Nitrogen-Doped Porous Carbons Derived from Peanut Shells as Efficient Electrodes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
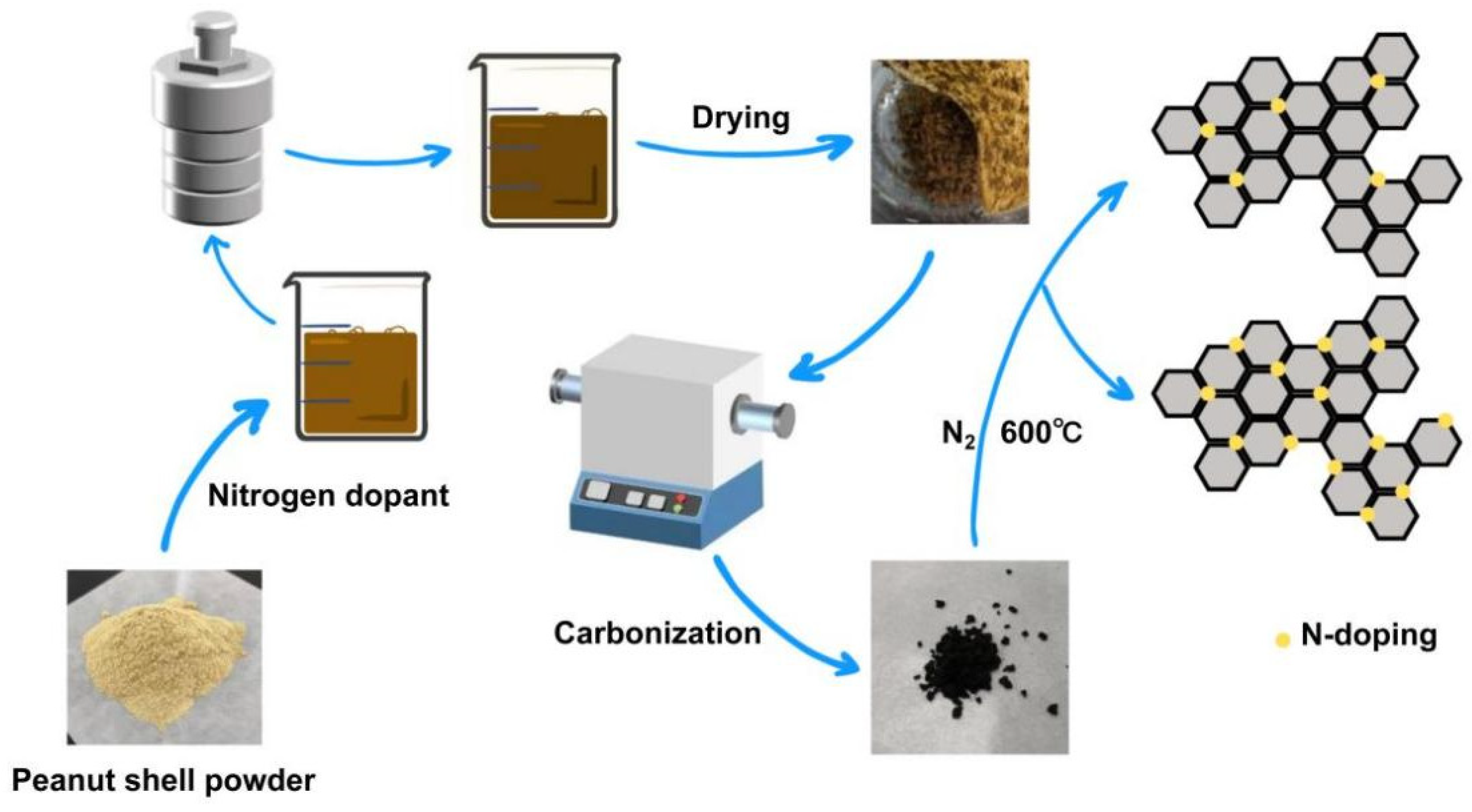
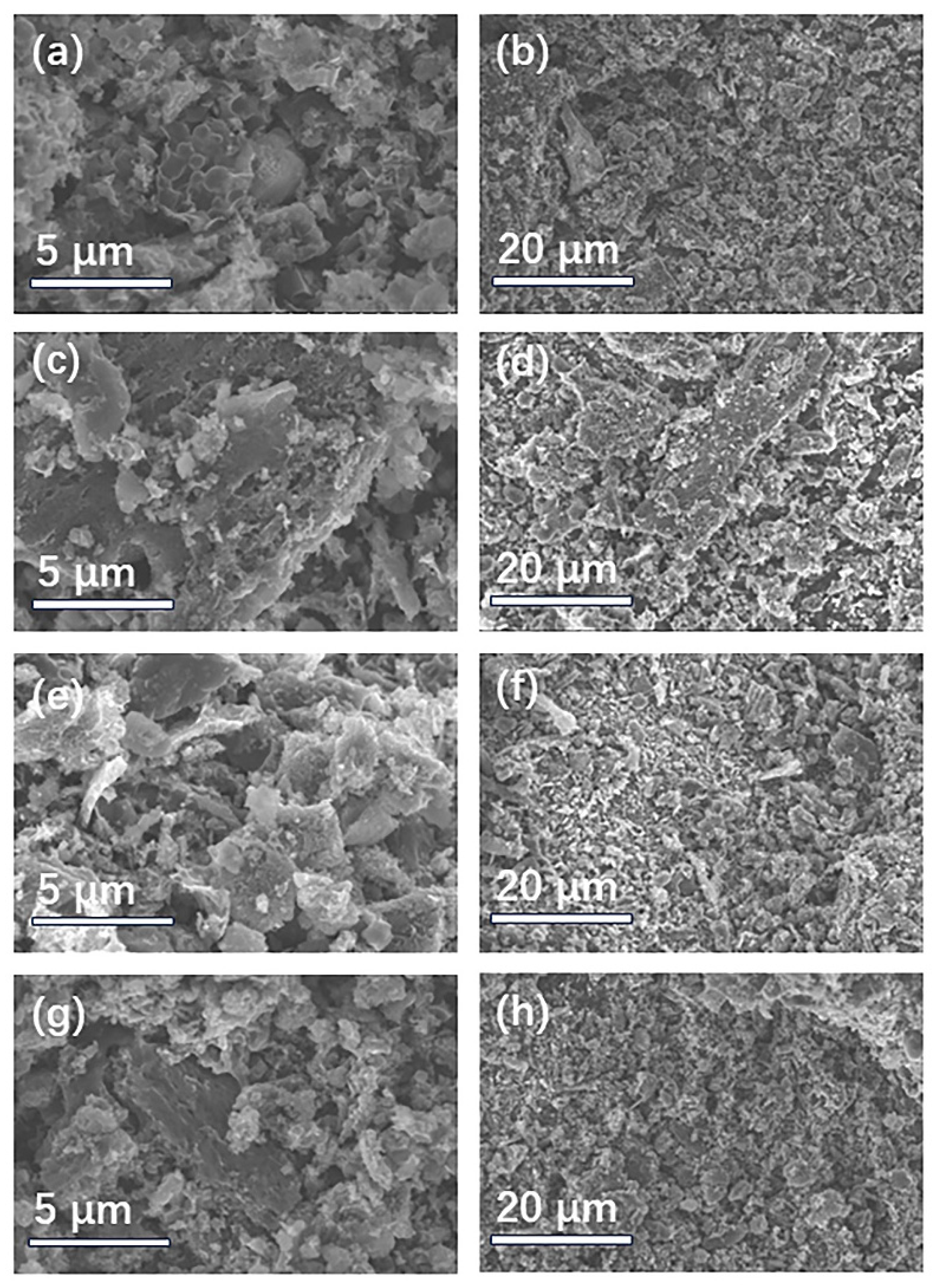
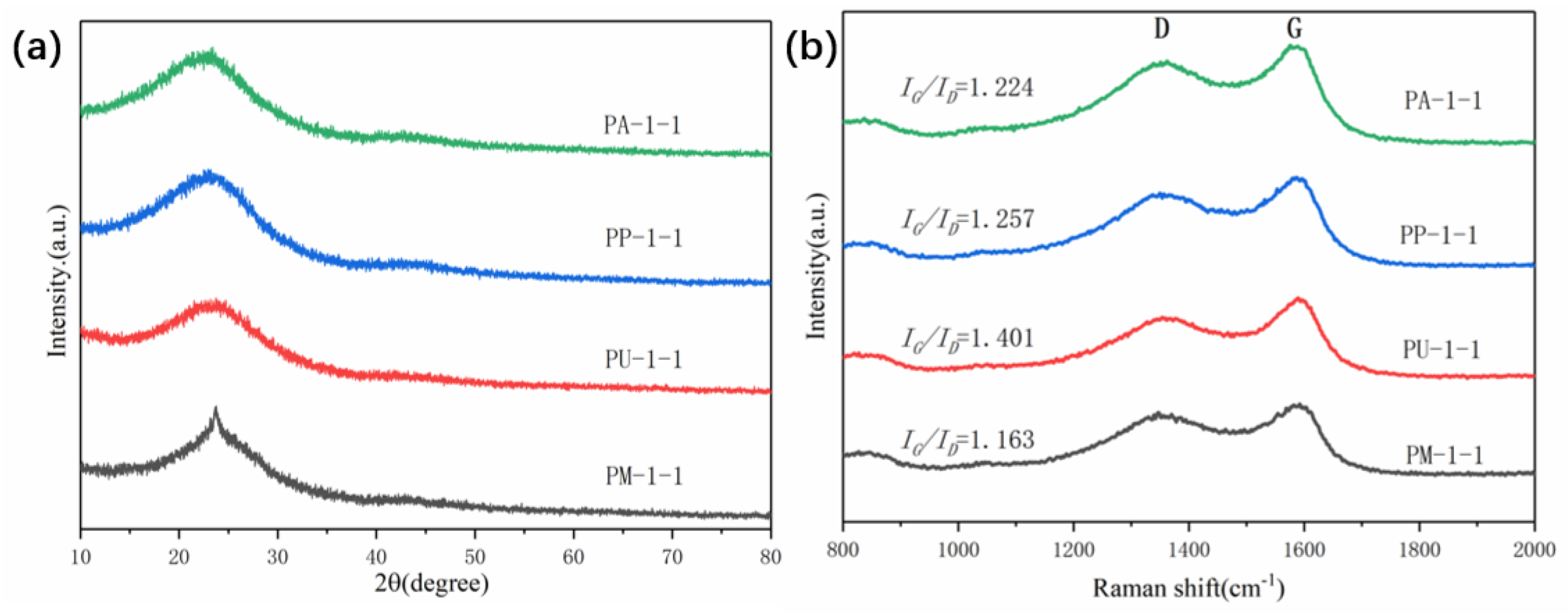
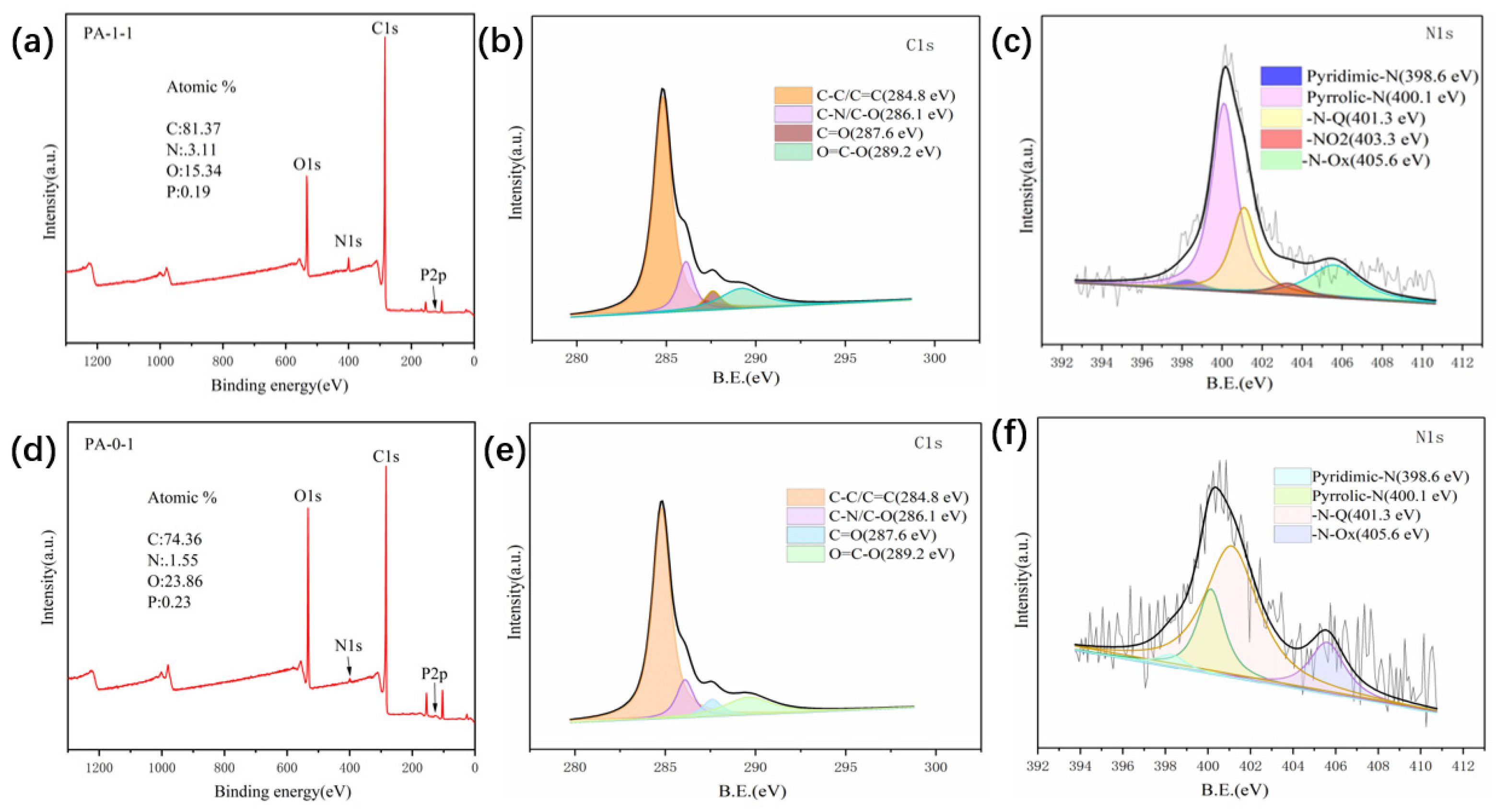
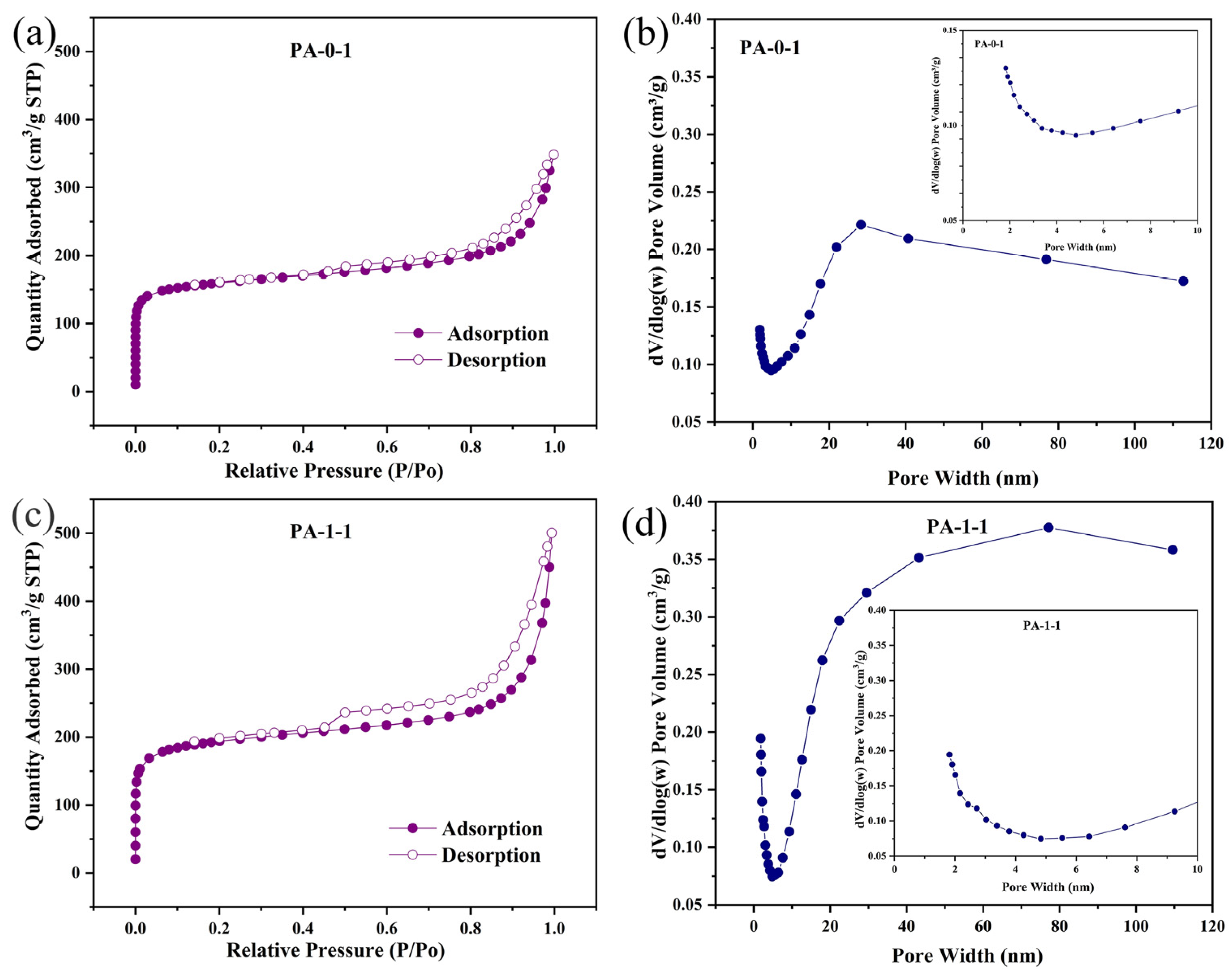
2.1. Characterization of Materials
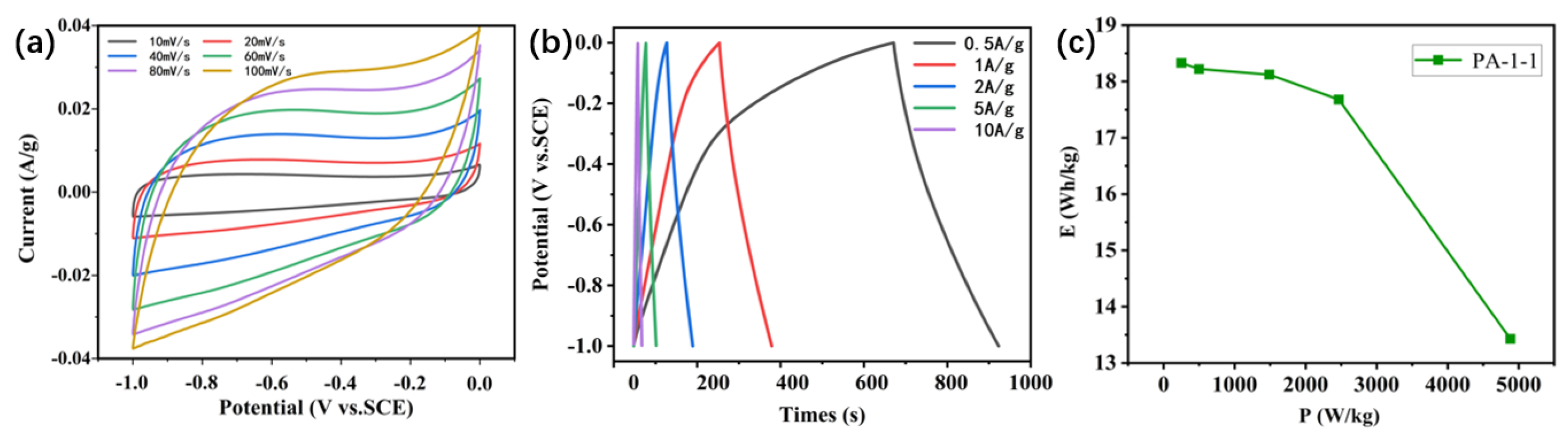
2.2. Electrochemical Performance
3. Experimental Methods
3.1. Materials
3.2. Preparation of Electrode Material
3.2.1. Preparation of Biochar
3.2.2. Activation Process
3.3. Materials Characterizations
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Li, J.; Wu, Q. Nanoporous materials derived from metal-organic framework for supercapacitor application. J. Energy Storage 2020, 30, 101525. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Zheng, X.; Tian, X.; Yuan, S. Enhancing energy storage via confining sulfite anions onto iron oxide/poly(3,4-ethylenedioxythiophene) heterointerface. ACS Appl. Mater. Interfaces 2023, 15, 