SGLT2 Inhibitors Empagliflozin and Canagliflozin Ameliorate Allergic Asthma Responses in Mice
Abstract
:1. Introduction
2. Results
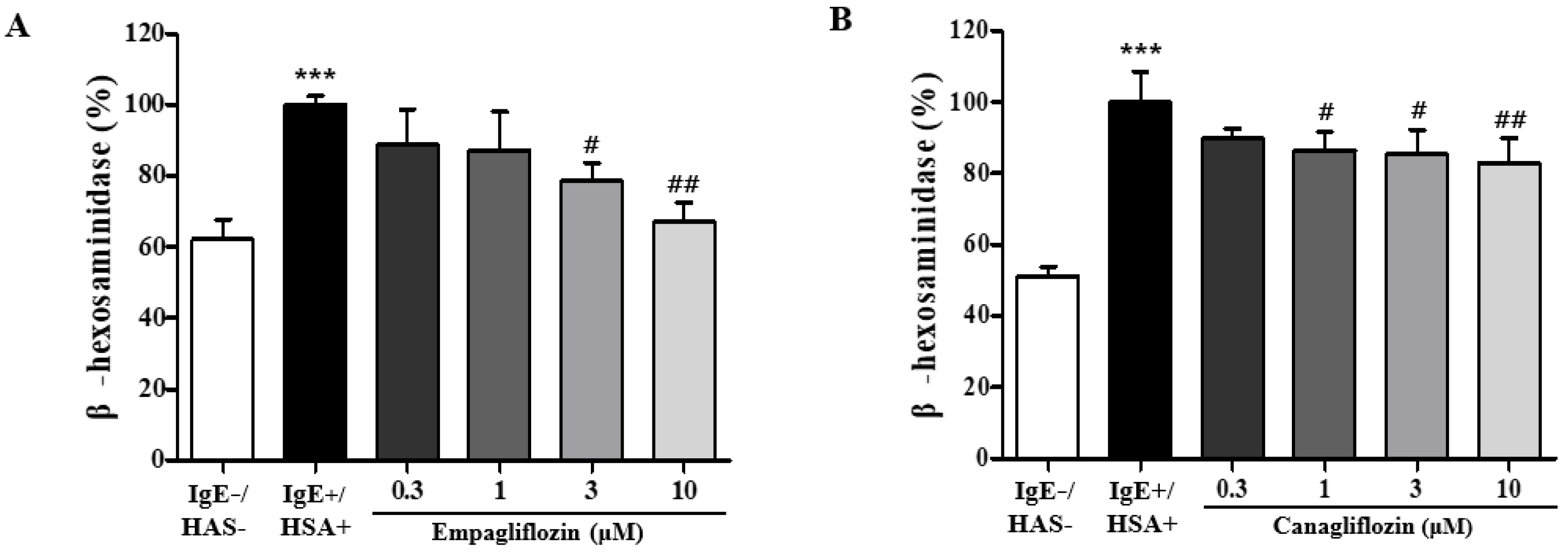
2.1. Empagliflozin and Canagliflozin Suppresses Mast Cell Degranulation
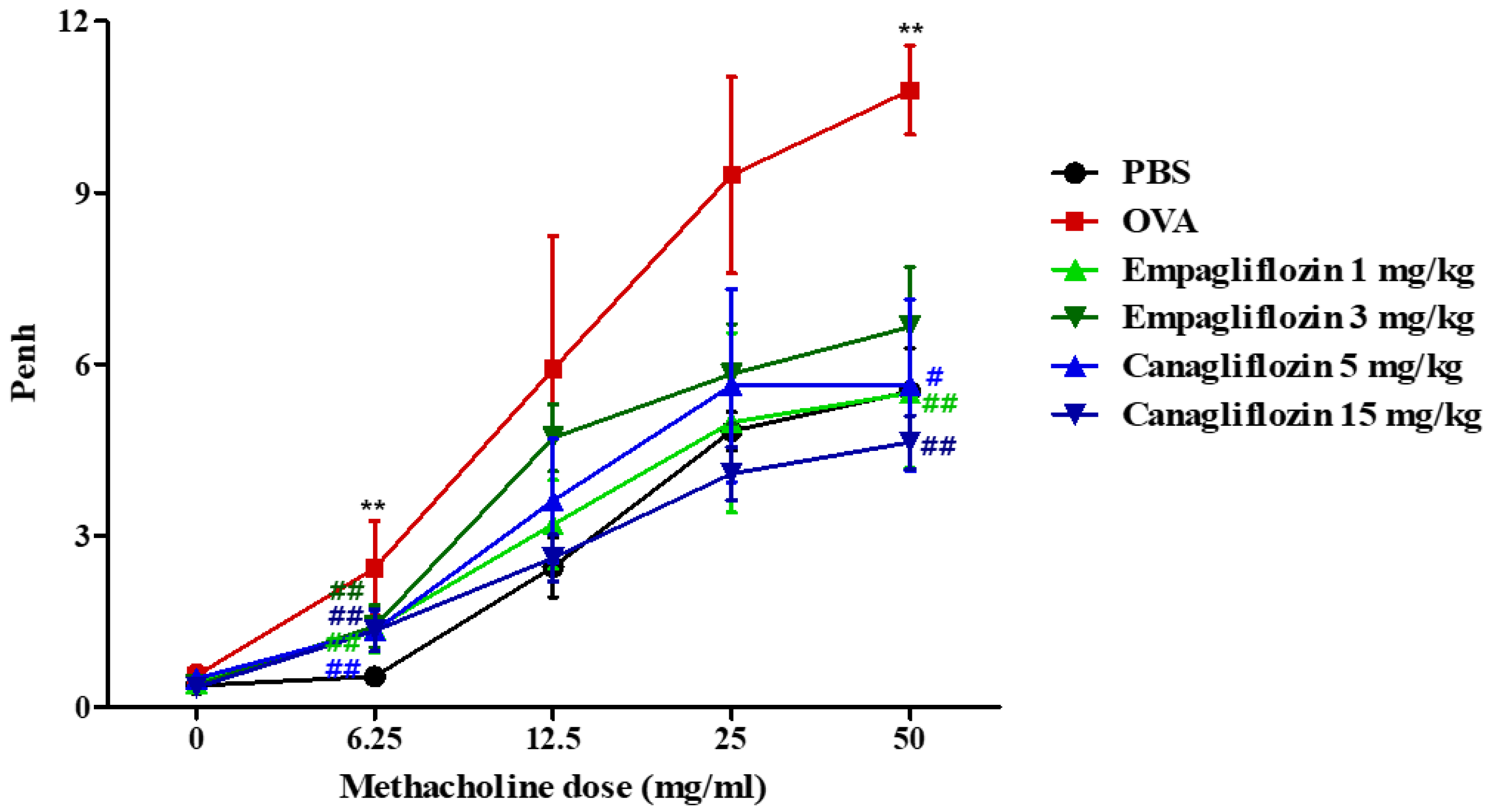
2.2. Empagliflozin and Canagliflozin Suppressed Ovalbumin-Induced Airway Hyper-Responsiveness in Mice
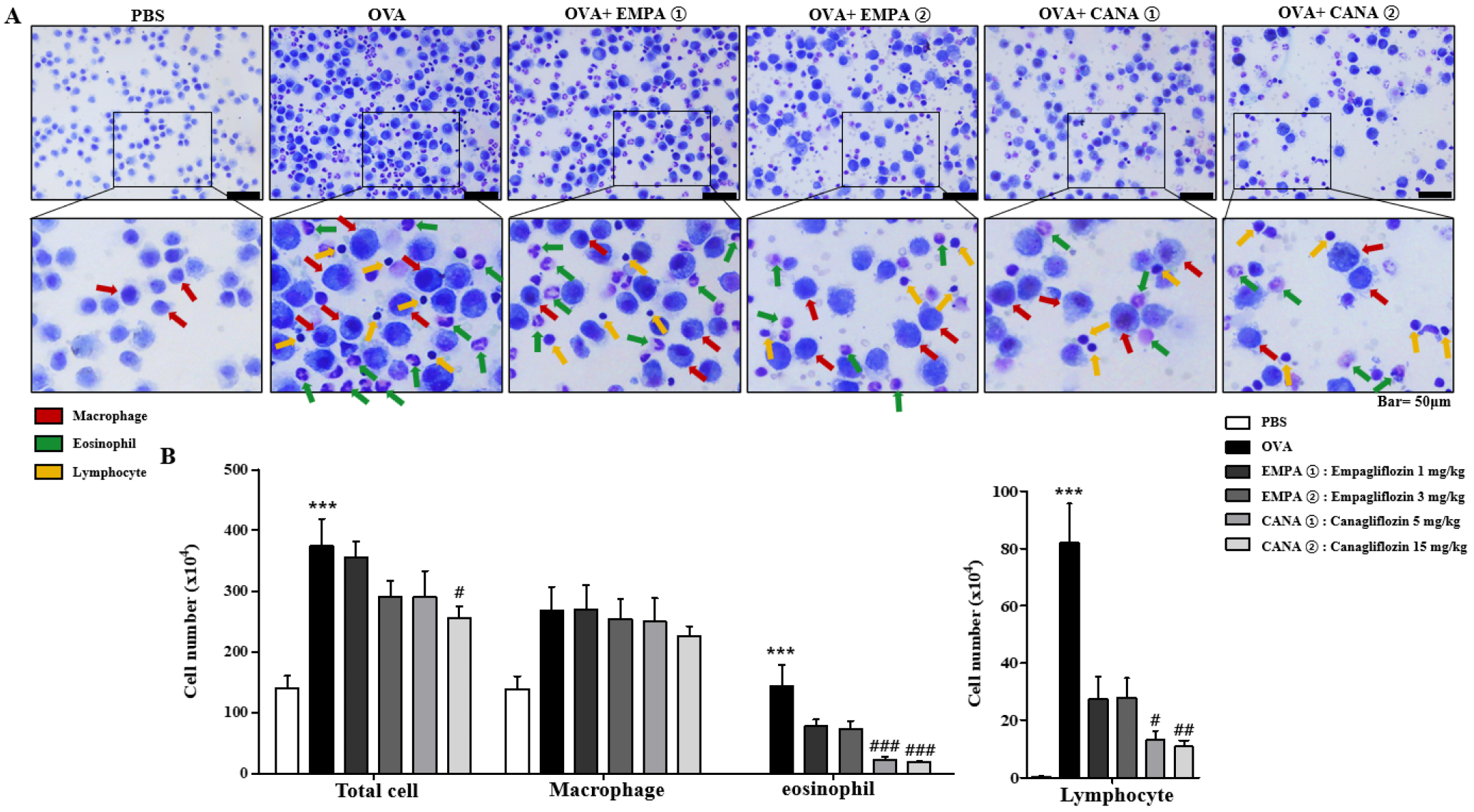
2.3. Empagliflozin and Canagliflozin Suppressed the OVA-Induced Increase in Immune Cell Counts and mRNA Expression Levels of Pro-Inflammatory Cytokines in Bronchoalveolar Lavage Fluid
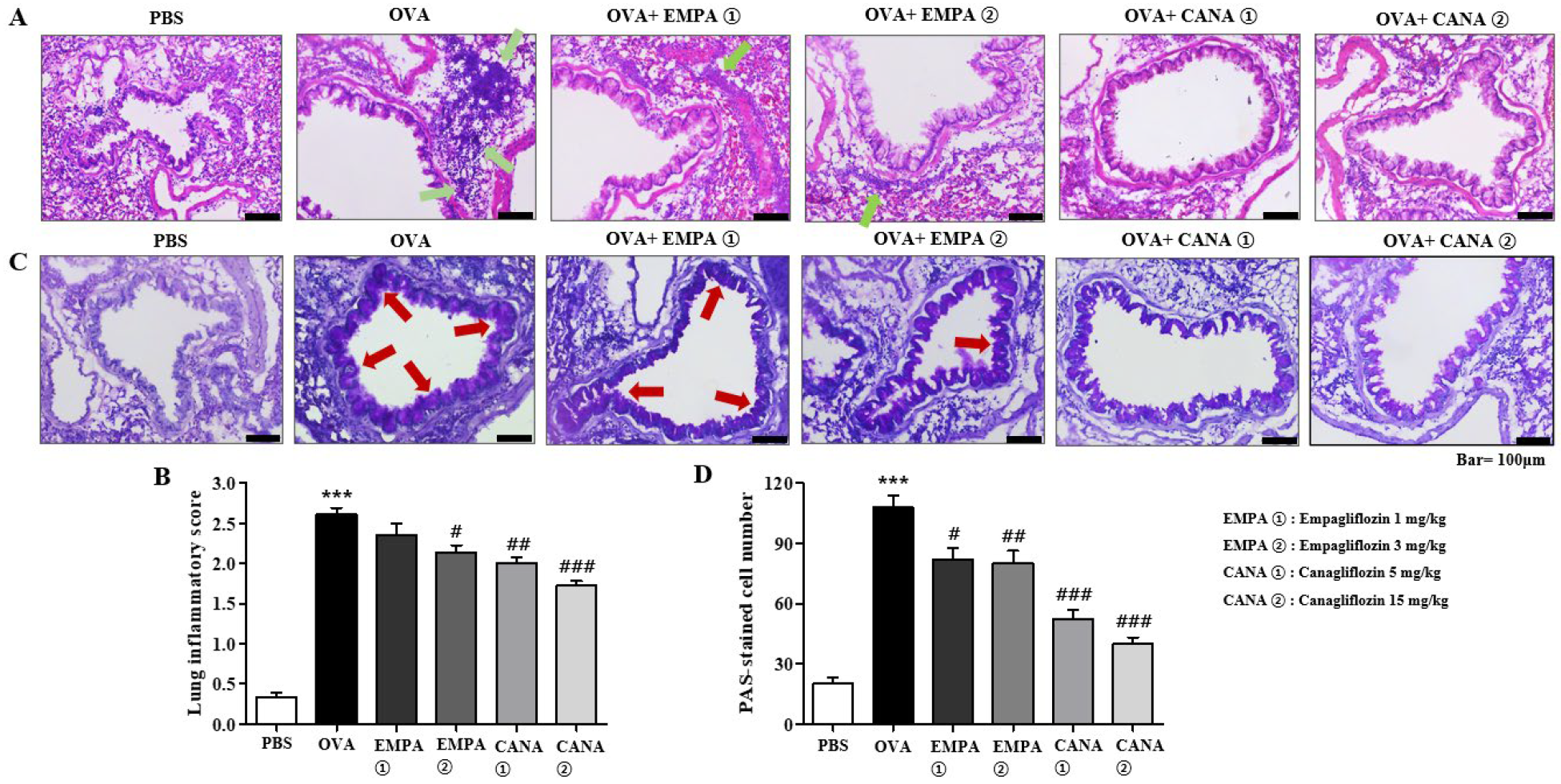
2.4. Empagliflozin and Canagliflozin Suppressed Histopathological Changes in the Airways
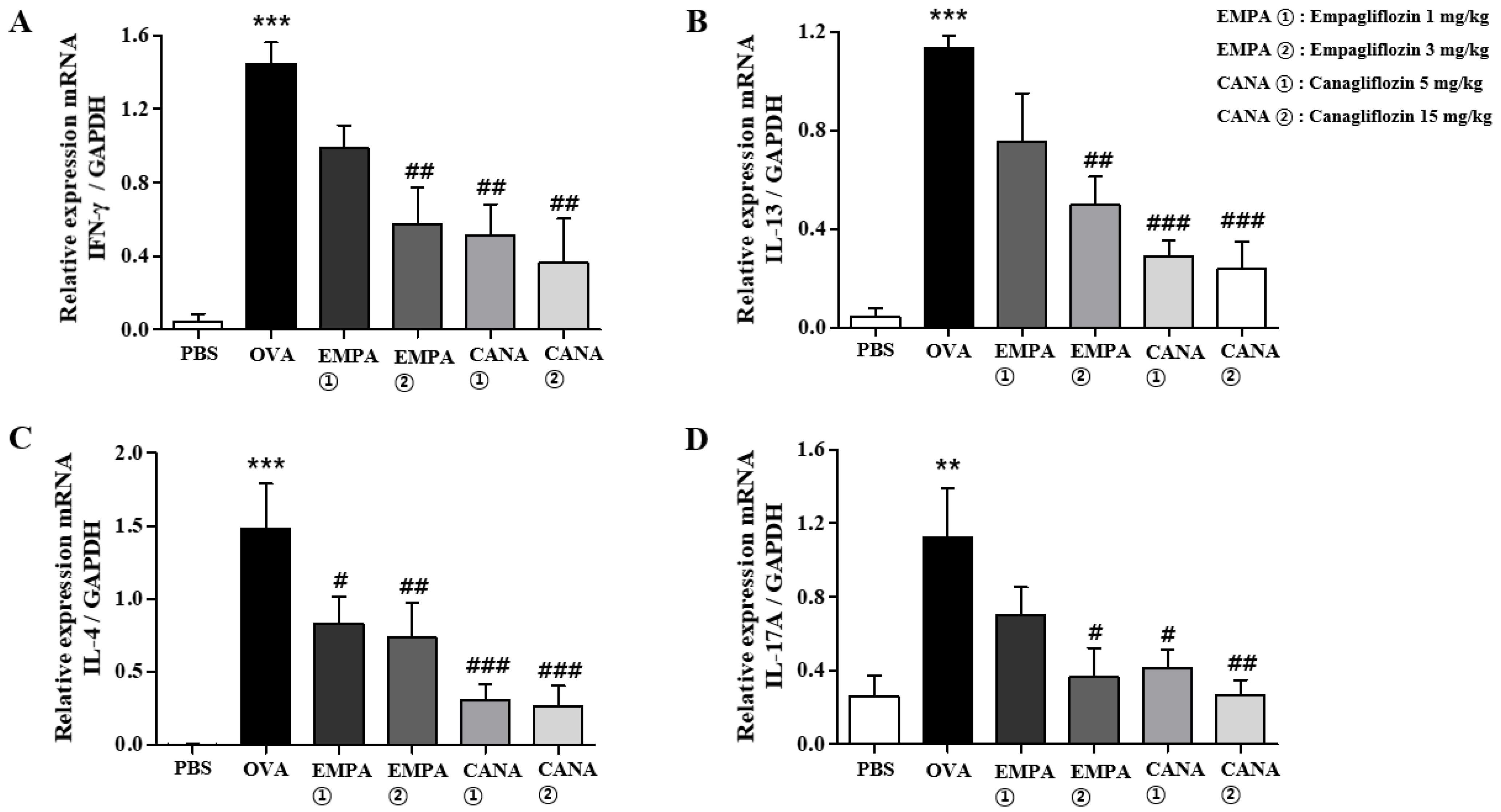
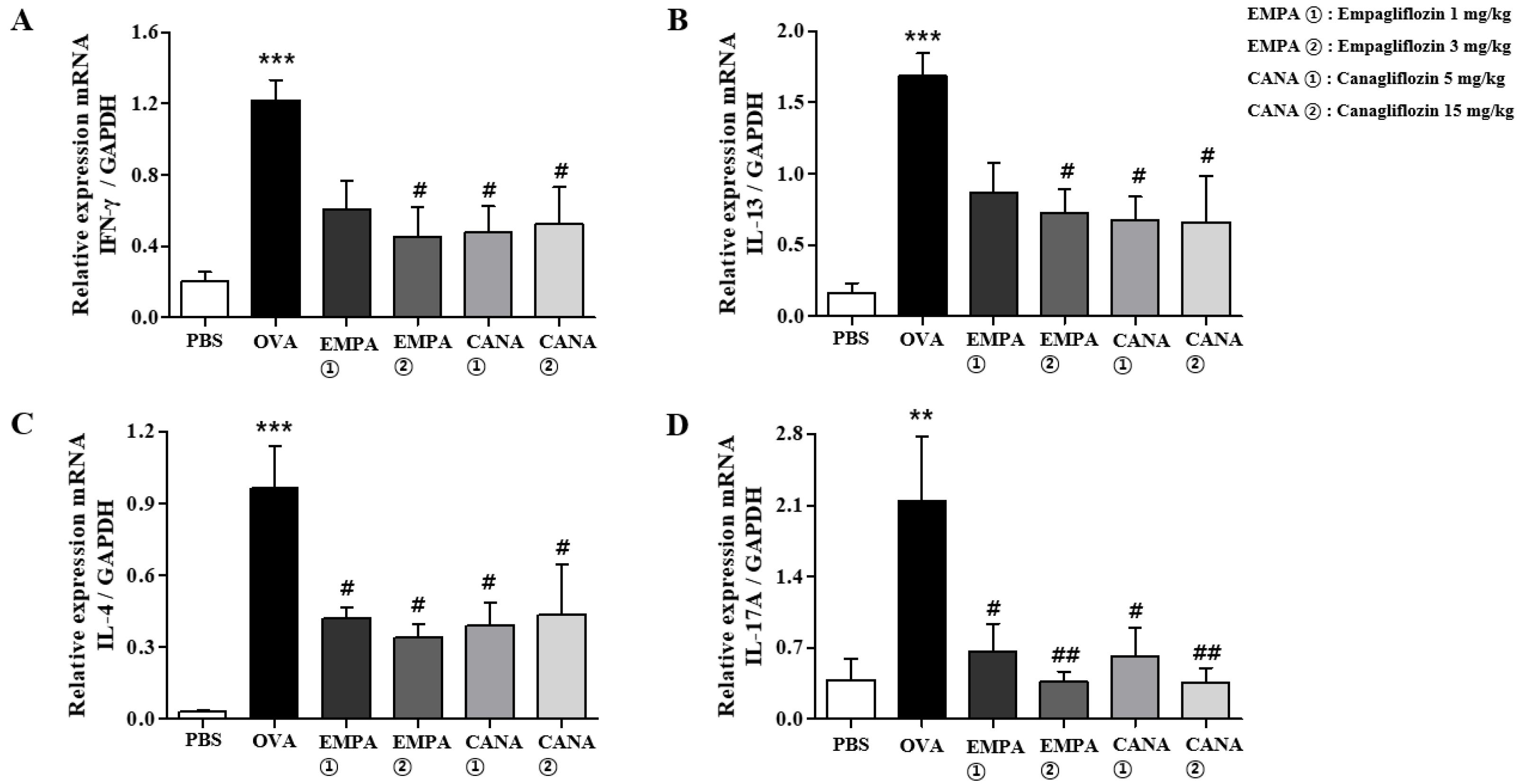
2.5. Empagliflozin and Canagliflozin Suppressed the OVA-Induced Increase in Expression Levels of Pro-Inflammatory Cytokines mRNAs in the Lungs
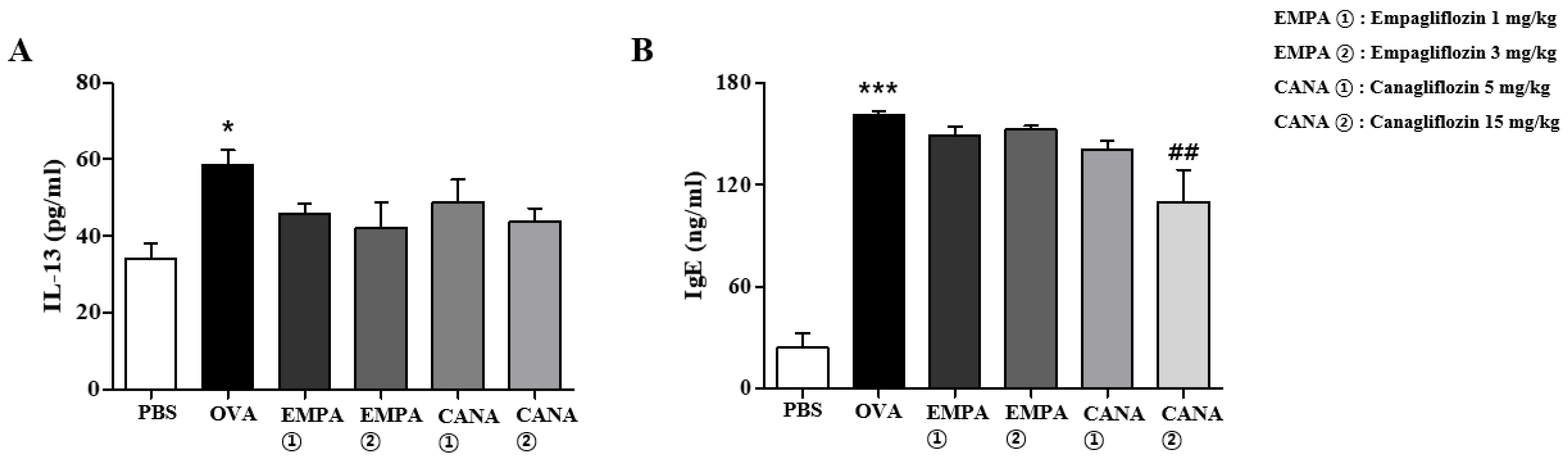
2.6. Empagliflozin and Canagliflozin Suppressed the Increase in Serum IgE Levels and BALF IL-13 Levels Induced by OVA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. RBL-2H3 Mast Cells
