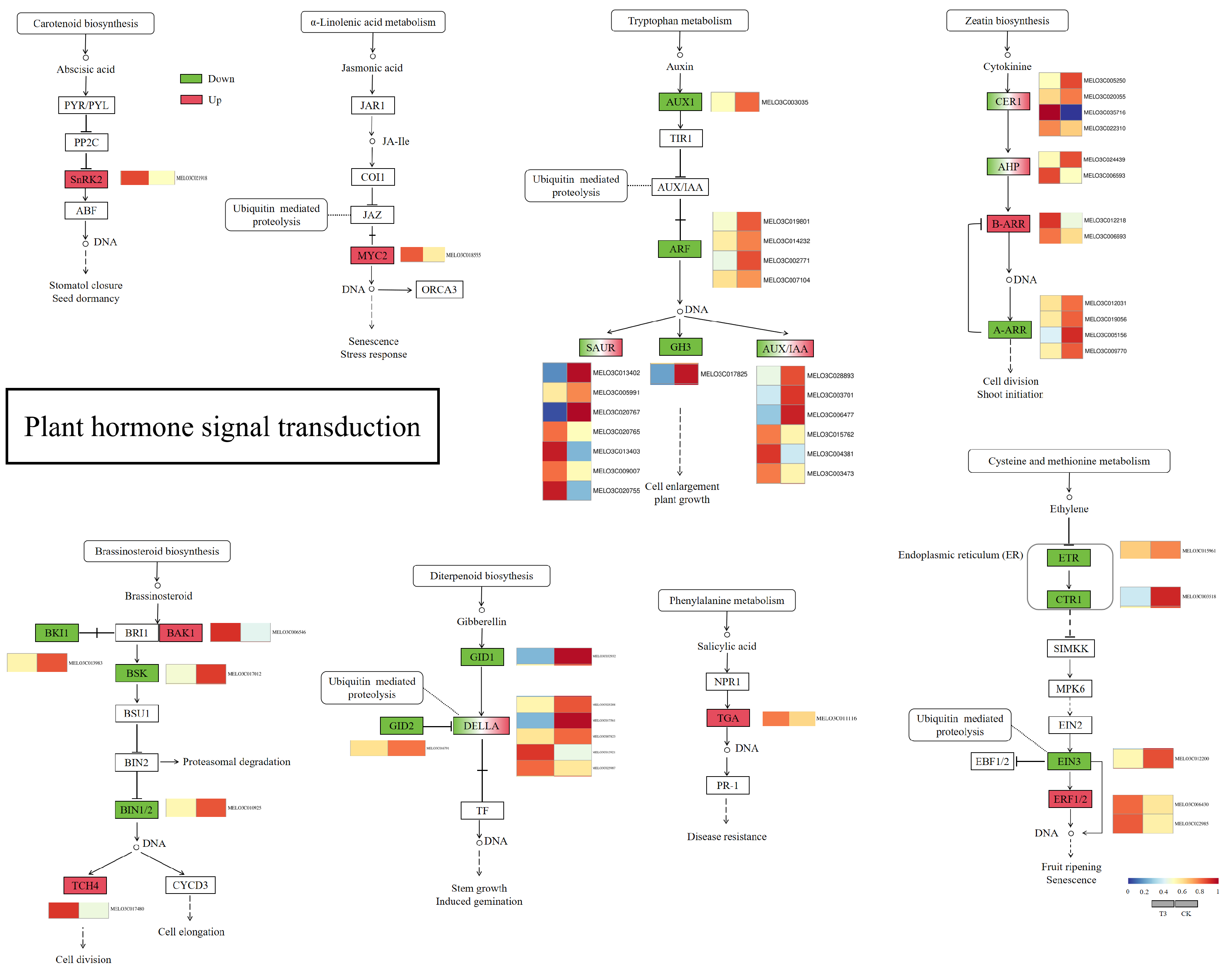
2.6.5. Analysis of Plant Hormone Synthesis Pathways
2.6.6. qRT-PCR Validation
2.7. Metabolomic Analysis of Leaf Metabolism of Melon Seedlings under Drought Stress by Replacing Part of Red and Blue Light with Green Light
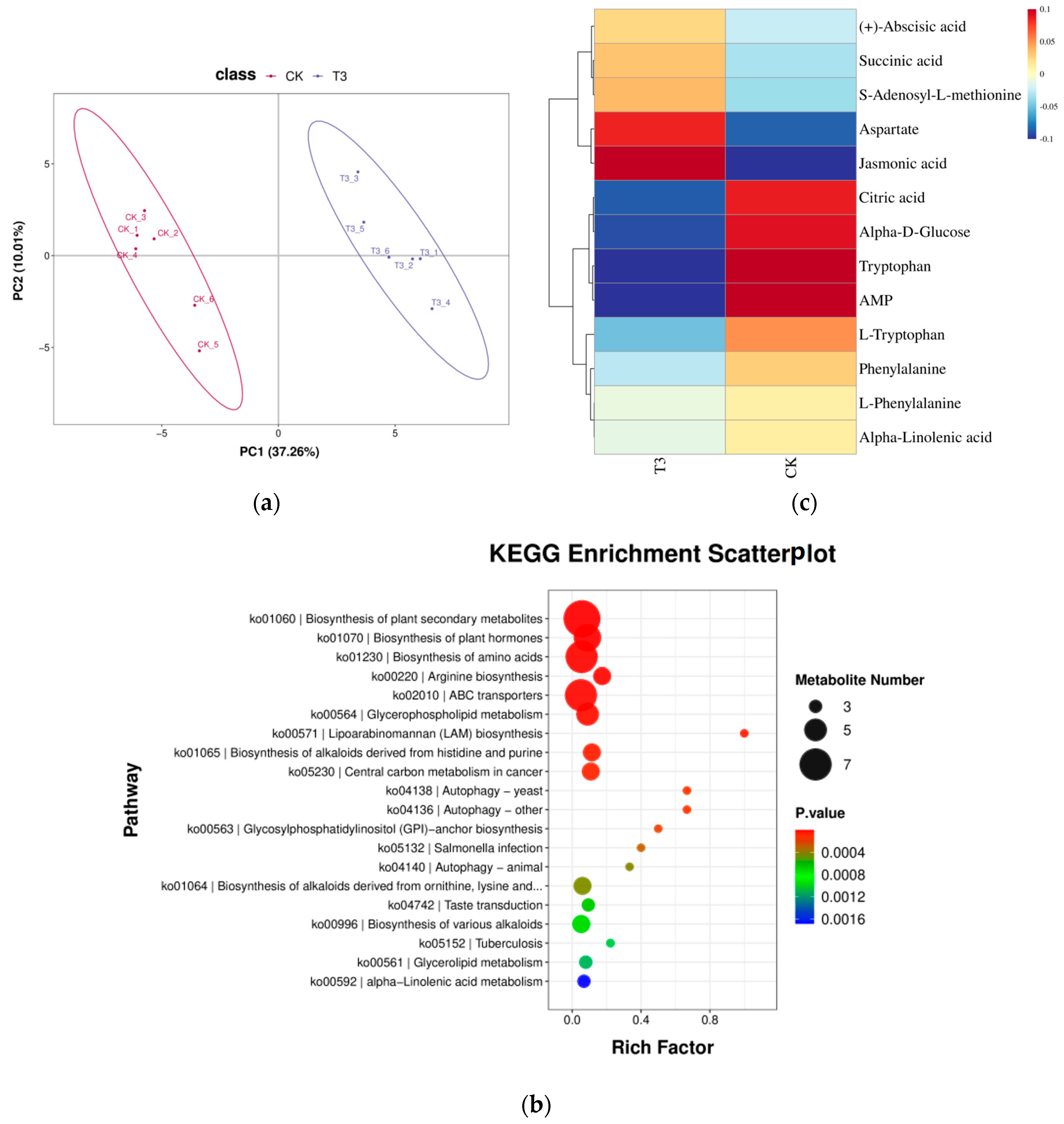
2.7.1. Differential Metabolite Statistics
2.7.2. Differential Metabolites KEGG Functional Annotation
2.7.3. Analysis of Differential Metabolites for Plant Hormone Synthesis
3. Discussion
3.1. Effects of Green Light Replacing Some Red and Blue Light on the Morphology and Photosynthesis of Melon Seedlings under Drought Stress
3.2. Effect of Green Light Replacing Some Red and Blue Light on Antioxidant System of Melon Seedlings under Drought Stress
3.3. Effect of Green Light Replacing Some Red and Blue Light on Stomatal Morphology of Melon Seedlings under Drought Stress
3.4. Effect of Green Light Percentage on Transcriptome of Phytohormones in Melon Seedlings under Drought Stress
3.5. Effect of Green Light Percentage on Phytohormone Metabolome of Melon Seedlings under Drought Stress
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Measuring Items and Methods
4.3.1. Morphometric Indicators
4.3.2. Measurement of Photosynthetic Properties
4.3.3. Stomatal Morphology and Electrolyte Permeability (REC)
4.3.4. Measurement of Malondialdehyde (MDA) and Superoxide Dismutase (SOD) Enzyme Activity
4.3.5. Determination of Hydrogen Peroxide (H2O2) and Superoxide Anion (O2∙−) Content
4.3.6. RNA Extraction and Transcriptome Sequencing
4.3.7. Metabolomics Analysis and Differential Metabolite Identification
4.3.8. Real-Time Quantitative RT-PCR Assay
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, Z.; Hu, C.; Gan, Y.; Zhou, J.; Tian, G.; Gao, L. Role of microbes in alleviating crop drought stress: A Review. Plants 2024, 13, 384. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Samarah, N.H.; Mullen, R.E. Drought Stress Effect on Crop Pollination, Seed Set, Yield and Quality. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 193–213. [Google Scholar]
- Zhang, J.; Chen, X.; Song, Y.; Gong, Z. Integrative regulatory mechanisms of stomatal movements under changing climate. J. Integr. Plant Biol. 2024, 66, 368–393. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.-I.; Kinoshita, T. Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.; Blatt, M. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.; Vialet-Chabrand, S.; Lawson, T. Role of blue and red light in stomatal dynamic behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Lawson, T.; Terashima, I.; Fujita, T.; Wang, Y. Coordination between photosynthesis and stomatal behavior. In The Leaf: A Platform for Performing Photosynthesis; Springer International Publishing: Cham, Switzerland, 2018; pp. 141–161. [Google Scholar]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef]
