Bioluminescent Systems for Theranostic Applications
Abstract
:1. Introduction
2. Bioluminescence-Based Systems
2.1. Luciferases and Luciferins
2.2. Luciferase and Luciferin Delivery

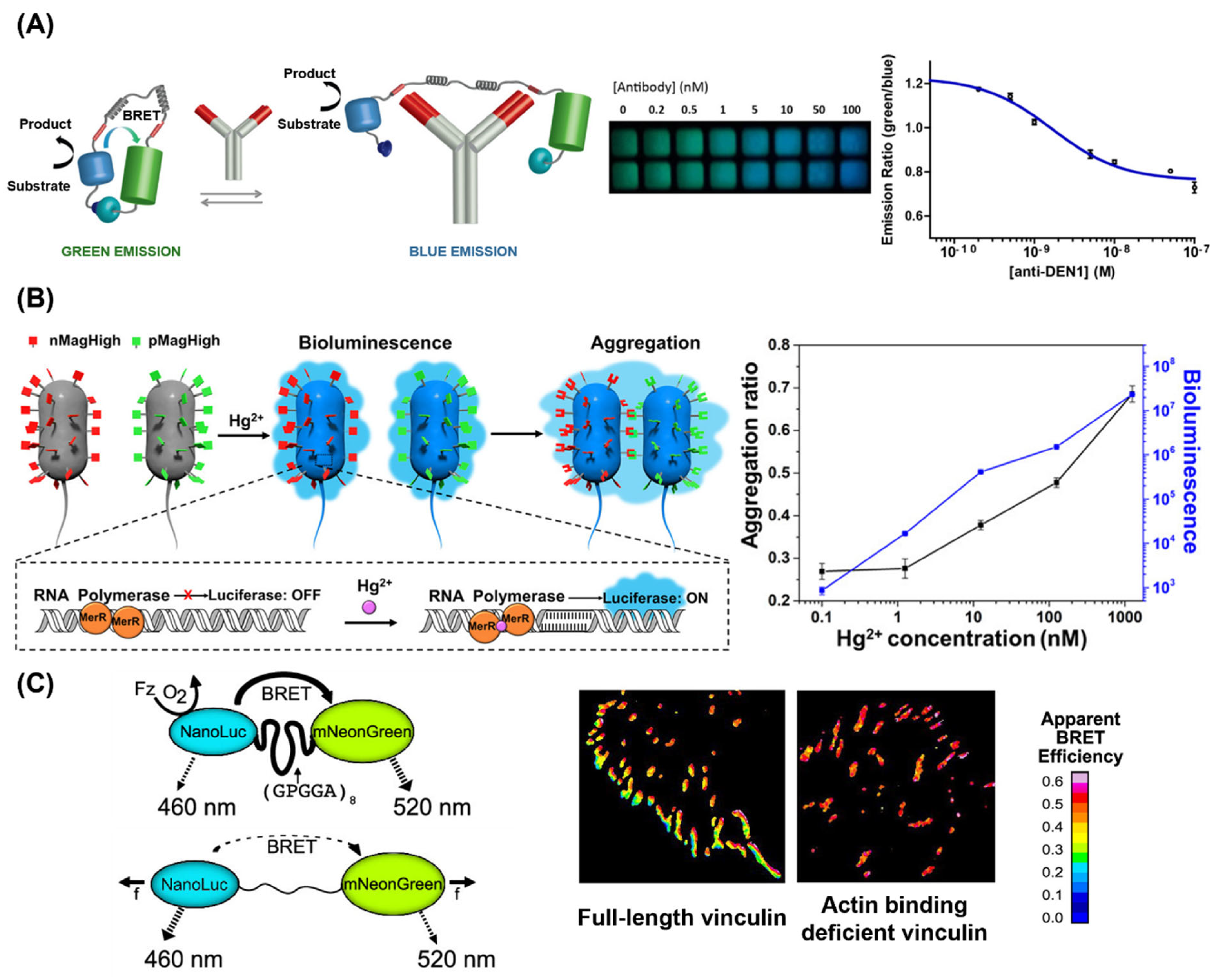
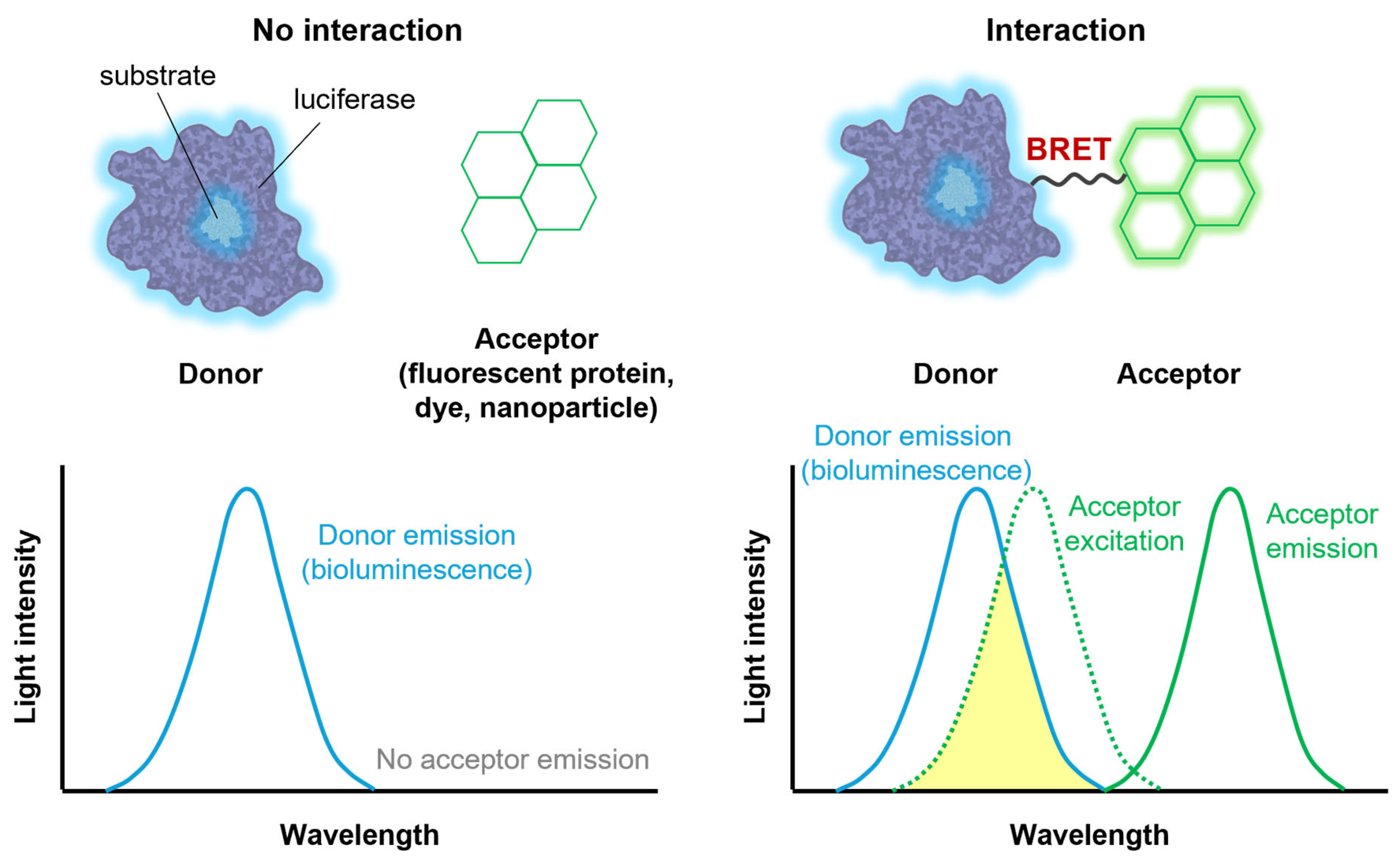
2.3. Bioluminescence Resonance Energy Transfer (BRET)
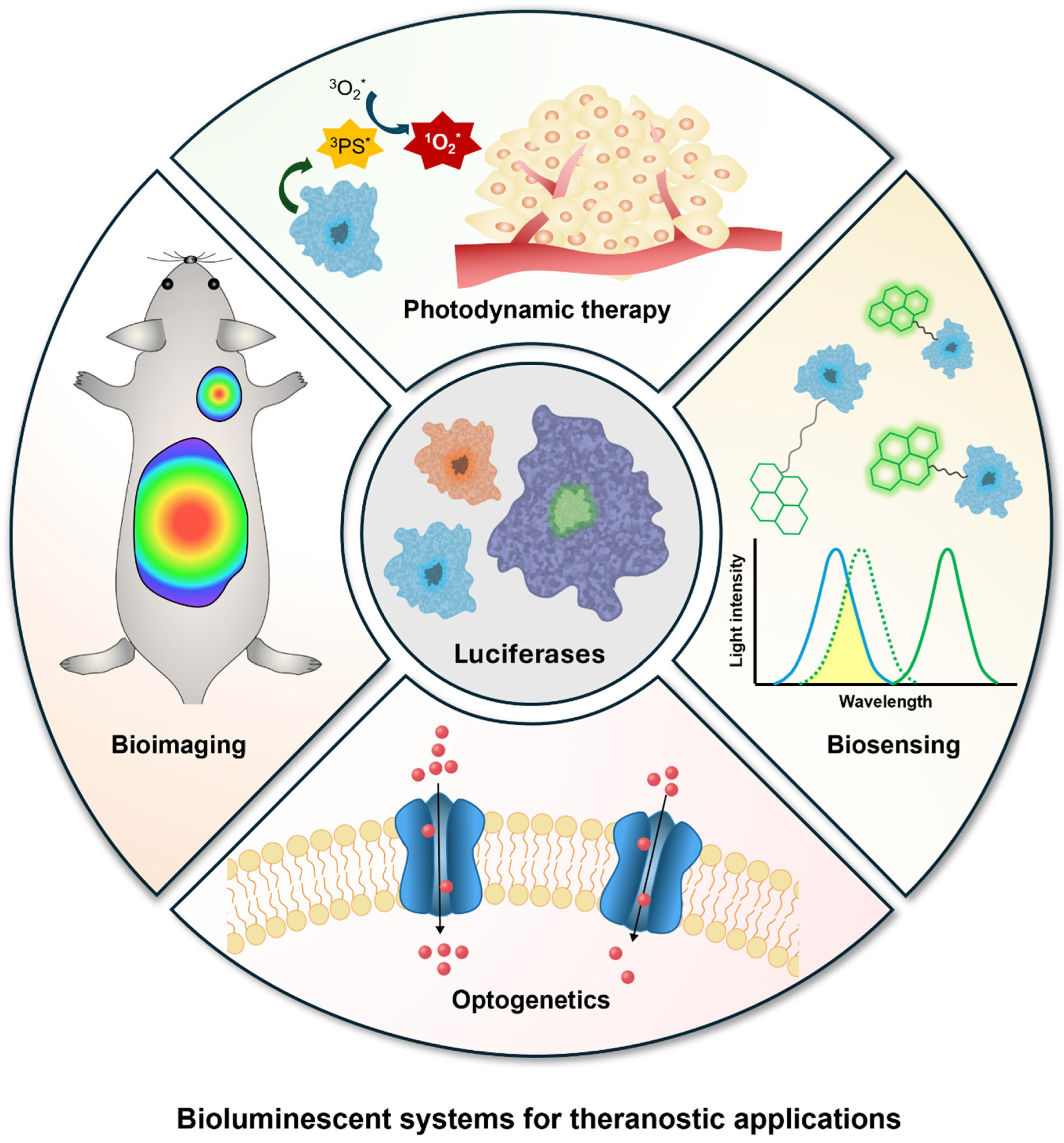
3. Theranostic Applications of Bioluminescence
3.1. Bioimaging
3.2. Biosensors
3.3. Photodynamic Therapy
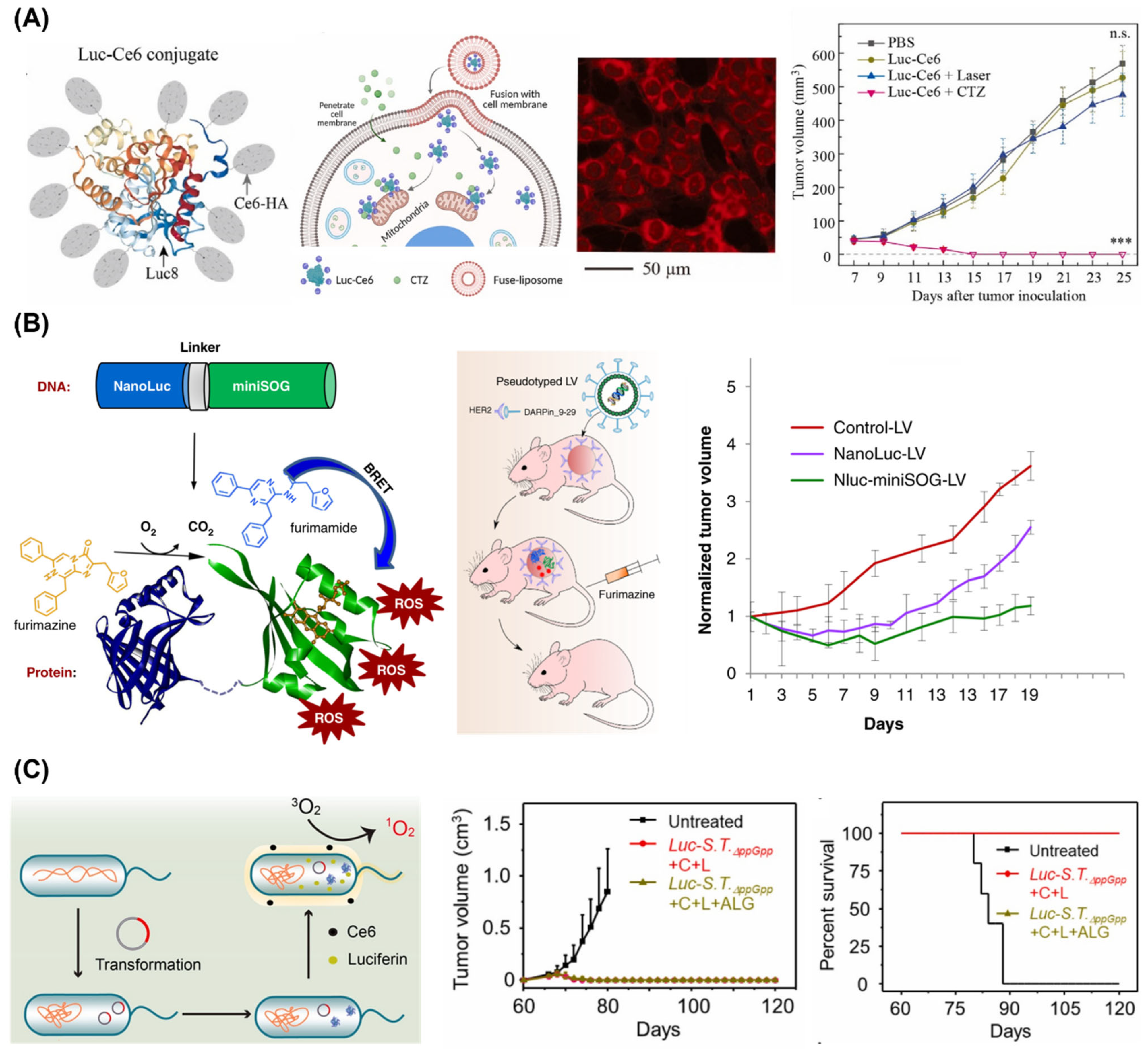
3.4. Optogenetic Applications
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, B.; Zhang, F. Molecular fluorophores for deep-tissue bioimaging. ACS Central. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, X.; Liu, H. Two-dimensional nanomaterials for photothermal therapy. Angew. Chem. Int. Ed. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 12, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rao, J.; Wang, M.; Li, X.; Liu, K.; Naylor, M.F.; Nordquist, R.E.; Chen, W.R.; Zhou, F. Cancer photo-immunotherapy: From bench to bedside. Theranostics 2021, 11, 2218–2231. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, Y.; Zhu, J.; Zhang, J.; Song, X.; Wang, W.; Shao, J.; Huang, W.; Chen, P.; Dong, X. Photothermal-pH-hypoxia responsive multifunctional nanoplatform for cancer photo-chemo therapy with negligible skin phototoxicity. Biomaterials 2019, 221, 119422. [Google Scholar] [CrossRef]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.-H.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Tang, Q.; Wang, J.; Song, W.; Lu, X.; Huang, X.; Chen, G.; Zheng, W.; Zhang, L.; et al. Low intensity near-infrared light promotes bone regeneration via circadian clock protein cryptochrome 1. Int. J. Oral Sci. 2022, 14, 53. [Google Scholar] [CrossRef]
- Sordillo, L.A.; Pu, Y.; Pratavieria, S.; Budansky, Y.; Alfano, R.R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 2014, 19, 056004. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, I.S.; Lee, J.S.; Jeon, S.; Yun, W.S.; Yang, S.; Moon, Y.; Kim, J.; Kim, J.; Choy, S.; et al. Implantable micro-scale LED device guided photodynamic therapy to potentiate antitumor immunity with mild visible light. Biomater. Res. 2022, 26, 56. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Xu, Z.; Chen, M.; Wang, W.; Wu, Y.; Cai, J.; Long, J.; Chen, Z.-S.; Zhang, D.; Guan, B.-Q. Fiber-optic theranostics (FOT): Interstitial fiber-optic needles for cancer sensing and therapy. Adv. Sci. 2022, 9, 2200456. [Google Scholar] [CrossRef]
- Wang, D.; Kuzma, M.L.; Tan, X.; He, T.-C.; Dong, C.; Liu, Z.; Yang, J. Phototherapy and optical waveguides for the treatment of infection. Adv. Drug Deliv. Rev. 2021, 179, 114036. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Alsawaftah, N.; Farooq, A.; Dhou, S.; Majdalawieh, A.F. Bioluminescence imaging applications in cancer: A comprehensive review. IEEE Rev. Biomed. Eng. 2021, 14, 307–326. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. [Google Scholar] [CrossRef]
- Syed, A.J.; Anderson, J.C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 2021, 50, 5668–5705. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.T.; Miller, S.C. Enzymatic promiscuity and the evolution of bioluminescence. FEBS J. 2020, 287, 1369–1680. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, Y.; Lin, M.Z.; Ronald, J.A. Brightening up biology: Advances in luciferase systems for in vivo imaging. ACS Chem. Biol. 2021, 16, 2707–2718. [Google Scholar] [CrossRef]
- Li, S.; Ruan, Z.; Zhang, H.; Xu, H. Recent achievements of bioluminescence imaging based on firefly luciferin-luciferase system. Eur. J. Med. Chem. 2021, 211, 113111. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Niwa, K.; Yamada, N.; Enomoto, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission. Nat. Photon. 2008, 2, 44–47. [Google Scholar] [CrossRef]
