Redox Enzymes P4HB and PDIA3 Interact with STIM1 to Fine-Tune Its Calcium Sensitivity and Activation
Abstract
:1. Introduction
2. Results and Discussion
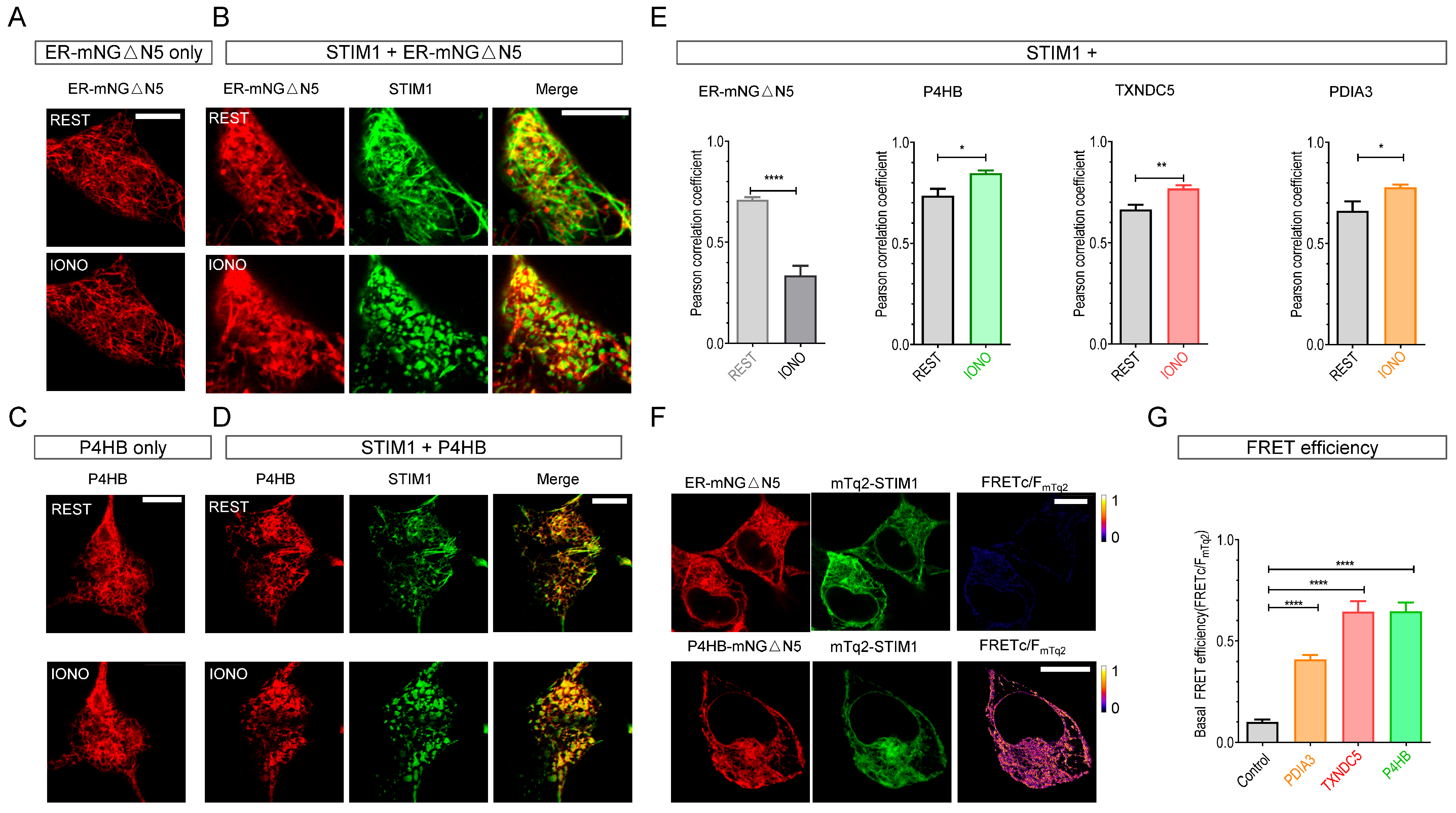
2.1. STIM Proteins Dynamically Interact with Three Protein Disulfide Isomerases (PDIs) (P4HB, TXNDC5, and PDIA3)
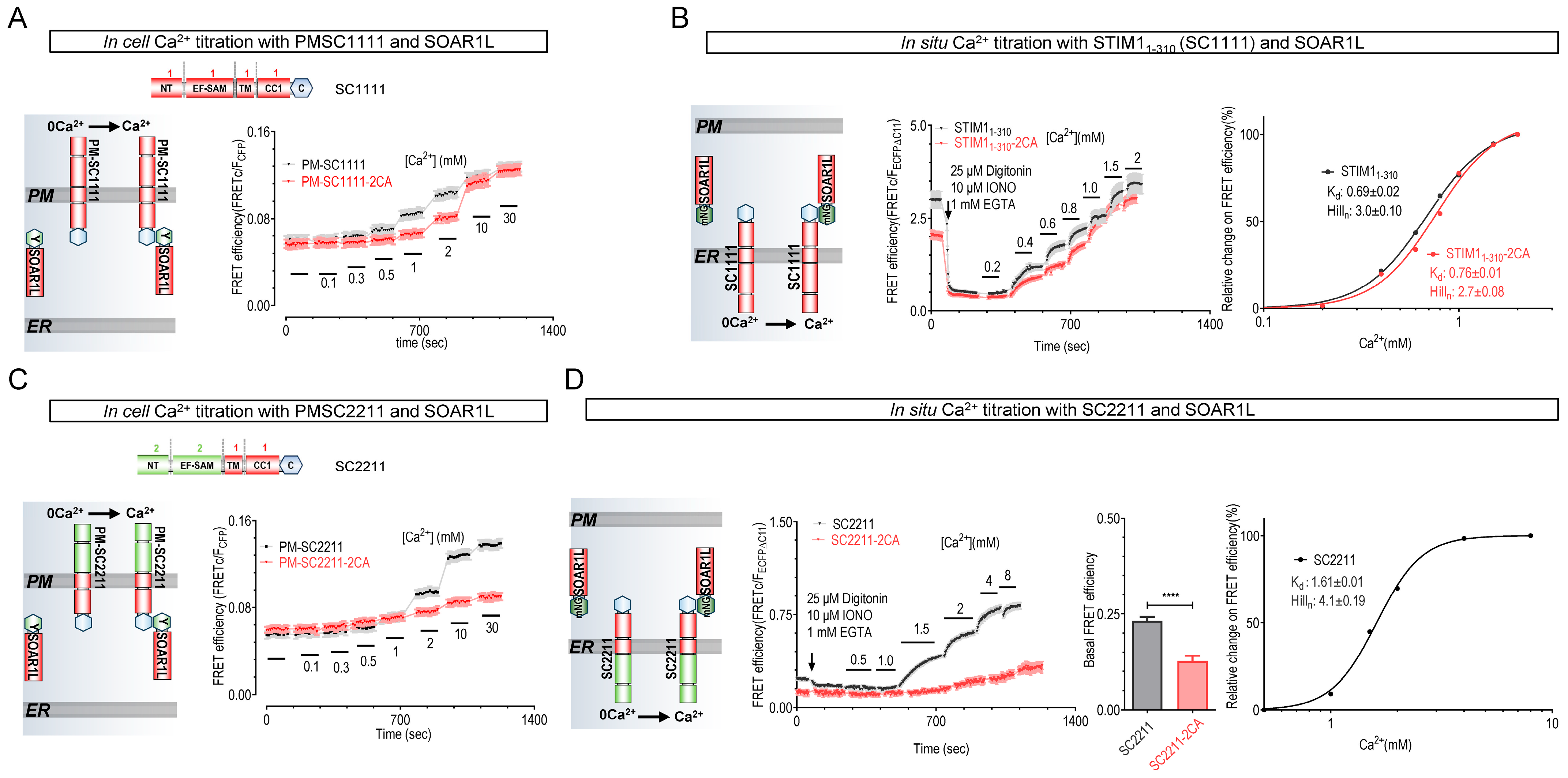
2.2. STIM Mutants Lacking Disulfide-Bond-Forming Ability Exhibited Lower Ca2+-Binding Affinity
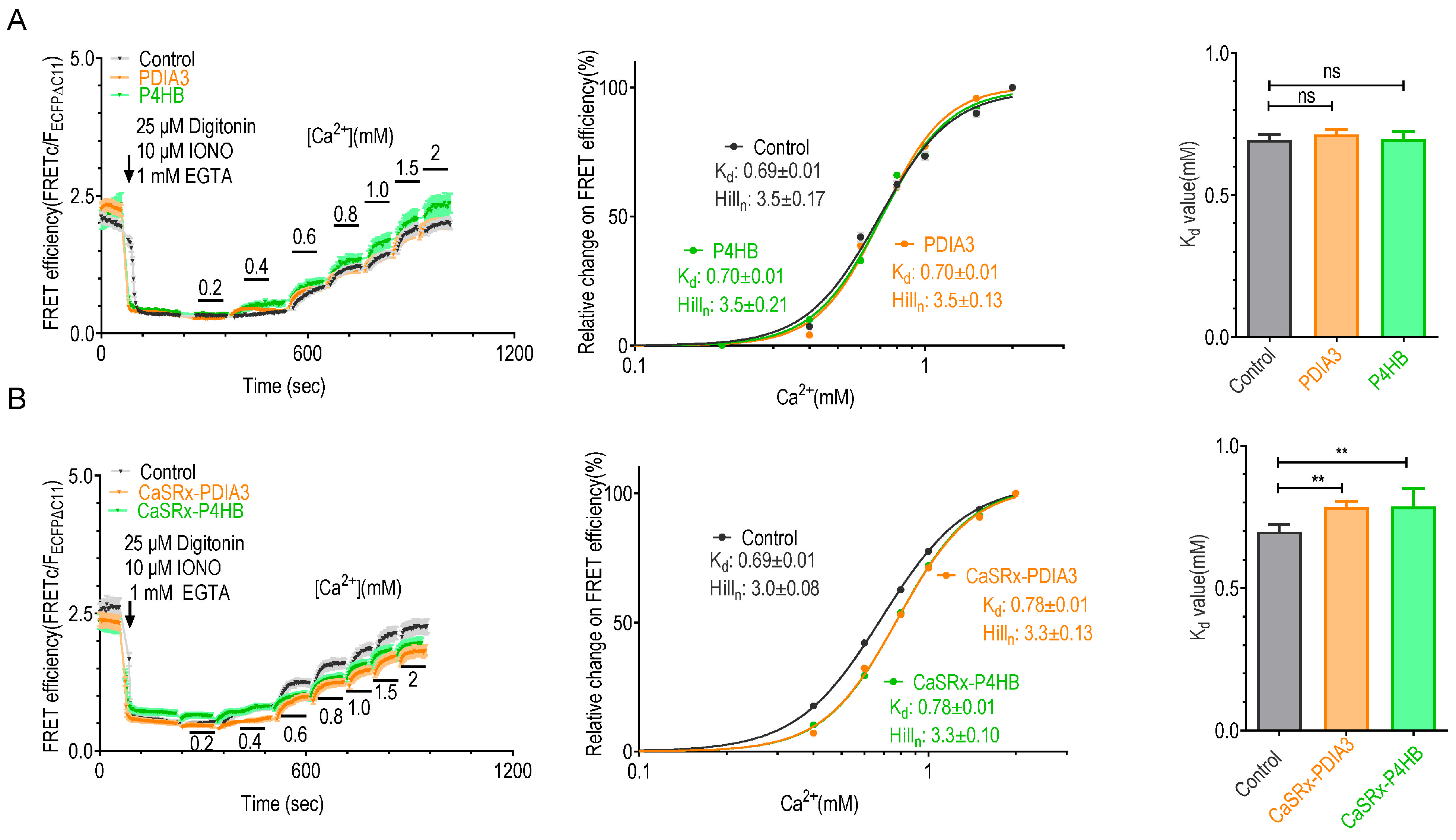
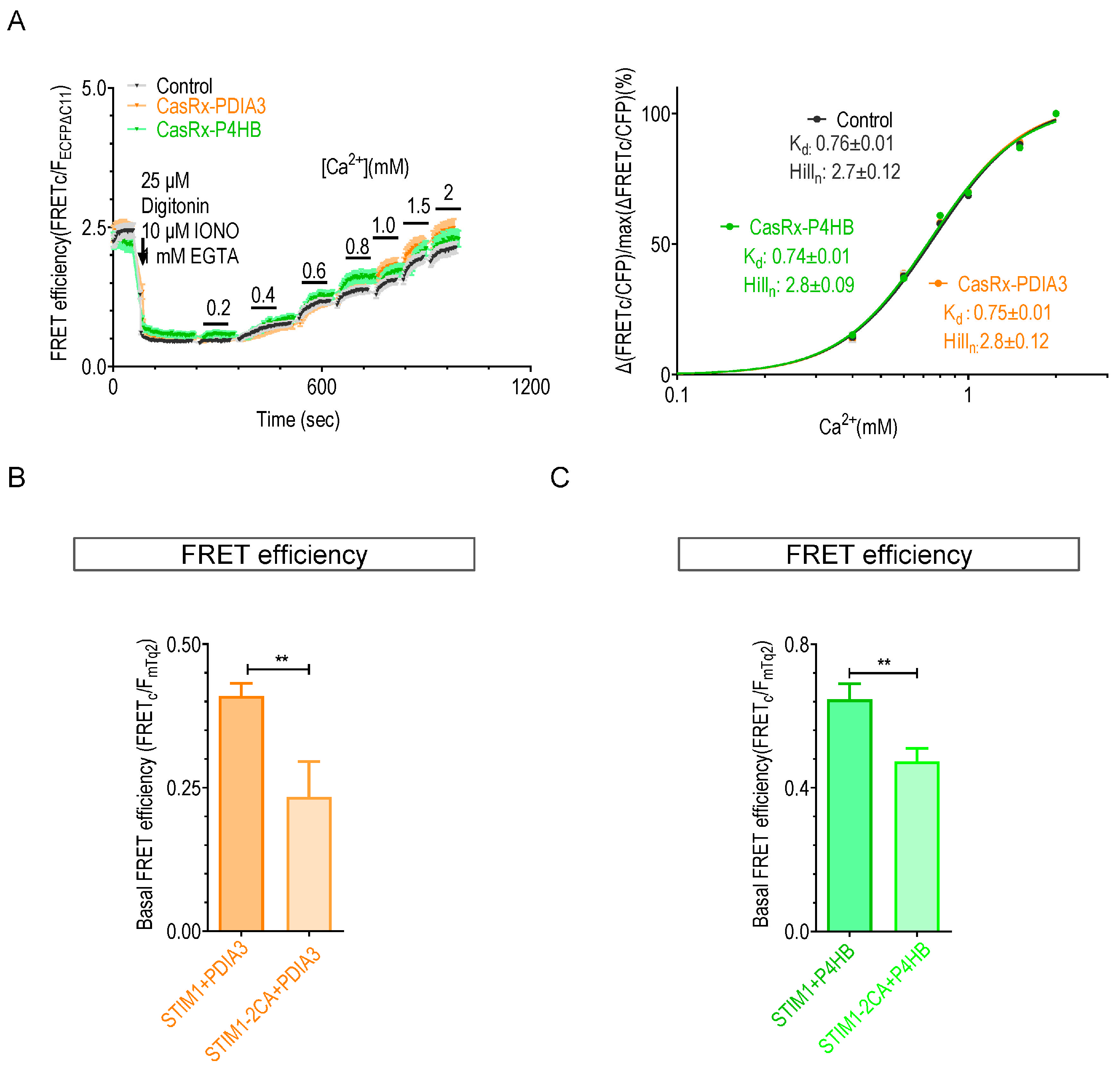
2.3. Knocking down P4HB or PDIA3 Reduce the Ca2+ Affinity of STIM1
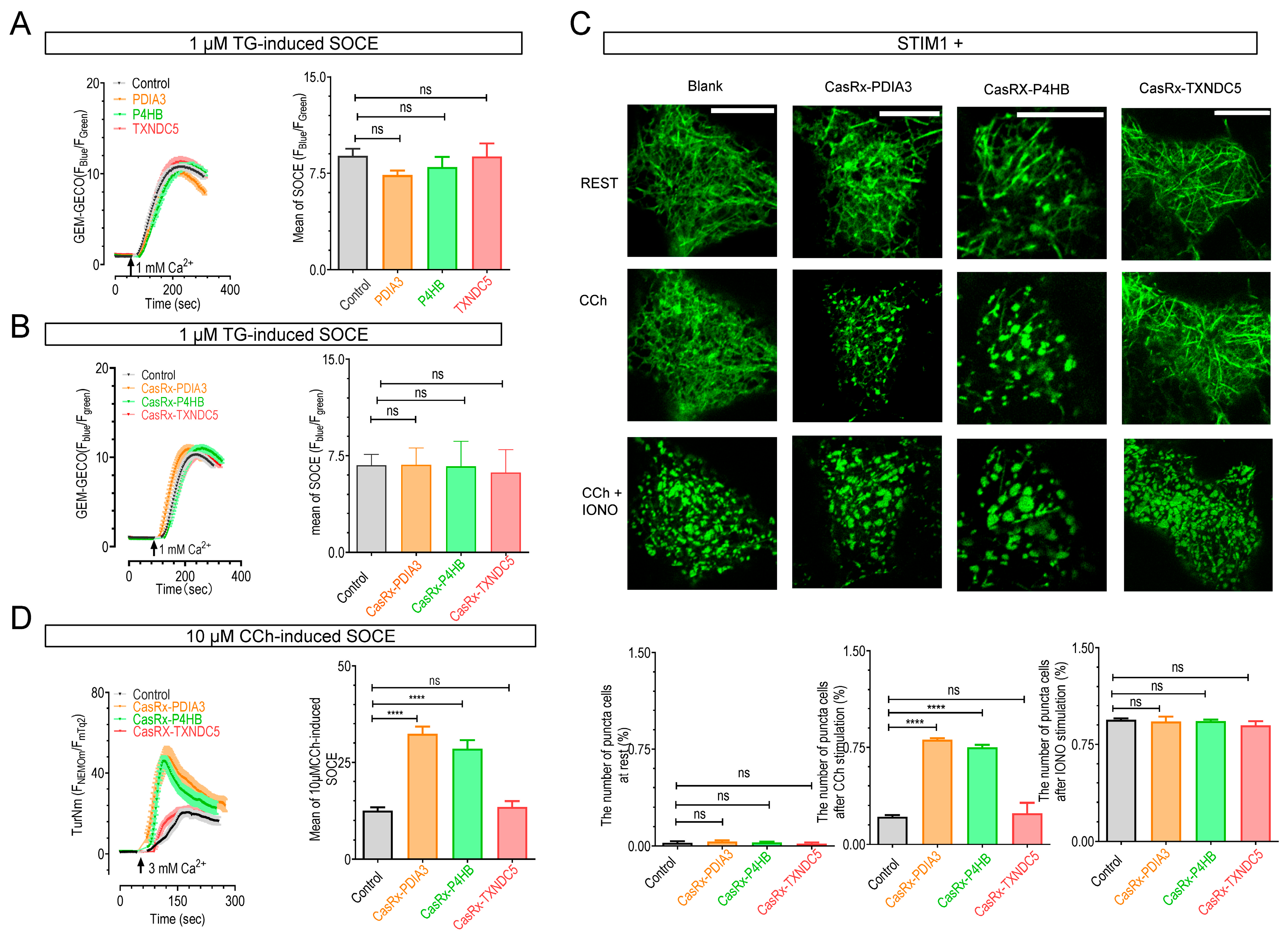
2.4. The Knockdown of P4HB or PDIA3 Promotes STIM1 Activation and SOCE
3. Materials and Methods
3.1. Plasmids Construction
3.2. Gene Knockdown by CasRx
3.3. Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
3.4. Cell Culture and Transfection
3.5. Ca2+ Imaging in Living Cells
3.6. Förster Resonance Energy Transfer (FRET) Imaging and Measurements of Ca2+ Affinity of STIM1
3.7. Confocal Microscopy
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancarella, S.; Wang, Y.J.; Gill, D.L. Calcium Signals: STIM Dynamics Mediate Spatially Unique Oscillations. Curr. Biol. 2009, 19, R950–R952. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Soboloff, J.; Rothberg, B.S.; Madesh, M.; Gill, D.L. STIM proteins: Dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012, 13, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Deng, X.X.; Hewavitharana, T.; Soboloff, J.; Gill, D.L. STIM, Orai and TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Stathopulos, P.B.; Zheng, L.; Li, G.Y.; Plevin, M.J.; Ikura, M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 2008, 135, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Luik, R.M.; Wang, B.; Prakriya, M.; Wu, M.M.; Lewis, R.S. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008, 454, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Muik, M.; Fahrner, M.; Schindl, R.; Stathopulos, P.; Frischauf, I.; Derler, I.; Plenk, P.; Lackner, B.; Groschner, K.; Ikura, M.; et al. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. Embo J. 2011, 30, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jennette, M.R.; Ma, G.; Kazzaz, S.A.; Baraniak, J.H.; Nwokonko, R.M.; Groff, M.L.; Velasquez-Reynel, M.; Huang, Y.; Wang, Y.; et al. An apical Phe-His pair defines the Orai1-coupling site and its occlusion within STIM1. Nat. Commun. 2023, 14, 6921. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, C.; Grabmayr, H.; Maltan, L.; Horvath, F.; Krobath, H.; Muik, M.; Tiffner, A.; Renger, T.; Romanin, C.; Fahrner, M.; et al. Defects in the STIM1 SOARα2 domain affect multiple steps in the CRAC channel activation cascade. Cell. Mol. Life Sci. 2021, 78, 6645–6667. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Weidinger, C.; Zee, I.; Feske, S. Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels 2013, 7, 379–391. [Google Scholar] [CrossRef]