- Scoffoni, C.; Albuquerque, C.; Cochard, H.; Buckley, T.N.; Fletcher, L.R.; Caringella, M.A.; Bartlett, M.; Brodersen, C.R.; Jansen, S.; McElrone, A.J. The causes of leaf hydraulic vulnerability and its influence on gas exchange in Arabidopsis thaliana. Plant Physiol. 2018, 178, 1584–1601. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Vuong, C.; Diep, S.; Cochard, H.; Sack, L. Leaf shrinkage with dehydration: Coordination with hydraulic vulnerability and drought tolerance. Plant Physiol. 2014, 164, 1772–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, T.; Huang, J.; Peng, S.; Xiong, D. Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. J. Exp. Bot. 2018, 69, 4033–4045. [Google Scholar] [CrossRef] [PubMed]
- Gollan, T.; Richards, R.; Rawson, H.; Passioura, J.; Johnson, D.; Munns, R. Soil water status affects the stomata1. Funct. Plant Biol. 1986, 13, 459–464. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.; Holbrook, N.; Gutierrez, M.V. Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ. 2002, 25, 1435–1444. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, Y.-C.; Sang, Y.; Li, Q.-H.; Yang, H.-Q. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 2005, 102, 12270–12275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, Y.-G.; Li, X.; Ni, M. Light-regulated stomatal aperture in Arabidopsis. Mol. Plant 2012, 5, 566–572. [Google Scholar] [CrossRef]
- Bouly, J.-P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Nikolova, G.; Ortiz, A.; Shmayevich, I.; Zeiger, E. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. Am. J. Bot. 2002, 89, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Hammad, J.W.; Harn, L.C.; Nguyen, V.H.; Patel, J.; Zeiger, E. Reversal by green light of blue light-stimulated stomatal opening in intact, attached leaves of Arabidopsis operates only in the potassium-dependent, morning phase of movement. Plant Cell Physiol. 2006, 47, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Chen, X.; Wu, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Foyer, C.H. SlHY5 integrates temperature, light, and hormone signaling to balance plant growth and cold tolerance. Plant Physiol. 2019, 179, 749–760. [Google Scholar] [CrossRef]
