Influence of BMI, Cigarette Smoking and Cryopreservation on Tyrosine Phosphorylation during Sperm Capacitation
Abstract
1. Introduction
2. Results
2.1. Influence of BMI on TP
2.2. Influence of Cigarette Smoking Habit on TP
2.3. Influence of BMI and Cigarette Smoking Habit on TP
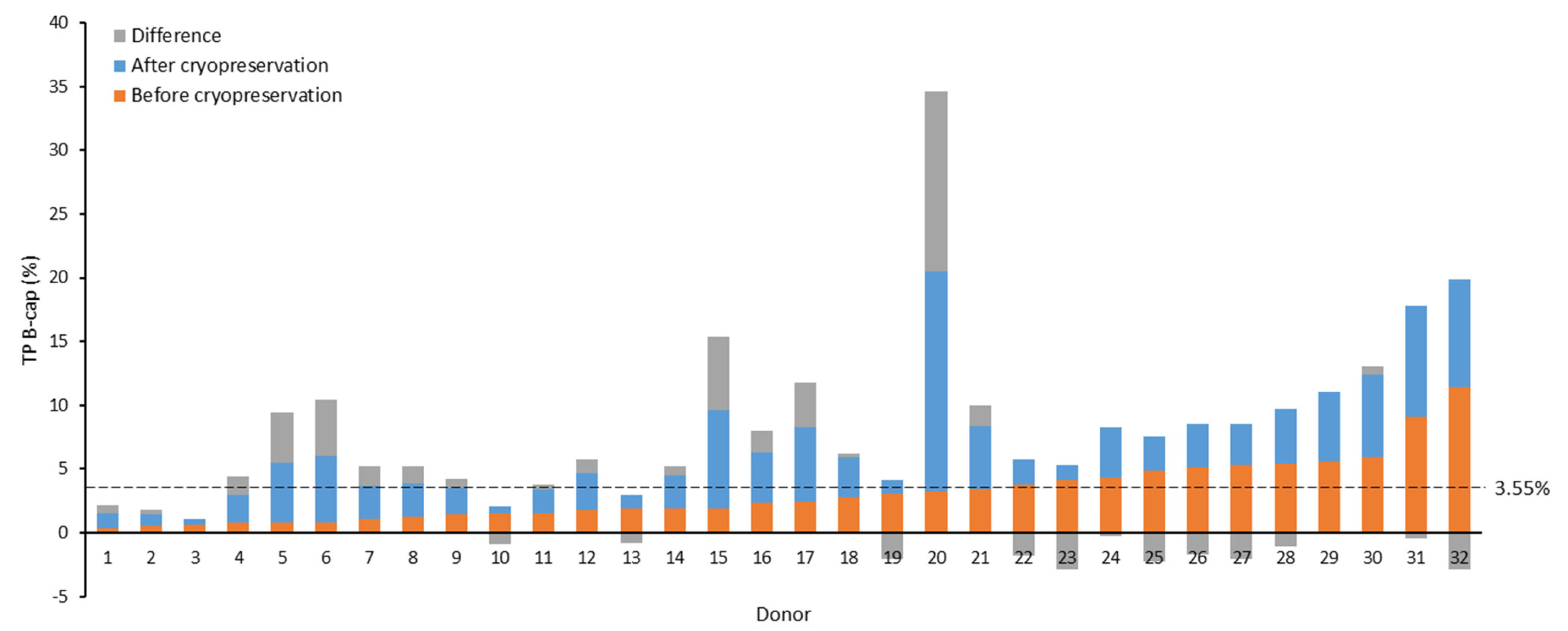
2.4. Influence of Cryopreservation on TP
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Semen Analysis and Sperm Capacitation
4.3. Sperm Cryopreservation and Intrauterine Insemination
4.4. Tyrosine Phosphorylation (TP) Assessment
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin, C.R. The ‘Capacitation’ of the Mammalian Sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the Penetration of the Sperm into the Mammalian Egg. Aust. J. Biol. Sci. 1951, 4, 581. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Development of Fertilizing Capacity of Rabbit Spermatozoa in the Uterus. Nature 1955, 175, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa. 1. Correlation between the Capacitation State and Protein Tyrosine Phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Westbrook, V.A.; Chertihin, O.; Demarco, I.; Sleight, S.; Diekman, A.B. Novel Signaling Pathways Involved in Sperm Acquisition of Fertilizing Capacity. J. Reprod. Immunol. 2002, 53, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Kopf, G.S. Regulation of Protein Phosphorylation during Sperm Capacitation1. Biol. Reprod. 1998, 59, 1–6. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789241547789. [Google Scholar]
- Boomsma, C.M.; Cohlen, B.J.; Farquhar, C. Semen Preparation Techniques for Intrauterine Insemination. Cochrane Database Syst. Rev. 2019, 2019, CD004507. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R. The “Switching on” of Mammalian Spermatozoa: Molecular Events Involved in Promotion and Regulation of Capacitation. Mol. Reprod. Dev. 2010, 77, 197–208. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s Meaning of Capacitation: A Molecular Perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm Capacitation: A Distant Landscape Glimpsed but Unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Yang, W.X. Factors and Pathways Involved in Capacitation: How Are They Regulated? Oncotarget 2017, 8, 3600–3627. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; De Lamirande, E.; Gagnon, C. Cyclic Adenosine 3’,5’monophosphate-Dependent Regulation of Protein Tyrosine Phosphorylation in Relation to Human Sperm Capacitation and Motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Galantino-Homer, H.L.; Visconti, P.E.; Kopf, G.S. Regulation of Protein Tyrosine Phosphorylation during Bovine Sperm Capacitation by a Cyclic Adenosine 3’,5’-Monophosphate-Dependent Pathway. Biol. Reprod. 