- Trebak, M. STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol. 2012, 590, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, A.; Trebak, M.; Motiani, R.K. STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol.-Cell Physiol. 2015, 309, C457–C469. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, L.S.; Graham, S.J.L.; Dziadek, M.A. STIM proteins: Integrators of signalling pathways in development, differentiation and disease. J. Cell. Mol. Med. 2010, 14, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, P.; Tauseef, M.; Kruse, K.; Amin, M.R.; Sheikh, R.; Feske, S.; Komarova, Y.; Mehta, D. STIM1 Phosphorylation at Y361 Recruits Orai1 to STIM1 Puncta and Induces Ca2+ Entry. Sci. Rep. 2017, 7, 42758. [Google Scholar] [CrossRef] [PubMed]
- Kodakandla, G.; West, S.J.; Wang, Q.; Tewari, R.; Zhu, M.X.; Akimzhanov, A.M.; Boehning, D. Dynamic S-acylation of the ER-resident protein stromal interaction molecule 1 (STIM1) is required for store-operated Ca(2+) entry. J. Biol. Chem. 2022, 298, 102303. [Google Scholar] [CrossRef] [PubMed]
- West, S.J.; Kodakandla, G.; Wang, Q.; Tewari, R.; Zhu, M.X.; Boehning, D.; Akimzhanov, A.M. S-acylation of Orai1 regulates store-operated Ca2+ entry. J. Cell Sci. 2022, 135, jcs258579. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.T.; Senior, P.V.; Van Stekelenburg, L.; Layton, J.E.; Smith, P.J.; Dziadek, M.A. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Et Biophys. Acta 2002, 1596, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Zhao, Y.; Bhattacharya, M.; Stathopulos, P.B. Structural perturbations induced by Asn131 and Asn171 glycosylation converge within the EFSAM core and enhance stromal interaction molecule-1 mediated store operated calcium entry. Biochimica Et Biophys. Acta-Mol. Cell Res. 2017, 1864, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Blackman, R.; Gross, S.; Soboloff, J. Control of STIM and Orai function by post-translational modifications. Cell Calcium 2022, 103, 102544. [Google Scholar] [CrossRef]