- Morse, D.; Tannous, B.A. A water-soluble coelenterazine for sensitive in vivo imaging of coelenterate luciferases. Mol. Ther. 2012, 20, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, L.; Li, M. Lighting up bioluminescence with coelenterazine: Strategies and applications. Photochem. Photobiol. Sci. 2016, 15, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Markova, S.V.; Larionova, M.D.; Vysotski, E.S. Shining light on the secreted luciferases of marine copepods: Current knowledge and applications. Photochem. Photobiol. 2019, 95, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.A.; Moutsiopoulou, A.; Ioannou, S.; Ahern, K.; Woodward, K.; Dikici, E.; Daunert, S.; Deo, S.K. Truncated variants of Gaussia luciferase with tyrosine linker for site-specific bioconjugate applications. Sci. Rep. 2016, 6, 26814. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, C.; Murai, A.; Inouye, S.; Shimomura, O. Efficient bioluminescence of bisdeoxycoelenterazine with the luciferase of a deep-sea shrimp OpZophorus. Tetrahedron Lett. 1997, 38, 6405–6406. [Google Scholar] [CrossRef]
- Inouye, S.; Sasaki, S. Overexpression, purification and characterization of the catalytic component of Oplophorus luciferase in the deep-sea shrimp, Oplophorus gracilirostris. Protein Expr. Purif. 2007, 56, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Sahara-Miura, Y.; Sato, J.; Iimori, R.; Yosida, S.; Hosoya, T. Expression, purification and luminescence properties of coelenterazine-utilizing luciferases from Renilla, Oplophorus and Gaussia: Comparison of substrate specificity for C2-modified coelenterazines. Protein Expr. Purif. 2013, 88, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Close, D.; Xu, T.; Smartt, A.; Rogers, A.; Crossley, R.; Price, S.; Ripp, S.; Sayler, G. The evolution of bacterial luciferase gene cassette (lux) as a real-time reporter. Sensors 2012, 12, 732–752. [Google Scholar] [CrossRef]
- Tinikul, R.; Chunthaboon, P.; Phonbuppha, J.; Paladkong, T. Chapter fourteen—Bacterial luciferase: Molecular mechanisms and applications. Enzymes 2020, 47, 427–455. [Google Scholar] [PubMed]
- Pozzo, T.; Akter, F.; Nomura, Y.; Louie, A.Y.; Yokobayashi, Y. Firefly luciferase mutant with enhanced activity and thermostability. ACS Omega 2018, 3, 2628–2633. [Google Scholar] [CrossRef]
- Colee, C.M.; Oberlag, N.M.; Simon, M.; Chapman, O.S.; Flanagan, L.C.; Reid-McLaughlin, E.S.; Gewing-Mullins, J.A.; Maiche, S.; Patel, D.F.; Cavalcanti, A.R.O.; et al. Discovery of red-shifting mutations in firefly luciferase using high-throughput biochemistry. Biochemistry 2024, 63, 733–742. [Google Scholar] [CrossRef]
- Jathoul, A.P.; Grounds, H.; Anderson, J.C.; Pule, M.A. A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew. Chem. Int. Ed. 2014, 53, 13059–13063. [Google Scholar] [CrossRef]
- Gupta, R.; Kasturi, P.; Bracher, A.; Loew, C.; Zheng, M.; Villella, A.; Garza, D.; Hartl, F.U.; Raychaudhuri, S. Firefly luciferase mutants as sensors of proteome stress. Nat. Methods 2011, 8, 879–884. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Bhaumik, S.; Gambhir, S.S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad Sci. USA 2002, 99, 377–382. [Google Scholar] [CrossRef]
- Badr, C.E.; Tannous, B.A. Bioluminescence imaging: Progress and applications. Trends Biotechnol. 2011, 29, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Baklaushev, V.P.; Kilpeläinen, A.; Petkov, S.; Abakumov, M.A.; Grinenko, N.F.; Yusubalieva, G.M.; Latanova, A.A.; Gubskiy, I.L.; Zabozlaev, F.G.; Starodubova, E.S.; et al. Bioluminescence imaging but imposes limitations on the orthotopic mouse (4T1) model of breast cancer. Sci. Rep. 2017, 7, 7715. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.V.; Lin, L.; Huang, X.; Yang, Y. Generation of luciferase-expressing tumor cell lines. Bio-Protocol 2018, 8, e2817. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Xiong, Z.; Cheng, Z.; Cai, W.; Gambhir, S.S.; Xing, L.; Chen, X. In vivo bioluminescence tumor imaging of RGD peptide-modified adenoviral vector encoding firefly luciferase reporter gene. Mol. Imaging Biol. 2007, 9, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Dai, J.; Moursi, A.M. Comparison of transfection efficiency of nonviral gene transfer reagents. Mol. Biotechnol. 2010, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 250–302. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Gao, Y.-G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.-J.; Jiang, S.-F.; Qadir, A.; Qian, A.-R. The development of functional non-viral vectors for gene delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed]