1997, 56, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Pommer, A.C.; Rutllant, J.; Meyers, S.A. Phosphorylation of Protein Tyrosine Residues in Fresh and Cryopreserved Stallion Spermatozoa under Capacitating Conditions. Biol. Reprod. 2003, 68, 1208–1214. [Google Scholar] [CrossRef]
- Naz, R.K.; Rajesh, P.B. Role of Tyrosine Phosphorylation in Sperm Capacitation/Acrosome Reaction. Reprod. Biol. Endocrinol. 2004, 2, 1–12. [Google Scholar] [CrossRef][Green Version]
- Visconti, P.E.; Galantino-Homer, H.; Moore, G.D.; Bailey, J.L.; Ning, X.; Fornes, M.; Kopf, G.S. The Molecular Basis of Sperm Capacitation. J. Androl. 1998, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male Infertility. Nat. Rev. Dis. Prim. 2023, 9, 49. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male Infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Balawender, K.; Orkisz, S. The Impact of Selected Modifiable Lifestyle Factors on Male Fertility in the Modern World. Cent. Eur. J. Urol. 2020, 73, 563–568. [Google Scholar] [CrossRef]
- Antonouli, S.; Di Nisio, V.; Messini, C.; Daponte, A.; Rajender, S.; Anifandis, G. A Comprehensive Review and Update on Human Fertility Cryopreservation Methods and Tools. Front. Vet. Sci. 2023, 10, 1151254. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal Obesity Negatively Affects Male Fertility and Assisted Reproduction Outcomes: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2015, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Shukla, K.K.; Chambial, S.; Dwivedi, S.; Misra, S.; Sharma, P. Recent Scenario of Obesity and Male Fertility. Andrology 2014, 2, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z. Obesity, a Serious Etiologic Factor for Male Subfertility in Modern Society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e136171. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of Obesity on Male Fertility, Sperm Function and Molecular Composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.E.; Brannigan, R.E. Obesity and Male Infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Ameratunga, D.; Gebeh, A.; Amoako, A. Obesity and Male Infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102393. [Google Scholar] [CrossRef] [PubMed]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and Male Infertility: An Evidence-Based Review. World J. Mens. Health 2015, 33, 143. [Google Scholar] [CrossRef]
- Mostafa, T. Cigarette Smoking and Male Infertility. J. Adv. Res. 2010, 1, 179–186. [Google Scholar] [CrossRef]