- Niemeyer, B.A. The STIM-Orai Pathway: Regulation of STIM and Orai by Thiol Modifications. Adv. Exp. Med. Biol. 2017, 993, 99–116. [Google Scholar] [CrossRef]
- Du, Y.C. Identification of Glucosidase II that regulates STIM1 activation dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Prins, D.; Groenendyk, J.; Touret, N.; Michalak, M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011, 12, 1182–1188. [Google Scholar] [CrossRef]
- Hirve, N.; Rajanikanth, V.; Hogan, P.G.; Gudlur, A. Coiled-Coil Formation Conveys a STIM1 Signal from ER Lumen to Cytoplasm. Cell Rep. 2018, 22, 72–83. [Google Scholar] [CrossRef]
- Gui, L.; Zhu, J.H.; Lu, X.R.; Sims, S.M.; Lu, W.Y.; Stathopulos, P.B.; Feng, Q.P. S-Nitrosylation of STIM1 by Neuronal Nitric Oxide Synthase Inhibits Store-Operated Ca2+ Entry. J. Mol. Biol. 2018, 430, 1773–1785. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Irrinki, K.M.; Mallilankaraman, K.; Lien, Y.C.; Wang, Y.J.; Bhanumathy, C.D.; Subbiah, R.; Ritchie, M.F.; Soboloff, J.; Baba, Y.; et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010, 190, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Gibhardt, C.S.; Cappello, S.; Bhardwaj, R.; Schober, R.; Kirsch, S.A.; Bonilla Del Rio, Z.; Gahbauer, S.; Bochicchio, A.; Sumanska, M.; Ickes, C.; et al. Oxidative Stress-Induced STIM2 Cysteine Modifications Suppress Store-Operated Calcium Entry. Cell Rep. 2020, 33, 108292. [Google Scholar] [CrossRef]