- Hao, Y.; Zeng, Z.; Zhang, X.; Xie, D.; Li, X.; Ma, L.; Liu, M.; Liu, H. Green means go: Green light promotes hypocotyl elongation via brassinosteroid signaling. Plant Cell 2023, 35, 1304–1317. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, X.; Wang, Y.; Lu, C. Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L.) seedlings under drought conditions. Environ. Exp. Bot. 2019, 167, 103844. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, L.; Wu, Y.; Tang, Z.; Xiao, X.; Lyu, J.; Xie, J.; Yu, J. Green light partial replacement of red and blue light improved drought tolerance by regulating water use efficiency in cucumber seedlings. Front. Plant Sci. 2022, 13, 878932. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Seth, C.S.; Roychoudhury, A. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Biochem. 2023, 206, 108259. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Lu, N.; Sun, Y.; Lv, W.; Luo, Z.; Zhang, H.; Ji, Q.; Yang, Q.; Chen, S.; Zhang, W. Heterologous expression of the DREB transcription factor AhDREB in Populus tomentosa Carrière confers tolerance to salt without growth reduction under greenhouse conditions. Forests 2019, 10, 214. [Google Scholar] [CrossRef]
- Ballard, T.; Peak, D.; Mott, K. Blue and red light effects on stomatal oscillations. Funct. Plant Biol. 2019, 46, 146. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.J.; Huang, S.; Hedrich, R.; Geilfus, C.; Roelfsema, M.R. The Green Light Gap: A window of opportunity for optogenetic control of Stomatal Movement. New Phytol. 2022, 236, 1237–1244. [Google Scholar] [CrossRef]
- Ehonen, S.; Hölttä, T.; Kangasjärvi, J. Systemic signaling in the regulation of stomatal conductance. Plant Physiol. 2020, 182, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Umar, S.; Sharma, S. Mechanism of free radical scavenging and role of phytohormones in plants under abiotic stresses. In Plant Adaptation and Phytoremediation; Springer: New York, NY, USA, 2010; pp. 99–118. [Google Scholar]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of phytohormones in plant defense: Signaling and cross talk. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Singapore, 2018; pp. 159–184. [Google Scholar]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Kharal, M.A. Role of phytohormones in stress tolerance of plants. In Plant, Soil and Microbes: Volume 2: Mechanisms and Molecular Interactions; Springer: Berlin/Heidelberg, Germany, 2016; pp. 385–421. [Google Scholar]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Zhao, Z.-Q.; Song, D.-D.; Yuan, Y.-X.; Sun, H.-J.; Zhao, J.-F.; Chen, Y.-L.; Zhang, C.-G. SnRK2. 6 interacts with phytochrome B and plays a negative role in red light-induced stomatal opening. Plant Signal. Behav. 2021, 16, 1913307. [Google Scholar] [CrossRef]