- Alahmar, A. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm Cryopreservation: A Review on Current Molecular Cryobiology and Advanced Approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef]
- Naresh, S.; Atreja, S.K. The Protein Tyrosine Phosphorylation during in Vitro Capacitation and Cryopreservation of Mammalian Spermatozoa. Cryobiology 2015, 70, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xu, W.; Zhao, X.; Zhang, M.; Zhang, D.; Nie, D.; Bao, M.; Wang, Z.; Wang, L.; Qiao, Z. Protein Profile Screening: Reduced Expression of Sord in the Mouse Epididymis Induced by Nicotine Inhibits Tyrosine Phosphorylation Level in Capacitated Spermatozoa. Reproduction 2016, 151, 227–237. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Q.; Xu, B.; Jiang, X.; Dai, Y.; Zhang, C.Y.; Zen, K. Sustained High Protein-Tyrosine Phosphatase 1B Activity in the Sperm of Obese Males Impairs the Sperm Acrosome Reaction. J. Biol. Chem. 2014, 289, 8432–8441. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Xu, Y.; Tang, M.; Fang, J.; Sun, H.; Sun, Y.; Gu, M.; Liu, Z.; Zhang, Z.; et al. Proteomic Characteristics of Human Sperm Cryopreservation. Proteomics 2014, 14, 298–310. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Pang, M.G. Diagnosis and Prognosis of Male Infertility in Mammal: The Focusing of Tyrosine Phosphorylation and Phosphotyrosine Proteins. J. Proteome Res. 2014, 13, 4505–4517. [Google Scholar] [CrossRef]
- Forman, H.M.; Fissore, R.A. Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction; Plant, T.M., Zeleznik, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 149–196. ISBN 9780123971753. [Google Scholar]
- Bernecic, N.C.; Gadella, B.M.; Leahy, T.; de Graaf, S.P. Novel Methods to Detect Capacitation-Related Changes in Spermatozoa. Theriogenology 2019, 137, 56–66. [Google Scholar] [CrossRef]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen Cryopreservation in Domestic Animals: A Damaging and Capacitating Phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [CrossRef]
- Huang, C.; Lei, L.; Wu, H.L.; Gan, R.X.; Yuan, X.B.; Fan, L.Q.; Zhu, W.B. Long-Term Cryostorage of Semen in a Human Sperm Bank Does Not Affect Clinical Outcomes. Fertil. Steril. 2019, 112, 663–669.e1. [Google Scholar] [CrossRef]
- Ortega-Ferrusola, C.; Gil, M.C.; Rodríguez-Martínez, H.; Anel, L.; Peña, F.J.; Martín-Muñoz, P. Flow Cytometry in Spermatology: A Bright Future Ahead. Reprod. Domest. Anim. 2017, 52, 921–931. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Cinque, B.; Antonangelo, C.; Sciarretta, F.; Santucci, R.; D’Angeli, A.; Francavilla, S.; Francavilla, F. Dynamics of the Global Tyrosine Phosphorylation during Capacitation and Acquisition of the Ability to Fuse with Oocytes in Human Spermatozoa. Biol. Reprod. 2008, 79, 649–656. [Google Scholar] [CrossRef][Green Version]