- Zhu, J.H.; Lu, X.R.; Feng, Q.P.; Stathopulos, P.B. A charge-sensing region in the stromal interaction molecule 1 luminal domain confers stabilization-mediated inhibition of SOCE in response to S-nitrosylation. J. Biol. Chem. 2018, 293, 8900–8911. [Google Scholar] [CrossRef]
- Sirko, C.; Novello, M.J.; Stathopulos, P.B. An S-glutathiomimetic Provides Structural Insights into Stromal Interaction Molecule-1 Regulation. J. Mol. Biol. 2022, 434, 167874. [Google Scholar] [CrossRef]
- Matsusaki, M.; Kanemura, S.; Kinoshita, M.; Lee, Y.H.; Inaba, K.; Okumura, M. The Protein Disulfide Isomerase Family: From proteostasis to pathogenesis. Biochim. Et Biophys. Acta. Gen. Subj. 2020, 1864, 129338. [Google Scholar] [CrossRef]
- Hettinghouse, A.; Liu, R.; Liu, C.J. Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacol. Ther. 2018, 181, 34–48. [Google Scholar] [CrossRef]
- Salgania, H.K.; Metz, J.; Jeske, M. ReLo is a simple and rapid colocalization assay to identify and characterize direct protein-protein interactions. Nat. Commun. 2024, 15, 2875. [Google Scholar] [CrossRef]
- Mancarella, S.; Wang, Y.J.; Deng, X.X.; Landesberg, G.; Scalia, R.; Panettieri, R.A.; Mallilankaraman, K.; Tang, X.D.; Madesh, M.; Gill, D.L. Hypoxia-induced Acidosis Uncouples the STIM-Orai Calcium Signaling Complex. J. Biol. Chem. 2011, 286, 44788–44798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Ma, G.L.; He, L.; Zhang, T.; Li, J.; Yuan, X.M.; Nguyen, N.T.; Huang, Y.; Zhang, X.Y.; Gao, P.; et al. Identification of molecular determinants that govern distinct STIM2 activation dynamics. PLoS Biol. 2018, 16, e2006898. [Google Scholar] [CrossRef]