- Tarantal, A.F.; Lee, C.C.I. Long-term luciferase expression monitored by bioluminescence imaging after adeno-associated virus-mediated fetal gene delivery in Rhesus monkeys (Macaca mulatta). Hum. Gene Ther. 2010, 21, 143–148. [Google Scholar] [CrossRef]
- Li, J.Z.; Holman, D.; Li, H.; Liu, A.-H.; Beres, B.; Hankins, G.R.; Helm, G.A. Long-term tracing of adenoviral expression in rat and rabbit using luciferase imaging. J. Gene Med. 2005, 7, 792–802. [Google Scholar] [CrossRef]
- Tannous, B.A. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat. Protoc. 2009, 4, 582–591. [Google Scholar] [CrossRef]
- Griesenbach, U.; Vicente, C.C.; Roberts, M.J.; Meng, C.; Soussi, S.; Xenariou, S.; Tennant, P.; Baker, A.; Baker, E.; Gordon, C.; et al. Secreted Gaussia luciferase as a sensitive reporter gene for in vivo and ex vivo studies of airway gene transfer. Biomaterials 2011, 32, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Higuchi, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. piggyBac Transposom-mediated long-term gene expression in mice. Mol. Ther. 2010, 18, 707–714. [Google Scholar] [CrossRef] [PubMed]
- El-Amouri, S.S.; Cao, P.; Miao, C. Secreted luciferase for in vivo enhancement of systemic protein delivery in mice. Mol. Biotechnol. 2013, 53, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-J.; Wei, Y.-F.; Wan, Y.-Y.; Jiang, L.-P.; Zhang, J.-F.; Xin, H.-B. Development of a novel liposomal nanodelivery system for bioluminescence imaging and targeted drug delivery in ErbB2-overexpressing metastatic ovarian carcinoma. Int. J. Mol. Med. 2014, 34, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Mery, C.E.; Craciun, I.; Schoenenberger, C.-A.; Wehr, R.; Palivan, C.R. Catalytic polymersomes to produce strong and long-lasting bioluminescence. Nanoscale 2021, 13, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yeow, J.; Najer, A.; Kit-Anan, W.; Wang, R.; Rafaie-Graham, O.; Thanapongpibul, C.; Stevens, M.M. Microliter scale synthesis of luciferase-encapsulated polymersomes as artificial organelles for optogenetic modulation of cardiomyocyte beating. Adv. Sci. 2022, 9, 2200239. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Lin, H.-H.; Wu, M.-S.; Pan, L.; Yadav, K.; Hsu, H.-H.; Ling, T.-Y.; Chen, Y.-T.; Chang, H.-C. Intracellular delivery of luciferase with fluorescent nanodiamonds for dual-modality imaging of human stem cells. Bioconjug. Chem. 2019, 30, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Kheirolomoom, A.; Kruse, D.E.; Qin, S.; Watson, K.E.; Lai, C.-Y.; Young, L.J.; Cardiff, R.D.; Ferrera, K.W. Enhanced in vivo bioluminescence imaging using liposomal luciferin delivery system. J. Control Release 2010, 141, 128–136. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Sideratou, Z.; Tsiourvas, D.; Paleos, C.M. A novel mitotropic oligolysine nanocarrier: Targeted delivery of covalently bound D-Luciferin to cell mitochondria. Mitochondrion 2011, 11, 982–986. [Google Scholar] [CrossRef]
- Chandran, S.S.; Williams, S.A.; Denmeade, S.R. Extended-release PEG-luciferin allows for long-term imaging of firefly luciferase activity in vivo. Luminescence 2009, 24, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kiryu, S.; Izawa, K.; Watanabe, M.; Tojo, A.; Ohtomo, K. Comparison of subcutaneous and intraperitoneal injection of D-luciferin for in vivo bioluminescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Byun, S.S.; Paik, J.-Y.; Lee, S.Y.; Song, S.H.; Choe, Y.S.; Kim, B.-T. Cell uptake and tissue distribution of radioiodine labelled D-luciferin: Implications for luciferase based gene imaging. Nucl. Med. Commun. 