- Sidhu, K.S.; Mate, K.E.; Gunasekera, T.; Veal, D.; Hetherington, L.; Baker, M.A.; Aitken, R.J.; Rodger, J.C. A Flow Cytometric Assay for Global Estimation of Tyrosine Phosphorylation Associated with Capacitation of Spermatozoa from Two Marsupial Species, the Tammar Wallaby (Macropus Eugenii) and the Brushtail Possum (Trichosurus Vulpecula). Reproduction 2004, 127, 95–103. [Google Scholar] [CrossRef][Green Version]
- Roldan, E.R.S. Assessments of Sperm Quality Integrating Morphology, Swimming Patterns, Bioenergetics and Cell Signalling. Theriogenology 2020, 150, 388–395. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Moreno-Irusta, A.; Torres-Rodriguez, P.; Giojalas, L.; Gervasi, M.G.; Visconti, P.E.; Treviño, C.L. Semi-Automatized Segmentation Method Using Image-Based Flow Cytometry to Study Sperm Physiology: The Case of Capacitation-Induced Tyrosine Phosphorylation. Mol. Hum. Reprod. 2018, 24, 64–73. [Google Scholar] [CrossRef]
- World Health Organization WHO. Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- González-Ravina, C.; Santamaría-López, E.; Pacheco, A.; Ramos, J.; Carranza, F.; Murria, L.; Ortiz-Vallecillo, A.; Fernández-Sánchez, M. Effect of Sperm Selection by Magnetic-Activated Cell Sorting in D-IUI: A Randomized Control Trial. Cells 2022, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; De La Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion Channels, Phosphorylation and Mammalian Sperm Capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Candenas, L.; Pinto, F.M.; Cejudo-Román, A.; González-Ravina, C.; Fernández-Sánchez, M.; Pérez-Hernández, N.; Irazusta, J.; Subirán, N. Veratridine-Sensitive Na+ Channels Regulate Human Sperm Fertilization Capacity. Life Sci. 2018, 196, 48–55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000; pp. i–xii, 1–253. [Google Scholar]

| Classification According to BMI (n = 40) | Classification According to Cigarette Smoking Habit (n = 40) | Overall (n = 40) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Normal Weight (n = 26) | Overweight (n = 14) | p-Value | Non-Smokers (n = 28) | Smokers (n = 12) | p-Value | |
| Age | 24.8 (22.7–26.8) | 26.1 (21.3–30.9) | 0.539 | 24.4 (22.2–26.6) | 27.2 (22.4–31.9) | 0.220 | 25.3 (23.4–27.3) |
| BMI | 21.8 (21.2–22.5) | 27.0 (26.1–27.9) | <0.001* | 23.7 (22.5–24.8) | 23.7 (21.6–25.7) | 1.000 | 23.7 (22.7–24.6) |
| Cigarettes smoked daily (cig/day) | 1.5 (0.3–2.7) | 2.1 (−0.3–4.6) | 0.580 | 0.0 (0.0–0.0) | 5.7 (3.2–8.4) | <0.001 * | 1.73 (0.6–2.8) |