- Lee, S.C.; Lim, C.W.; Lan, W.; He, K.; Luan, S. ABA signaling in guard cells entails a dynamic protein–protein interaction relay from the PYL-RCAR family receptors to ion channels. Mol. Plant 2013, 6, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, X.; Chen, D.; Wang, F.; Zhang, H. The Brassica napus MYC2 regulates drought tolerance by monitoring stomatal closure. Eur. J. Hortic. Sci. 2020, 85, 226–231. [Google Scholar]
- Xia, Y.; Jiang, S.; Wu, W.; Du, K.; Kang, X. MYC2 regulates stomatal density and water use efficiency via targeting EPF2/EPFL4/EPFL9 in poplar. New Phytol. 2024, 241, 2506–2522. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, A.K.; Roy, D.; Mansi, M.; Gough, C.; Bhagat, P.K.; Zhang, C.; Sadanandom, A. The conjugation of SUMO to the transcription factor MYC2 functions in blue light-mediated seedling development in Arabidopsis. Plant Cell 2022, 34, 2892–2906. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Chattopadhyay, S. MYC2, a bHLH transcription factor, modulates the adult phenotype of SPA1. Plant Signal. Behav. 2010, 5, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in Aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Tari, I. Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Funct. Plant Biol. 2012, 39, 1028–1037. [Google Scholar] [CrossRef]
- McLachlan, D.H.; Lan, J.; Geilfus, C.-M.; Dodd, A.N.; Larson, T.; Baker, A.; Horak, H.; Kollist, H.; He, Z.; Graham, I. The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr. Biol. 2016, 26, 707–712. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and regulation of cytokinins in plant response to drought stress. Plants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Ptošková, K.; Szecówka, M.; Jaworek, P.; Tarkowská, D.; Petřík, I.; Pavlović, I.; Novák, O.; Thomas, S.G.; Phillips, A.L.; Hedden, P. Changes in the concentrations and transcripts for gibberellins and other hormones in a growing leaf and roots of wheat seedlings in response to water restriction. BMC Plant Biol. 2022, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Han, L.; Xu, L.; Zhang, X. Ethephon seed treatment impacts on drought tolerance of kentucky bluegrass seedlings. HortTechnology 2018, 28, 319–326. [Google Scholar] [CrossRef]
- Zelená, E. The effect of light on IAA metabolism in different parts of maize seedlings in correlation with their growth. Plant Growth Regul. 2000, 32, 239–243. [Google Scholar] [CrossRef]