2003, 24, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Jameson, M.J.; Broaddus, W.; Chung, T.D.; Golding, S.E.; Dever, S.M.; Rosenberg, E.; Valerie, K. Subcutaneous administration of D-luciferin is an effective alternative to intraperitoneal injection in bioluminescence imaging of xenograft tumors in nude mice. ISRN Mol. Imaging 2013, 2013, 689279. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.M.; Howe, S.J.; Rahim, A.A.; Burning, H.; Mcintosh, J.; Wong, S.-P.; Baker, A.H.; Nathwani, A.; Thrasher, A.J.; Coutelle, C.; et al. Luciferin detection after intranasal vector delivery is improved by intranasal rather than intraperitoneal luciferin administration. Hum. Gene Ther. 2008, 19, 1050–1056. [Google Scholar] [CrossRef]
- Kobayashi, H.; Picard, L.-P.; Schönegge, A.-M.; Bouvier, M. Bioluminescence resonance energy transfer-based imaging of protein-protein interactions in living cells. Nat. Protoc. 2019, 14, 1084–1107. [Google Scholar] [CrossRef] [PubMed]
- Hiblot, J.; Yu, Q.; Sabbadini, M.D.B.; Reymond, L.; Xue, L.; Schena, A.; Sallin, O.; Hill, N.; Griss, R.; Johnsson, K. Luciferases with tunable emission wavelengths. Angew. Chem. Int. Ed. 2017, 129, 14748–14752. [Google Scholar] [CrossRef]
- Weihs, F.; Wang, J.; Pfleger, K.D.G.; Dacres, H. Experimental determination of the bioluminescence resonance energy transfer (BRET) Förster distances of NanoBRET and red-shifted BRET pairs. Anal. Chim. Acta X 2020, 6, 100059. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, L.; Edenhofer, A.; Barck, L.; Huppertz, M.-C.; Frei, M.S.; Tarnawski, M.; Bergner, A.; Koch, B.; Johnsson, K.; Hiblot, J. A general method for the development of multicolor biosensors with large dynamic ranges. Nat. Chem. Biol. 2022, 19, 1147–1157. [Google Scholar] [CrossRef]
- Pfleger, K.D.G.; Eidne, K.A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 2006, 3, 165–174. [Google Scholar] [CrossRef]
- Breton, B.; Sauvageau, É.; Zhou, J.; Bonin, H.; Gouill, C.L.; Bouvier, M. Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys. J. 2010, 99, 4039–4046. [Google Scholar] [CrossRef]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E.I.; et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar] [CrossRef]
- Saito, K.; Chang, Y.-F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef]
- Takahiro, K.; Suka, T.; Hirota, K.; Kadonosono, T.; Kizaka-Kondoh, S. A novel injectable BRET-based in vivo imaging probe for detecting the activity of hypoxia-inducible factor regulated by the ubiquitin-proteasome system. Sci. Rep. 2016, 6, 34311. [Google Scholar]
- Wu, C.; Kawasaki, K.; Ohgiya, S.; Ohmiya, Y. Chemical studies on the BRET system between the bioluminescence of Cyprindina and quantum dots. Photochem. Photobiol. Sci. 2011, 10, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Shuhendler, A.J.; Rao, J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumor imaging. Nat. Commun. 2012, 3, 1193. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, B.S.; Steinhardt, R.C.; Mills, J.H.; Prescher, J.A. Multicomponent bioluminescence imaging with a π-extended luciferin. J. Am. Chem. Soc. 2020, 142, 14080–14089. [Google Scholar] [CrossRef]
- Tamaki, S.; Kitada, N.; Kiyama, M.; Fujii, R.; Hirano, T.; Kim, S.B.; Maki, S. Color-tunable bioluminescence imaging portfolio for cell imaging. Sci. Rep. 2021, 11, 2219. [Google Scholar] [CrossRef]
- Evans, M.S.; Chaurette, J.P.; Adams, S.T.; Reddy, G.R.; Paley, M.A.; Aronin, N.; Prescher, J.A.; Miller, S.C. A synthetic luciferin improves bioluminescence imaging in live mice. Nat. Methods 2014, 11, 393–395. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, K.; Dong, H.; Yang, S.; Li, J.; Luo, J.; Li, Y.; Yang, R. Long-lasting bioluminescence imaging of the fibroblast activation protein by an amphiphilic block copolymer-based probe. Anal. Chem. 2021, 93, 3726–3732. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, X.; Li, G.; Zhao, X.; Sun, T.; Müller, R.; Wang, H.; Li, M.; Zhang, Y. Bacteria-based live vehicle for in vivo bioluminescence imaging. Anal. Chem. 2021, 93, 15687–15695. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, Q.; Deng, R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X.; et al. In vitro and in vivo uncaging and bioluminescence imaging by using photocaged upconversion nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Park, H.M.; Au-Yeung, H.Y.; Van de Bittner, G.C.; Ackerman, C.M.; Stahl, A.; Chang, C.J. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2016, 113, 14219–14224. [Google Scholar] [CrossRef]