4.3. Determination of Mast Cell Degranulation
4.4. BALB/c Mice
4.5. OVA-Induced Asthma Model and Canagliflozin Treatment
4.6. BALF Cell Counting and Analysis
4.7. Measuring Airway Hyper-Responsiveness to Methacholine
4.8. Histopathological Analysis of the Lung
4.9. Measurement of the Protein Levels of IgE in Serum and IL-13 in BALF
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulet, L.P.; Boulay, M.; Côté, A.; FitzGerald, J.M.; Bergeron, C.; Lemière, C.; Lougheed, M.D.; Vandemheen, K.L.; Aaron, S.D. Airway inflammation and hyperresponsiveness in subjects with respiratory symptoms and normal spirometry. Eur. Respir. J. 2023, 61, 2201194. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Davis, B.E. Mechanisms of airway hyperresponsiveness. J. Allergy Clin. Immunol. 2006, 118, 551–559; quiz 560–561. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef] [PubMed]
- Albertson, T.E.; Chenoweth, J.A.; Adams, J.Y.; Sutter, M.E. Muscarinic antagonists in early stage clinical development for the treatment of asthma. Expert Opin. Investig. Drugs 2017, 26, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Spina, D. Current and novel bronchodilators in respiratory disease. Curr. Opin. Pulm. Med. 2014, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Steinhilber, D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006, 112, 701–718. [Google Scholar] [CrossRef]
- Poff, C.D.; Balazy, M. Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer. Curr. Drug Targets Inflamm. Allergy 2004, 3, 19–33. [Google Scholar] [CrossRef]
- Papierniak, E.S.; Lowenthal, D.T.; Harman, E. Novel therapies in asthma: Leukotriene antagonists, biologic agents, and beyond. Am. J. Ther. 2013, 20, 79–103. [Google Scholar] [CrossRef]
- Manka, L.A.; Wechsler, M.E. New biologics for allergic diseases. Expert Rev. Clin. Immunol. 2018, 14, 285–296. [Google Scholar] [CrossRef]
- Morita, H.; Matsumoto, K.; Saito, H. Biologics for allergic and immunologic diseases. J. Allergy Clin. Immunol. 2022, 150, 766–777. [Google Scholar] [CrossRef]
- Tan, H.T.; Sugita, K.; Akdis, C.A. Novel Biologicals for the Treatment of Allergic Diseases and Asthma. Curr. Allergy Asthma Rep. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Im, D.S. Alisol B 23-Acetate Ameliorates Ovalbumin-Induced Allergic Asthma during Sensitization and Challenge Periods. Biomol. Ther. 2023, 31, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Im, D.S. Selonsertib, an ASK1 Inhibitor, Ameliorates Ovalbumin-Induced Allergic Asthma during Challenge and Sensitization Periods. Biomol. Ther. 2024, 32, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Im, D.S. Elafibranor PPARα/δ Dual Agonist Ameliorates Ovalbumin-Induced Allergic Asthma. Biomol. Ther. 2024, 32, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Son, S.E.; Im, D.S. Free fatty acid 3 receptor agonist AR420626 reduces allergic responses in asthma and eczema in mice. Int. Immunopharmacol. 2024, 127, 111428. [Google Scholar] [CrossRef]
- Lee, J.-H.; Son, S.-H.; Kim, N.-J.; Im, D.-S. NJK14047 Suppression of the p38 MAPK Ameliorates OVA-Induced Allergic Asthma during Sensitization and Challenge Periods. Biomol. Ther. 2023, 31, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Novac, N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013, 34, 267–272. [Google Scholar] [CrossRef]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Jarada, T.N.; Rokne, J.G.; Alhajj, R. A review of computational drug repositioning: Strategies, approaches, opportunities, challenges, and directions. J. Cheminform 2020, 12, 46. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Song, C.; Zeng, Y.; Li, Y.; Li, H.; Liu, B.; Dai, M.; Pan, P. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. Int. Immunopharmacol. 2020, 88, 106969. [Google Scholar] [CrossRef]
- Abdollahi, E.; Keyhanfar, F.; Delbandi, A.A.; Falak, R.; Hajimiresmaiel, S.J.; Shafiei, M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-κB activation in human endothelial cells and differentiated macrophages. Eur. J. Pharmacol. 2022, 918, 174715. [Google Scholar] [CrossRef]
- Osataphan, S.; Macchi, C.; Singhal, G.; Chimene-Weiss, J.; Sales, V.; Kozuka, C.; Dreyfuss, J.M.; Pan, H.; Tangcharoenpaisan, Y.; Morningstar, J.; et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 2019, 4, e123130. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Soni, S.; Dyck, J.R.B. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology 2021, 29, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, M.; Xu, B.; Kang, L. Empagliflozin Alleviates Atherosclerosis Progression by Inhibiting Inflammation and Sympathetic Activity in a Normoglycemic Mouse Model. J. Inflamm. Res. 2021, 14, 2277–2287. [Google Scholar] [CrossRef]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Efentakis, P.; Balafas, E.; Togliatto, G.; Davos, C.H.; Varela, A.; Dimitriou, C.A.; Nikolaou, P.E.; Maratou, E.; Lambadiari, V.; et al. Empagliflozin Limits Myocardial Infarction in Vivo and Cell Death in Vitro: Role of STAT3, Mitochondria, and Redox Aspects. Front. Physiol. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res. 2015, 47, 686–692. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of SGLT2 inhibitors: Part 3. Effects on diabetic complications in type 2 diabetic mice. Eur. J. Pharmacol. 2017, 809, 163–171. [Google Scholar] [CrossRef]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Renal. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, W.; Zhong, J.; Lei, F.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem. Pharmacol. 2018, 152, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, H.; Zhang, N.; Feng, X. Association between novel Glucose-Lowering drugs and risk of Asthma: A network Meta-Analysis of cardiorenal outcome trials. Diabetes Res. Clin. Pract. 2022, 183, 109080. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Liu, D.; Jiang, H.; Cai, C.; Li, G.; Yu, G. Canagliflozin Prevents Lipid Accumulation, Mitochondrial Dysfunction, and Gut Microbiota Dysbiosis in Mice with Diabetic Cardiovascular Disease. Front. Pharmacol. 2022, 13, 839640. [Google Scholar] [CrossRef]
- Munitz, A.; Brandt, E.B.; Mingler, M.; Finkelman, F.D.; Rothenberg, M.E. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.A.; Borish, L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011, 32, 83–94. [Google Scholar] [CrossRef]
- Luo, W.; Hu, J.; Xu, W.; Dong, J. Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma. Front. Immunol. 2022, 13, 974066. [Google Scholar] [CrossRef]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 cells: New players in asthma pathogenesis. Allergy 2011, 66, 989–998. [Google Scholar] [CrossRef]
- Holgate, S.T.; Djukanović, R.; Wilson, J.; Roche, W.; Britten, K.; Howarth, P.H. Allergic inflammation and its pharmacological modulation in asthma. Int. Arch. Allergy Appl. Immunol. 1991, 94, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sabolic, I.; Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.; Breljak, D.; Ljubojevic, M.; Brzica, H.; Sebastiani, A.; Thal, S.C.; et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol. 2012, 302, C1174–C1188. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac, I.; Balen Eror, D.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. Eur. J. Physiol. 2015, 467, 1881–1898. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Huang, B.P.; Lin, C.H.; Chen, H.M.; Lin, J.T.; Cheng, Y.F.; Kao, S.H. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF-κB signaling in murine macrophages. DNA Cell Biol. 2015, 34, 133–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-E.; Im, D.-S. SGLT2 Inhibitors Empagliflozin and Canagliflozin Ameliorate Allergic Asthma Responses in Mice. Int. J. Mol. Sci. 2024, 25, 7567. https://doi.org/10.3390/ijms25147567
Lee Y-E, Im D-S. SGLT2 Inhibitors Empagliflozin and Canagliflozin Ameliorate Allergic Asthma Responses in Mice. International Journal of Molecular Sciences. 2024; 25(14):7567. https://doi.org/10.3390/ijms25147567
Chicago/Turabian StyleLee, Ye-Eul, and Dong-Soon Im. 2024. "SGLT2 Inhibitors Empagliflozin and Canagliflozin Ameliorate Allergic Asthma Responses in Mice" International Journal of Molecular Sciences 25, no. 14: 7567. https://doi.org/10.3390/ijms25147567