- Marchetti, C.F.; Škrabišová, M.; Galuszka, P.; Novák, O.; Causin, H.F. Blue light suppression alters cytokinin homeostasis in wheat leaves senescing under shading stress. Plant Physiol. Biochem. 2018, 130, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zeng, B.; Tang, D.; Yang, J.; Qu, L.; Yan, J.; Wang, X.; Li, X.; Liu, X.; Zhao, X. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Cao, J.; Yuan, J.; Liang, Y.; Li, J. Integration of light and brassinosteroid signaling during seedling establishment. Int. J. Mol. Sci. 2021, 22, 12971. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Q.; Yang, Y.; Jiang, Q.; Cao, H.; Zhang, Z. Light influences the effect of exogenous ethylene on the phenolic composition of Cabernet Sauvignon grapes. Front. Plant Sci. 2024, 15, 1356257. [Google Scholar] [CrossRef]
- Ghafari, H.; Hassanpour, H.; Jafari, M.; Besharat, S. Physiological, biochemical and gene-expressional responses to water deficit in Apple subjected to partial root-zone drying (PRD). Plant Physiol. Biochem. 2020, 148, 333–346. [Google Scholar] [CrossRef]
- Talaat, N.B. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef]
- Good, A.G.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar] [CrossRef]
- Jia, X.; Sun, C.; Zuo, Y.; Li, G.; Li, G.; Ren, L.; Chen, G. Integrating transcriptomics and metabolomics to characterise the response of astragalus membranaceus BGE. var. mongolicus (BGE.) to progressive drought stress. BMC Genom. 2016, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Yüce, M.; Neslihan Öztürk Gökçe, Z.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Vitor, S.C.; do Amarante, L.; Sodek, L. Are phloem-derived amino acids the origin of the elevated malate concentration in the xylem sap following mineral n starvation in soybean? Planta 2018, 248, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Paidi, M.K.; Agarwal, P.; More, P.; Agarwal, P.K. Chemical derivatization of metabolite mass profiling of the recretohalophyte aeluropus lagopoides revealing salt stress tolerance mechanism. Mar. Biotechnol. 2017, 19, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef]
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Stroiński, A.; Kubiś, J. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- Wu, H.; Wu, X.; Li, Z.; Duan, L.; Zhang, M. Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J. Plant Growth Regul. 2011, 31, 113–123. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Ball, E.; Lüttge, U. Differential effects of drought and light levels on accumulation of citric and malic acids during cam in clusia. Plant Cell Environ. 1992, 15, 821–829. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Pornsiriwong, W.; Estavillo, G.M.; Chan, K.X.; Tee, E.E.; Ganguly, D.; Crisp, P.A.; Phua, S.Y.; Zhao, C.; Qiu, J.; Park, J.; et al. A chloroplast retrograde signal, 3′-phosphoadenosine 5′-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. eLife 2017, 6, e23361. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-J.; Min, J.-H.; Van Nguyen, T.; Kim, Y.-M.; Kim, C.S. Loss of Arabidopsis Halotolerance 2-like (AHL), a 3′-phosphoadenosine-5′-phosphate phosphatase, suppresses insensitive response of Arabidopsis thaliana ring zinc finger 1 (ATRZF1) mutant to abiotic stress. Plant Mol. Biol. 2019, 99, 363–377. [Google Scholar] [CrossRef]