- Conway, M.; Xu, T.; Kirkpatrick, A.; Ripp, S.; Sayler, G.; Close, D. Real-time tracking of stem cell viability, proliferation, and differentiation with autonomous bioluminescence imaging. BMC Biol. 2020, 18, 79. [Google Scholar] [CrossRef]
- Tögel, F.; Yang, Y.; Zhang, P.; Hu, Z.; Westenfelder, C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am. J. Physiol. Renal Physiol. 2008, 295, F315–F321. [Google Scholar] [CrossRef]
- Jiang, T.; Bai, X.; Li, M. Advances in the development of bacterial bioluminescence imaging. Annu. Rev. Anal. Chem. 2024, 17, 16.1–16.24. [Google Scholar] [CrossRef]
- Niu, J.; Shen, L.; Huang, B.; Ye, F.; Zhao, L.; Wang, H.; Deng, Y.; Tan, W. Non-invasive bioluminescence imaging of HCoV-OC43 infection and therapy in the central nervous system of live mice. Antiviral Res. 2020, 173, 104646. [Google Scholar] [CrossRef]
- Mehle, A. Fiat Luc: Bioluminescence imaging reveals in vivo viral replication dynamics. PLoS Pathog. 2015, 11, e1005081. [Google Scholar] [CrossRef]
- Gupta, D.; Liang, X.; Pavlova, S.; Wiklander, O.P.B.; Corso, G.; Zhao, Y.; Saher, O.; Bost, J.; Zickler, A.M.; Piffko, A.; et al. Quantification of extracellular vesicles in vitro and in vivo using sensitie bioluminescence imaging. J. Extracell. Vesicles 2020, 9, 1800222. [Google Scholar] [CrossRef]
- Gangadaran, P.; Li, X.J.; Lee, H.W.; Oh, J.M.; Kalimuthu, S.; Rajendran, R.L.; Son, S.H.; Baek, S.H.; Singh, T.D.; Zhu, L.; et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget 2017, 8, 109894–109914. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Yoshimura, H.; Kim, S.B. Advances in fluorescence and bioluminescence imaging. Anal. Chem. 2013, 85, 590–609. [Google Scholar] [CrossRef]
- Karimi, M.A.; Lee, E.; Bachmann, M.H.; Salicioni, A.M.; Behrens, E.M.; Kambayashi, T.; Baldwin, C.L. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assy. PLoS ONE 2014, 9, e89357. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.; Hartog, I.; Zijlema, S.E.; Thijssen, V.; van der Beelen, S.H.E.; Merkx, M. Detection of antibodies in blood plasma using bioluminescent sensor proteins and a smartphone. Anal. Chem. 2016, 88, 4525–4532. [Google Scholar] [CrossRef]
- Gräwe, A.; Merkx, M. Bioluminescence goes dark: Boosting the performance of bioluminescent sensor proteins using complementation inhibitors. ACS Sens. 2022, 7, 3800–3808. [Google Scholar] [CrossRef]
- Tomimuro, K.; Tenda, K.; Ni, Y.; Hiruta, Y.; Merkx, M.; Citterio, D. Thread-based bioluminescent sensor for detecting multiple antibodies in a single drop of whole bold. ACS Sens. 2020, 5, 1786–1794. [Google Scholar] [CrossRef]
- Chen, L.; Bao, Y.; Denstedt, J.; Zhang, J. Nanostructured bioluminescent sensor for rapidly detecting thrombin. Biosens. Bioelectron. 2016, 77, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-D.; Jin, Q.; Zou, L.-W.; Hou, J.; Lv, X.; Lei, W.; Cheng, H.-L.; Ge, G.-B.; Yang, L. A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples. Chem. Commun. 2016, 52, 3183. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Arts, R.; Merkx, M. Ratiometric bioluminescent sensor proteins based on intramolecular split luciferase complementation. ACS Sens. 2019, 4, 20–25. [Google Scholar] [CrossRef]
- Schihada, H.; Vandenabeele, S.; Zabel, U.; Frank, M.; Lohse, M.J.; Maiellaro, I. A universal bioluminescence resonance energy transfer sensor design enables high-sensitivity screening of GPCR activation dynamics. Commun. Biol. 2018, 1, 105. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, Q.; Lu, X.; Zhang, C. A label-free bioluminescent sensor for real-time monitoring polynucleotide kinase activity. Anal. Chem. 2014, 86, 8481–8488. [Google Scholar] [CrossRef] [PubMed]