| Fresh seminal sample | |||||||
| Sexual abstinence (day) | 3.5 (3.3–3.6) | 3.4 (3.2–3.7) | 0.886 | 3.4 (3.3–3.6) | 3.5 (3.2–3.7) | 0.885 | 3.4 (3.3–3.6) |
| Volume (mL) | 3.0 (2.6–3.5) | 3.0 (2.5–3.6) | 0.968 | 3.2 (2.8–3.6) | 2.7 (2.0–3.4) | 0.192 | 3.0 (2.7–3.4) |
| Progressive motility (%) | 66.0 (63.3–68.6) | 66.4 (62.7–70.1) | 0.850 | 66.2 (63.4–69.0) | 66.0 (63.4–68.7) | 0.944 | 66.1 (64.1–68.2) |
| Non-progressive motility (%) | 7.0 (6.2–7.7) | 6.1 (5.0–7.1) | 0.147 | 6.6 (5.9–7.3) | 6.7 (5.5–8.0) | 0.867 | 6.6 (6.0–7.2) |
| Immotile (%) | 27.1 (24.6–29.5) | 27.6 (24.0–31.1) | 0.807 | 27.2 (24.7–29.7) | 27.3 (24.1–30.5) | 0.982 | 27.2 (25.3–29.2) |
| Vitality (%) | 78.4 (75.9–81.0) | 76.6 (72.6–80.5) | 0.389 | 77.7 (75.1–80.3) | 78.1 (74.1–82.1) | 0.857 | 77.8 (75.7–79.9) |
| Normal forms (%) | 9.8 (9.0–10.5) | 9.9 (9.1–10.8) | 0.812 | 10.0 (9.3–10.7) | 9.4 (8.4–10.3) | 0.259 | 9.8 (9.3–10.4) |
| TSC (mill) | 219.5 (193.9–245.1) | 201.1 (164.4–237.8) | 0.388 | 222.2 (199.1–245.3) | 191.6 (147.8–235.5) | 0.164 | 213.0 (192.8–233.3) |
| TPMSC (mill) | 146.6 (127.7–165.5) | 135.5 (109.0–162.0) | 0.477 | 148.5 (131.7–265.3) | 129.3 (96.4–162.2) | 0.233 | 142.7 (127.9–157.5) |
| Capacitated seminal sample | |||||||
| Progressive motility (%) | 90.0 (88.6–91.4) | 90.6 (88.5–92.6) | 0.617 | 90.8 (89.5–92.2) | 88.7 (86.7–90.6) | 0.071 | 90.2 (89.1–91.3) |
| Non-progressive motility (%) | 3.0 (2.4–3.7) | 2.6 (2.1–3.1) | 0.293 | 2.8 (2.4–3.3) | 3.0 (1.8–4.1) | 0.795 | 2.9 (2.4–3.3) |
| Immotile (%) | 7.0 (5.8–8.2) | 6.1 (4.7–7.5) | 0.368 | 6.0 (4.9–7.0) | 8.4 (6.8–10.0) | 0.013 * | 6.7 (5.8–7.6) |
| Normal forms (%) | 11.5 (10.6–12.3) | 11.3 (10.6–11.9) | 0.778 | 11.8 (11.0–12.4) | 10.6 (9.6–11.6) | 0.064 | 11.4 (10.8–12.0) |
| TSC (mill) | 124.5 (103.6–145.3) | 96.7 (64.9–128.4) | 0.12 | 119.1 (97.1–141.2) | 104.5 (74.9–134.1) | 0.438 | 114.7 (97.5–131.9) |
| TPMSC (mill) | 113.2 (93.4–133.1) | 88.6 (59.1–118.2) | 0.145 | 109.3 (88.5–130.2) | 93.6 (66.8–120.5) | 0.375 | 104.6 (88.4–120.8) |
| Outcome Variable | Estimated Coefficient | Standard Error | p-Value |
|---|---|---|---|
| TP (%) | |||
| B-cap | 0.68 | 0.35 | 0.27 |
| A-cap | 0.49 | 1.34 | 0.72 |
| 1 h | −0.32 | 2.14 | 0.88 |
| 3 h | −1.26 | 3.85 | 0.74 |
| Absolute changes in TP (%) | |||
| B-cap to A-cap | −0.18 | 0.99 | 0.85 |
| A-cap to 1 h | −0.81 | 1.12 | 0.47 |
| A-cap to 3 h | −1.75 | 3.14 | 0.58 |
| 1 h to 3 h | −0.94 | 2.21 | 0.67 |
| Fold change in TP | |||
| B-cap to A-cap | −0.47 | 0.57 | 0.41 |
| A-cap to 1 h | −1.33 | 0.87 | 0.13 |