- Suzuki, J.; Kanemaru, K.; Ishii, K.; Ohkura, M.; Okubo, Y.; Iino, M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014, 5, 4153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Stathopulos, P.B.; Li, G.Y.; Ikura, M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 2008, 369, 240–246. [Google Scholar] [CrossRef]
- Brandman, O.; Liou, J.; Park, W.S.; Meyer, T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007, 131, 1327–1339. [Google Scholar] [CrossRef]
- Gu, W.J. highly Dmamic and Sensitive NEMOer Calcium Indicators suitable for lmaging ofEndoplasmic Reticulum Calcium Signals in Excitable Cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An expanded palette of genetically encoded Ca2+ indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676. [Google Scholar] [CrossRef]
- Shaner, N.C.; Lambert, G.G.; Chammas, A.; Ni, Y.; Cranfill, P.J.; Baird, M.A.; Sell, B.R.; Allen, J.R.; Day, R.N.; Israelsson, M.; et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 2013, 10, 407–409. [Google Scholar] [CrossRef]
- Bindels, D.S.; Haarbosch, L.; van Weeren, L.; Postma, M.; Wiese, K.E.; Mastop, M.; Aumonier, S.; Gotthard, G.; Royant, A.; Hink, M.A.; et al. mScarlet: A bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 2017, 14, 53–56. [Google Scholar] [CrossRef]