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176. [Google Scholar] [CrossRef]



| Treatment | Plant Height | Stem Diameter | Fresh Weight | Dry Weight | Total Leaf Area |
|---|---|---|---|---|---|
| CK | 13.633 ± 1.075 c | 4.060 ± 0.141 a | 6.503 ± 0.341 c | 0.723 ± 0.003 b | 138.563 ± 7.467 c |
| T1 | 13.827 ± 0.693 c | 4.237 ± 0.289 a | 6.259 ± 0.655 c | 0.853 ± 0.100 ab | 168.807 ± 6.289 b |
| T2 | 15.487 ± 0.365 b | 4.137 ± 0.065 a | 7.564 ± 0.138 b | 0.899 ± 0.090 ab | 170.253 ± 5.830 b |
| T3 | 17.573 ± 0.552 a | 4.420 ± 0.358 a | 10.112 ± 0.210 a | 0.941 ± 0.1306 a | 231.680 ± 15.790 a |
| Treatment | Pore Width/μm | Pore Length/μm | Stomatal Width/μm | Stomatal Length/μm | Pore Area/μm2 |
|---|---|---|---|---|---|
| CK | 13.633 ± 1.075 c | 4.060 ± 0.141 a | 6.503 ± 0.341 c | 0.723 ± 0.003 b | 138.563 ± 7.467 c |
| T1 | 13.827 ± 0.693 c | 4.237 ± 0.289 a | 6.259 ± 0.655 c | 0.853 ± 0.100 ab | 168.807 ± 6.289 b |
| T2 | 15.487 ± 0.365 b | 4.137 ± 0.065 a | 7.564 ± 0.138 b | 0.899 ± 0.090 ab | 170.253 ± 5.830 b |
| T3 | 17.573 ± 0.552 a | 4.420 ± 0.358 a | 10.112 ± 0.210 a | 0.941 ± 0.1306 a | 231.680 ± 15.790 a |
| Treatment | Red Light μmol/(m2·s) | Blue Light μmol/(m2·s) | Green Light μmol/(m2·s) | PPFD μmol/(m2·s) | R:B:G |
|---|---|---|---|---|---|
| CK | 200 | 50 | 0 | 250 | 4:1:0 |
| T1 | 182 | 45 | 23 | 250 | 4:1:0.5 |
| T2 | 160 | 40 | 50 | 250 | 4:1:1.25 |
| T3 | 143 | 36 | 71 | 250 | 4:1:2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, S.; Cao, Q.; Qiu, C.; Yang, Y.; Zhang, G.; Wu, Y.; Yang, Z. Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. Int. J. Mol. Sci. 2024, 25, 7561. https://doi.org/10.3390/ijms25147561
Li X, Zhao S, Cao Q, Qiu C, Yang Y, Zhang G, Wu Y, Yang Z. Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. International Journal of Molecular Sciences. 2024; 25(14):7561. https://doi.org/10.3390/ijms25147561
Chicago/Turabian StyleLi, Xue, Shiwen Zhao, Qianqian Cao, Chun Qiu, Yuanyuan Yang, Guanzhi Zhang, Yongjun Wu, and Zhenchao Yang. 2024. "Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress" International Journal of Molecular Sciences 25, no. 14: 7561. https://doi.org/10.3390/ijms25147561
APA StyleLi, X., Zhao, S., Cao, Q., Qiu, C., Yang, Y., Zhang, G., Wu, Y., & Yang, Z. (2024). Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. International Journal of Molecular Sciences, 25(14), 7561. https://doi.org/10.3390/ijms25147561