- den Hamer, A.; Dierickx, P.; Arts, R.; de Vries, J.S.P.M.; Brunsveld, L.; Merkx, M. Bright bioluminescent BRET sensor proteins for measuring intracellular caspase activity. ACS Sens. 2017, 2, 729–734. [Google Scholar] [CrossRef]
- Griss, R.; Schena, A.; Reymond, L.; Patiny, L.; Werner, D.; Tinberg, C.E.; Baker, D.; Johnsson, K. Bioluminescent sensor proteins for point-of-care therapeutic drug monitoring. Nat. Chem. Biol. 2014, 10, 598–603. [Google Scholar] [CrossRef]
- Xiong, M.; Wu, Y.; Kong, G.; Lewis, W.; Yang, Z.; Zhang, H.; Xu, L.; Liu, Y.; Liu, Q.; Zhao, X.; et al. A semisynthetic bioluminescence sensor for ratiometric imaging of metal ions in vivo using DNAzymes conjugated to an engineered nano-luciferase. Angew. Chem. Int. Ed. 2023, 62, e202308086. [Google Scholar] [CrossRef]
- Michielsen, C.M.S.; van Aalen, E.A.; Merkx, M. Ratiometric bioluminescent zinc sensor proteins to quantify serum and intracellular free Zn2+. ACS Chem. Biol. 2022, 17, 1567–1576. [Google Scholar] [CrossRef]
- Chen, F.; Warnock, R.L.; van der Meer, J.R.; Wegner, S.V. Bioluminescence-triggered photoswitchable bacterial adhesions enable higher sensitivity and dual-readout bacterial biosensors for mercury. ACS Sens. 2020, 5, 2205–2210. [Google Scholar] [CrossRef]
- Viviani, V.R.; Pelentir, G.F.; Bevilaqua, V.R. Bioluminescence color-tuning firefly luciferases: Engineering and prospects for real-time intracellular pH imaging and heavy metal biosensing. Biosensors 2022, 12, 400. [Google Scholar] [CrossRef]
- Aird, E.J.; Tompkins, K.J.; Ramirez, M.P.; Gordon, W.R. Enhanced molecular tension sensor based on bioluminescence resonance energy transfer (BRET). ACS Sens. 2020, 5, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.S.; Das, S.; Chen, J.-P. Photosensitizer-functionalized nanocomposites for light-activated cancer theranostics. Int. J. Mol. Sci. 2021, 22, 6658. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Deng, Q.-P.; Zheng, Q.-H.; Wang, Y.; Shen, J.; Zhou, J.-H. Hypericin nanoparticles for self-illuminated photodynamic cytotoxicity based on bioluminescence resonance energy transfer. Int. J. Pharm. 2022, 620, 121738. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, W.; Liu, S.; Sun, K.; Li, M.; Wu, C. Biodegradable polymer nanoparticles for photodynamic therapy by bioluminescence resonance energy transfer. Biomacromol. 2018, 19, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Forward, S.; Kim, K.-H.; Wu, Y.; Hui, J.; Kashiparekh, A.; Yun, S.-H. All-natural-molecule, bioluminescent photodynamic therapy results in complete tumor regression and prevents metastasis. Biomaterials 2023, 296, 122079. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.W.; Zhang, L.; Ferreira, L.; Turyanska, L.; Bradshaw, T.D.; Thomas, N.R. Listeria innocua Dps as a nanoplatform for bioluminescence based photodynamic therapy utilizing Gaussia princeps luciferase and zinc protoporphyrin IX. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102005. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Gong, J.; Fang, L.; Jiang, K. A photodynamic system based on endogenous bioluminescence for in vitro anticancer studies. Z. Anorg. Allg. Chem. 2019, 645, 1161–1164. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, S.; Kim, Y.K.; Moon, M.; Park, J.; Lee, K.J.; Lee, S.; Kim, Y.-P. Self-luminescent photodynamic therapy using breast cancer targeted proteins. Sci. Adv. 2020, 6, eaba3009. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Filimonova, V.P.; Frolova, A.Y.; Pichkur, E.B.; Fedotov, V.R.; Konevega, A.L.; Deyev, S.M.; Proshkina, G.M. HER-2-specific liposomes loaded with proteinaceous BRET pair as a promising tool for targeted self-excited photodynamic therapy. Eur. J. Pharm. Biopharm. 2023, 193, 208–217. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, C.-W.; Yu, H.-P.; Lin, Y.-F.; Lai, P.