- Jing, J.; He, L.; Sun, A.M.; Quintana, A.; Ding, Y.H.; Ma, G.L.; Tan, P.; Liang, X.W.; Zheng, X.L.; Chen, L.Y.; et al. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat. Cell Biol. 2015, 17, 1339–1347. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Chen, Y.; Yang, Y.; Liu, J.; Liu, K.; Lee, Y.T.; He, N.; Zhou, Y.; Wang, Y. Visible light excited ratiometric-GECIs for long-term in-cellulo monitoring of calcium signals. Cell Calcium 2020, 87, 102165. [Google Scholar] [CrossRef]
- Yue, L.; Wang, L.; Du, Y.; Zhang, W.; Hamada, K.; Matsumoto, Y.; Jin, X.; Zhou, Y.; Mikoshiba, K.; Gill, D.L.; et al. Type 3 Inositol 1,4,5-Trisphosphate Receptor is a Crucial Regulator of Calcium Dynamics Mediated by Endoplasmic Reticulum in HEK Cells. Cells 2020, 9, 275. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Zhou, Y.; Hendron, E.; Mancarella, S.; Ritchie, M.F.; Tang, X.D.; Baba, Y.; Kurosaki, T.; Mori, Y.; et al. STIM protein coupling in the activation of Orai channels. Proc. Natl. Acad. Sci. USA 2009, 106, 7391–7396. [Google Scholar] [CrossRef]
- Zal, T.; Gascoigne, N.R. Photobleaching-corrected FRET efficiency imaging of live cells. Biophys. J. 2004, 86, 3923–3939. [Google Scholar] [CrossRef]
- Navarro-Borelly, L.; Somasundaram, A.; Yamashita, M.; Ren, D.; Miller, R.J.; Prakriya, M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 2008, 586, 5383–5401. [Google Scholar] [CrossRef]
- Wei, M.; Zhou, Y.; Sun, A.; Ma, G.; He, L.; Zhou, L.; Zhang, S.; Liu, J.; Zhang, S.L.; Gill, D.L.; et al. Molecular mechanisms underlying inhibition of STIM1-Orai1-mediated Ca(2+) entry induced by 2-aminoethoxydiphenyl borate. Pflug. Arch. Eur. J. Physiol. 2016, 468, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