| A-cap to 3 h | −1.21 | 1.08 | 0.27 |
| 1 h to 3 h | −0.18 | 0.24 | 0.47 |
| Outcome Variable | Estimated Coefficient | Standard Error | p-Value |
|---|---|---|---|
| TP (%) | |||
| B-cap | −0.07 | 0.63 | 0.92 |
| A-cap | −0.4 | 1.4 | 0.78 |
| 1 h | −2.47 | 2.19 | 0.27 |
| 3 h | −5.67 | 3.9 | 0.15 |
| Absolute changes in TP (%) | |||
| B-cap to A-cap | −0.33 | 1.02 | 0.75 |
| A-cap to 1 h | −2.07 | 1.13 | 0.07 |
| A-cap to 3 h | −5.27 | 3.17 | 0.10 |
| 1 h to 3 h | −3.20 | 2.25 | 0.16 |
| Fold change in TP | |||
| B-cap to A-cap | 0.37 | 0.59 | 0.54 |
| A-cap to 1 h | 0.37 | 0.93 | 0.70 |
| A-cap to 3 h | 0.45 | 1.12 | 0.69 |
| 1 h to 3 h | −0.02 | 0.25 | 0.92 |
| Overweight | Cigarette Smoker | |||||
|---|---|---|---|---|---|---|
| Outcome Variable | Estimated Coefficient | Standard Error | p-Value | Estimated Coefficient | Standard Error | p-Value |
| TP (%) | ||||||
| B-cap | 0.11 | 0.10 | 0.27 | −0.07 | 0.63 | 0.92 |
| A-cap | 0.02 | 0.22 | 0.94 | −0.4 | 1.42 | 0.78 |
| 1 h | −0.04 | 0.35 | 0.92 | −2.47 | 2.22 | 0.27 |
| 3 h | −0.18 | 0.62 | 0.77 | −5.67 | 3.95 | 0.16 |
| Absolute changes in TP (%) | ||||||
| B-cap to A-cap | −0.09 | 0.16 | 0.57 | −0.33 | 1.03 | 0.75 |
| A-cap to 1 h | −0.05 | 0.18 | 0.77 | −2.07 | 1.14 | 0.08 |
| A-cap to 3 h | −0.2 | 0.51 | 0.69 | −5.27 | 3.21 | 0.11 |
| 1 h to 3 h | −0.15 | 0.36 | 0.68 | −3.2 | 2.27 | 0.17 |
| Fold change in TP | ||||||
| B-cap to A-cap | −0.12 | 0.09 | 0.19 | 0.37 | 0.59 | 0.54 |
| A-cap to 1 h | −0.15 | 0.15 | 0.32 | 0.36 | 0.93 | 0.70 |
| A-cap to 3 h | 0.01 | 0.18 | 0.95 | 0.45 | 1.13 | 0.69 |
| 1 h to 3 h | −0.01 | 0.04 | 0.85 | −0.02 | 0.25 | 0.92 |
| Non-Cryopreserved Sample (n = 32) | Cryopreserved Sample (n = 32) | p-Value | |
|---|---|---|---|
| Non-capacitated sample | |||
| Progressive motility (%) | 68.6 (65.7–71.5) | 36.0 (32.2–39.8) | <0.001 * |
| Non-progressive motility (%) | 6.2 (4.8–7.6) | 6.6 (4.6–8.5) | 0.758 |
| Immotile (%) | 25.2 (22.7–27.8) | 57.3 (52.4–62.1) | <0.001 * |
| Normal forms (%) | 10.6 (9.4–11.8) | 9.7 (8.8–10.7) | 0.274 |
| Capacitated sample | |||
| Progressive motility (%) | 91.1 (89.3–92.9) | 81.7 (78.7–84.7) | <0.001 * |
| Non-progressive motility (%) | 3.3 (2.2–4.4) | 4.4 (2.9–5.9) | 0.284 |
| Immotile (%) | 5.7 (4.4–6.9) | 13.8 (11.1–16.5) | <0.001 * |
| Normal forms (%) | 11.8 (10.7–12.9) | 11.6 (10.6–12.5) | 0.731 |
| TP (%) | |||
| B-cap | 3.1 (2.2–4.0) | 3.9 (2.7–5.1) | 0.180 |
| A-cap | 6.7 (4.9–8.4) | 8.9 (6.7–11.2) | 0.035 * |
| 1 h | 11.2 (8.5–13.9) | 13.3 (10.4–16.2) | 0.032 * |
| 3 h | 21.1 (16.3–25.9) | 24.8 (19.7–30.0) | 0.026 * |
| Absolute changes in TP (%) | |||