-S. Bioluminescence resonance energy transfer using luciferase-immobilized quantum dots for self-illuminated photodynamic therapy. Biomaterials 2013, 34, 1204–1212. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, S.; Choi, J.W.; Choi, S.Y.; Lee, S.-H.; Kim, H.; Hahn, S.K.; Koh, G.Y.; Yun, S.H. Bioluminescence-activated deep-tissue photodynamic therapy of cancer. Theranostics 2015, 5, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.; Liu, C.; Ding, S.; Tian, F.; Li, F. Bioluminescence-initiated photodynamic therapy bridged on high-luminescent carbon dots-conjugated protoporphyrin IX. J. Mater. Sci. 2019, 54, 3383–3391. [Google Scholar] [CrossRef]
- Fan, D.; Wang, T.; Hu, J.; Zhou, L.; Zhou, J.; Wei, S. Plasmid DNA-based bioluminescence-activated system for photodynamic therapy in cancer treatment. ChemMedChem 2021, 16, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Henriquez, N.; MacRobert, A.; Kitchen, N.; Williams, N.; Bown, S. Bioluminescence-activated photodynamic therapy for luciferase transfected, grade 4 astrocytoma cells in vitro. Photodiagn. Photodyn. Ther. 2022, 38, 102856. [Google Scholar] [CrossRef]
- Ng, J.; Henriquez, N.; Kitchen, N.; Williams, N.; Novelli, M.; Oukrif, D.; MacRobert, A.; Bown, S. Suppression of tumour growth from transplanted astrocytoma cells transfected with luciferase in mice by bioluminescence mediated, systemic, photodynamic therapy. Photodiagn. Photodyn. Ther. 2024, 45, 103923. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Chumakov, S.P.; Shipunova, V.O.; Ryabova, A.V.; Telegin, G.B.; Kabashin, A.V.; Deyev, S.M.; Proshkina, G.M. Genetically encoded BRET-activated photodynamic therapy for the treatment of deep-seated tumors. Light Sci. Appl. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, Y.; Dong, Z.; Hao, Y.; Wang, C.; Li, Q.; Wu, Y.; Feng, L.; Liu, Z. Engineering bioluminescent bacteria to boost photodynamic therapy and systemic anti-tumor immunity for synergistic cancer treatment. Biomaterials 2022, 281, 121332. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.M.; Roska, B.; Häusser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 2013, 16, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.R.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef]
- Berglund, K.; Birkner, E.; Augustine, G.J.; Hochgeschwender, U. Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons. PLoS ONE 2013, 8, e59759. [Google Scholar] [CrossRef]
- Berglund, K.; Clissold, K.; Li, H.E.; Wen, L.; Park, S.Y.; Gleixner, J.; Klein, M.E.; Lu, D.; Barter, J.W.; Rossi, M.A.; et al. Luminopsins integrate opto-and chemogenetics by using physical and biological light sources for opsin activation. Proc. Natl. Acad. Sci. USA 2016, 113, E358–E367. [Google Scholar] [CrossRef]
- Tung, J.K.; Gutekunst, C.-A.; Gross, R.E. Inhibitory luminopsins: Genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci. Rep. 2015, 5, 14366. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, M.; More, A.I.; Friedman, N.G.; Hochgeschwender, U.; Moore, C.I. The bioluminescent-optogenetic in vivo response to coelenterazine is proportional, sensitive, and specific in neocortex. J. Neurosci. Res. 2020, 98, 471–480. [Google Scholar] [CrossRef]
- Crepso, E.L.; Bjorefeldt, A.; Prakash, M.; Hochgeschwender, U. Bioluminescent optogenetics 2.0: Harnessing bioluminescence to activate photosensory proteins in vitro and in vivo. J. Vis. Exp. 2021, 174, e62850. [Google Scholar]
- Björefeldt, A.; Murphy, J.; Crespo, E.L.; Lambert, G.G.; Prakash, M.; Ikefuama, E.C.; Friedman, N.; Brown, T.M.; Lipscombe, D.; Moore, C.I.; et al. Efficient opto- and chemogenetic control in a single molecule driven by FRET-modified bioluminescence. Neurophotonics 2024, 11, 021005. [Google Scholar] [CrossRef] [PubMed]
- Slaviero, A.N.; Gorantla, N.; Simkins, J.; Crespo, E.L.; Ikefuama, E.C.; Tree, M.O.; Prakash, M.; Björefeldt, A.; Barnett, L.M.; Lambert, G.G.; et al. Engineering luminopsins with improved coupling efficiencies. Neurophotonics 2024, 11, 024208. [Google Scholar] [CrossRef]