| Target | Primers (5′→3′) | Sequences |
|---|---|---|
| PDIA3 | Forward | CAAGCAGCGGGTTAGTGGT |
| Reverse | TCTCGAAGTTGTCGTCCGTG | |
| P4HB | Forward | TGCCAAGCAGTTTTTGCAGG |
| Reverse | AATCTTCGGGGCTGTCTGCT | |
| TXNDC5 | Forward | GACATGTTCACGCACGGGAT |
| Reverse | GGCTTGAAAAGCTTTAAGGTGGG | |
| GAPDH | Forward | AACTGCTTAGCACCCCTGGC |
| Reverse | ATGACCTTGCCCACAGCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Wang, F.; Liu, P.; Zheng, S.; Li, J.; Huang, R.; Li, W.; Zhang, X.; Wang, Y. Redox Enzymes P4HB and PDIA3 Interact with STIM1 to Fine-Tune Its Calcium Sensitivity and Activation. Int. J. Mol. Sci. 2024, 25, 7578. https://doi.org/10.3390/ijms25147578
Du Y, Wang F, Liu P, Zheng S, Li J, Huang R, Li W, Zhang X, Wang Y. Redox Enzymes P4HB and PDIA3 Interact with STIM1 to Fine-Tune Its Calcium Sensitivity and Activation. International Journal of Molecular Sciences. 2024; 25(14):7578. https://doi.org/10.3390/ijms25147578
Chicago/Turabian StyleDu, Yangchun, Feifan Wang, Panpan Liu, Sisi Zheng, Jia Li, Rui Huang, Wanjie Li, Xiaoyan Zhang, and Youjun Wang. 2024. "Redox Enzymes P4HB and PDIA3 Interact with STIM1 to Fine-Tune Its Calcium Sensitivity and Activation" International Journal of Molecular Sciences 25, no. 14: 7578. https://doi.org/10.3390/ijms25147578
APA StyleDu, Y., Wang, F., Liu, P., Zheng, S., Li, J., Huang, R., Li, W., Zhang, X., & Wang, Y. (2024). Redox Enzymes P4HB and PDIA3 Interact with STIM1 to Fine-Tune Its Calcium Sensitivity and Activation. International Journal of Molecular Sciences, 25(14), 7578. https://doi.org/10.3390/ijms25147578