| B-cap to A-cap | 3.5 (2.3–4.7) | 5.0 (3.3–6.8) | 0.090 |
| A-cap to 1 h | 4.5 (2.9–6.2) | 4.4 (2.4–6.4) | 0.820 |
| A-cap to 3 h | 14.5 (10.5–18.4) | 15.9 (11.4–20.4) | 0.339 |
| 1 h to 3 h | 9.9 (7.3–12.6) | 11.5 (8.5–14.5) | 0.194 |
| Fold change in TP | |||
| B-cap to A-cap | 2.6 (2.1–3.2) | 3.1 (2.3–3.9) | 0.280 |
| A-cap to 1 h | 1.9 (1.6–2.2) | 1.7 (1.4–2.1) | 0.409 |
| A-cap to 3 h | 4.2 (3.0–5.4) | 3.7 (2.8–4.7) | 0.474 |
| 1 h to 3 h | 2.0 (1.7–2.3) | 2.3 (1.7–3.0) | 0.340 |
| B-Cap | A-Cap | 1 h | 3 h | Total | |
|---|---|---|---|---|---|
| Increase | 18 (56.3) | 22 (68.8) | 22 (68.8) | 20 (62.5) | 82 (64.1) |
| Decrease | 14 (43.7) | 9 (28.1) | 10 (31.2) | 12 (37.5) | 45 (35.2) |
| No change | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0 (0) | 1 (0.7) |
| Total | 32 (100) | 32 (100) | 32 (100) | 32 (100) | 128 (100) |
| Variable 1 | Variable 2 | Spearman’s Rho | p-Value |
|---|---|---|---|
| Cryostorage time (days) | TP B-cap (%) after F–T | −0.138 | 0.453 |
| TP A-cap (%) after F–T | −0.148 | 0.420 | |
| TP 1 h (%) after F–T | −0.175 | 0.337 | |
| TP 3 h (%) after F–T | 0.030 | 0.869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Vallecillo, A.; Santamaría-López, E.; García-Ruiz, D.; Martín-Lozano, D.; Candenas, L.; Pinto, F.M.; Fernández-Sánchez, M.; González-Ravina, C. Influence of BMI, Cigarette Smoking and Cryopreservation on Tyrosine Phosphorylation during Sperm Capacitation. Int. J. Mol. Sci. 2024, 25, 7582. https://doi.org/10.3390/ijms25147582
Ortiz-Vallecillo A, Santamaría-López E, García-Ruiz D, Martín-Lozano D, Candenas L, Pinto FM, Fernández-Sánchez M, González-Ravina C. Influence of BMI, Cigarette Smoking and Cryopreservation on Tyrosine Phosphorylation during Sperm Capacitation. International Journal of Molecular Sciences. 2024; 25(14):7582. https://doi.org/10.3390/ijms25147582
Chicago/Turabian StyleOrtiz-Vallecillo, Ana, Esther Santamaría-López, Diego García-Ruiz, David Martín-Lozano, Luz Candenas, Francisco M. Pinto, Manuel Fernández-Sánchez, and Cristina González-Ravina. 2024. "Influence of BMI, Cigarette Smoking and Cryopreservation on Tyrosine Phosphorylation during Sperm Capacitation" International Journal of Molecular Sciences 25, no. 14: 7582. https://doi.org/10.3390/ijms25147582
APA StyleOrtiz-Vallecillo, A., Santamaría-López, E., García-Ruiz, D., Martín-Lozano, D., Candenas, L., Pinto, F. M., Fernández-Sánchez, M., & González-Ravina, C. (2024). Influence of BMI, Cigarette Smoking and Cryopreservation on Tyrosine Phosphorylation during Sperm Capacitation. International Journal of Molecular Sciences, 25(14), 7582. https://doi.org/10.3390/ijms25147582