- Petersen, E.D.; Sharkey, E.D.; Pal, A.; Shafau, L.O.; Zenchak-Petersen, J.; Peña, A.J.; Aggarwal, A.; Prakash, M.; Hochgeschwender, U. Restoring function after severe spinal cord injury through bioluminescent-optogenetics. Front. Neurol. 2022, 12, 792643. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Berglund, K.; Carrasco, D.; Goebel, K.; Gross, R.E.; Isaacson, R.; Mistretta, O.C.; Wynans, C. Bioluminescent optogenetics: A novel experimental therapy to promote axon regeneration after peripheral nerve injury. Int. J. Mol. Sci. 2021, 22, 7217. [Google Scholar] [CrossRef]
- Ecanow, A.; Berglund, K.; Carrasco, D.; Isaacson, R.; English, A.W. Enhancing motor and sensory axon regeneration after peripheral nerve injury using bioluminescent optogenetics. Int. J. Mol. Sci. 2022, 23, 16084. [Google Scholar] [CrossRef]
- Zenchak, J.R.; Palmateer, B.; Dorka, N.; Brown, T.M.; Wagner, L.-M.; Medendorp, W.E.; Petersen, E.D.; Prakash, M.; Hochgeschwender, U. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neuro. Res. 2020, 98, 458–468. [Google Scholar] [CrossRef]
- Ding, J.; Lu, J.; Zhang, Q.; Xu, Y.; Xu, Y.; Song, B.; Wu, Y.; Shi, H.; Chu, B.; Wang, H.; et al. Camouflage nanoparticles enable in situ bioluminescence-driven optogenetic therapy of retinoblastoma. ACS Nano 2023, 17, 7750–7764. [Google Scholar] [CrossRef] [PubMed]
- Adir, O.; Albalak, M.R.; Abel, R.; Weiss, L.E.; Chen, G.; Gruber, A.; Staufer, O.; Kurman, Y.; Kaminer, I.; Shklover, J.; et al. Synthetic cells with self-activating optogenetic proteins communicate with natural cells. Nat. Commun. 2022, 13, 2328. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, Z.; Huang, K.; Chen, R.; Nguyen, N.T.; Wang, R.; Cai, X.; Huang, Z.; Siwko, S.; Walker, J.R.; et al. Optogenetic control of non-apoptotic cell death. Adv. Sci. 2021, 8, 2100424. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Gregucci, D.; Martínez-Pérez-Cejuela, H.; Michelini, E. A luciferase mutant with improved brightness and stability for whole-cell bioluminescent biosensors and in vitro biosensing. Biosensors 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.H.-W.; Norn, C.; Kipnis, Y.; Tischer, D.; Pellock, S.J.; Evans, D.; Ma, P.; Lee, G.R.; Zhang, J.Z.; Anishchenko, I.; et al. De novo design of luciferases using deep learning. Nature 2023, 614, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Liu, D.; Wang, X.; Zhang, A.; Wei, Q.; Nandi, A.; Dong, S.; Warshel, A. Enhancing luciferase activity and stability through generative modeling of natural enzyme sequences. Proc. Natl. Acad. Sci. USA 2023, 120, e2312848120. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
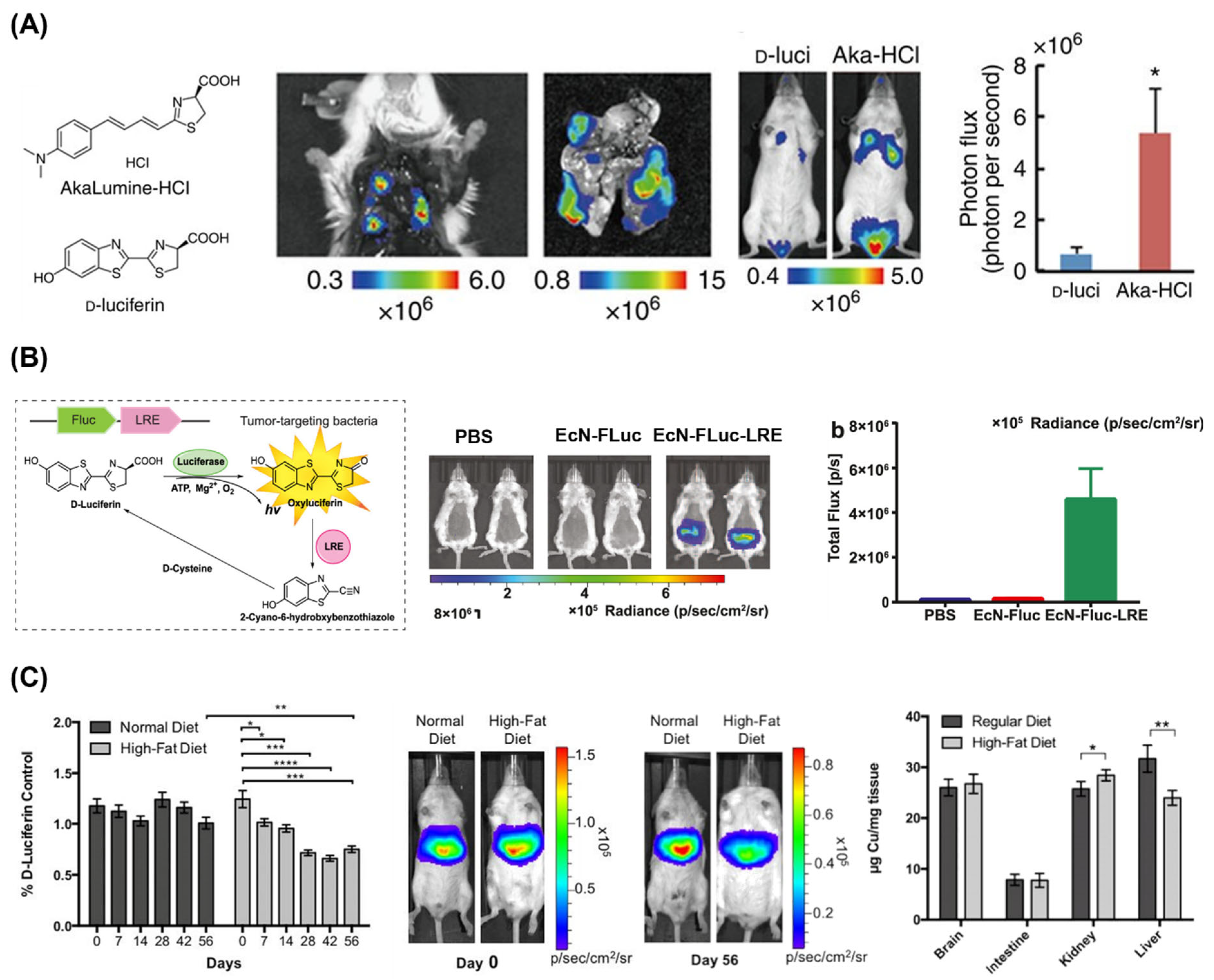
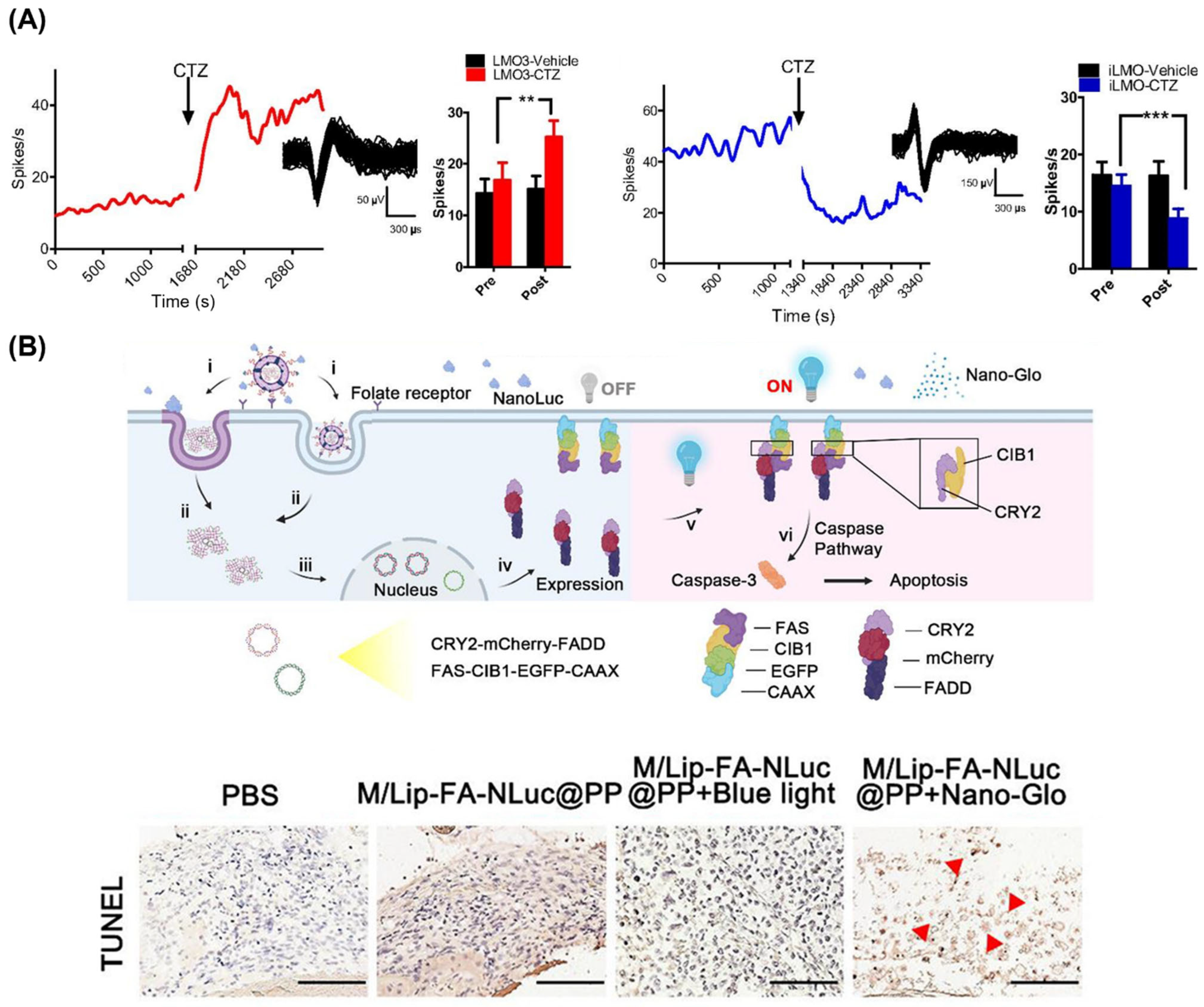
| Luciferins | Luciferases (Molecular Weight) | Requirements for Reactions | , nm) | Kinetics |
|---|---|---|---|---|
| D-luciferin | Firefly luciferase (62 kDa) | ATP, Mg2+, O2 | 558 | Glow |
| Coelenterazine | Renilla luciferase (36 kDa) | O2 | 480 | Flash |
| Gaussia luciferase (20 kDa) | O2 | 485 | Flash | |
| Oplophorus luciferase (106 kDa) | O2 | 454 | Flash | |
| Long-chain aldehyde | Bacterial luciferase (77 kDa) | FMNH2, O2 | 490 | Flash |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jung, S.O.; Lee, S.; Lee, Y. Bioluminescent Systems for Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 7563. https://doi.org/10.3390/ijms25147563
Kim H, Jung SO, Lee S, Lee Y. Bioluminescent Systems for Theranostic Applications. International Journal of Molecular Sciences. 2024; 25(14):7563. https://doi.org/10.3390/ijms25147563
Chicago/Turabian StyleKim, Hyemin, Seung Oh Jung, Seungchan Lee, and Yujin Lee. 2024. "Bioluminescent Systems for Theranostic Applications" International Journal of Molecular Sciences 25, no. 14: 7563. https://doi.org/10.3390/ijms25147563
APA StyleKim, H., Jung, S. O., Lee, S., & Lee, Y. (2024). Bioluminescent Systems for Theranostic Applications. International Journal of Molecular Sciences, 25(14), 7563. https://doi.org/10.3390/ijms25147563