59413–59421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-step preparation of ultrasmall iron oxide-embedded carbon nanotubes on carbon cloth with excellent superhydrophilicity and enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2021, 13, 45670–45678. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Huang, F.; Zhang, H.; Gong, J.; Miao, C.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J.; et al. High-performance asymmetric supercapacitor assembled with three-dimensional, coadjacent graphene-like carbon nanosheets and its composite. J. Electroanal. Chem. 2018, 823, 474–481. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y.; et al. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-Y.; Zhang, H.; Lin, H.; Guo, Q.; Lau, K.-T.; Jia, B. Specializing liquid electrolytes and carbon-based materials in EDLCs for low-temperature applications. J. Energy Chem. 2022, 68, 580–602. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, H.; Duan, Q.; Liu, Y.; Wang, Y.; Yuan, S. Phosphorus-functionalized low-crystallinity transition-metal oxide nanorod arrays grown on carbon cloth for high-performance asymmetric supercapacitors. Colloids Surf. A 2022, 654, 130189. [Google Scholar] [CrossRef]
- Zheng, L.-H.; Chen, M.-H.; Liang, S.-X.; Lü, Q.-F. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mater. 2021, 113, 108267. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous electrode materials for supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Frackowiak, E.; Jurewicz, K.; Friebe, M.; Parmentier, J.; Béguin, F. Electrochemical energy storage in ordered porous carbon materials. Carbon 2005, 43, 1293–1302. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, T.C.; Ji, X.; Yuan, S. Ti-doped iron phosphide nanoarrays grown on carbon cloth as a self-supported electrode for enhanced electrocatalytic nitrogen reduction. Nanoscale 2023, 15, 16219–16226. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. Biomass derived nitrogen-doped hierarchical porous carbon sheets for supercapacitors with high performance. J. Colloid Interface Sci. 2018, 523, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhou, Y.; Guo, M.; Zheng, Q.; Lin, D. Novel sustainable nitrogen, iodine-dual-doped hierarchical porous activated carbon as a superior host material for high performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2018, 43, 20022–20032. [Google Scholar] [CrossRef]
- Yu, C.; Hou, H.; Liu, X.; Yao, Y.; Liao, Q.; Dai, Z.; Li, D. Old-loofah-derived hard carbon for long cyclicity anode in sodium ion battery. Int. J. Hydrogen Energy 2018, 43, 3253–3260. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, D.; Xiao, J.; Zhang, W.; Xu, Y. Rice husk derived porous carbon decorated with hierarchical molybdenum disulfide microflowers: Synergistic lithium storage performance and lithiation kinetics. Int. J. Hydrogen Energy 2019, 44, 7438–7447. [Google Scholar] [CrossRef]
- Guo, Z.; Han, X.; Zhang, C.; He, S.; Liu, K.; Hu, J.; Yang, W.; Jian, S.; Jiang, S.; Duan, G. Activation of biomass-derived porous carbon for supercapacitors: A review. Chin. Chem. Lett. 2024, 35, 109007. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Liu, K.; Zhang, M.; Cai, X.-M.; Si, C. Biomass-based materials for advanced supercapacitor: Principles, progress, and perspectives. Aggregate 2024, 5, e428. [Google Scholar] [CrossRef]
- Mandala, R.; Hegde, G.; Kodali, D.; Kode, V.R. From Waste to Strength: Unveiling the mechanical properties of peanut-shell-based polymer composites. J. Compos. Sci. 2023, 7, 307. [Google Scholar] [CrossRef]
- Tang, X.; Liu, D.; Wang, Y.-J.; Cui, L.; Ignaszak, A.; Yu, Y.; Zhang, J. Research advances in biomass-derived nanostructured carbons and their composite materials for electrochemical energy technologies. Prog. Mater. Sci. 2021, 118, 100770. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Qian, L. Synthesis of nitrogen-doped hierarchical porous carbons from peanut shell as a promising electrode material for high-performance supercapacitors. J. Energy Storage 2020, 30, 101451. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, M.K.; Yadav, N.; Hashmi, S.A. High performance quasi-solid-state supercapacitors with peanut-shell-derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, X.; Jia, X.; Liang, S.; Qian, L.; Rao, Z. Synthesis of biomass carbon electrode materials by bimetallic activation for the application in supercapacitors. J. Electroanal. Chem. 2019, 844, 105. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, C.; Kong, F.; Zhao, Q.; Kong, A.; Shan, Y. Activated biochar derived from peanut shells as the electrode materials with excellent performance in Zinc-air battery and supercapacitance. Waste Manag. 2021, 125, 257. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of synthetic strategies and properties of heteroatoms-doped (N, P, S, O) carbon materials for supercapacitors. J. Energy Storage 2022, 56, 105995. [Google Scholar] [CrossRef]
- Hu, H.; Yan, M.; Jiang, J.; Huang, A.; Cai, S.; Lan, L.; Ye, K.; Chen, D.; Tang, K.; Zuo, Q.; et al. A state-of-the-art review on biomass-derived carbon materials for supercapacitor applications: From precursor selection to design optimization. Sci. Total Environ. 2024, 912, 169141. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wu, D.; Mu, Y.; Xing, L.; Zhang, W.; Gao, Z.; Xu, F.; Jiang, K. Boron and nitrogen Co-doped holey graphene aerogels with rich B–N motifs for flexible supercapacitors. Carbon 2020, 159, 94–101. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Song, Y.; Qu, W.; He, Y.; Yang, H.; Du, M.; Wang, A.; Yang, Q.; Chen, Y. Synthesis and processing optimization of N-doped hierarchical porous carbon derived from corncob for high performance supercapacitors. J. Energy Storage 2020, 32, 101877. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, K.; Cheng, B.-H.; Jiang, H. Preparation of N-doped supercapacitor materials by integrated salt templating and silicon hard templating by pyrolysis of biomass wastes. ACS Sustain. Chem. Eng. 2017, 5, 6682–6691. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. rGO/N-porous carbon composites for enhanced CO2 capture and energy storage performances. J. Alloys Compd. 2021, 857, 157534. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Ramadass, K.; Sathish, C.I.; Vinu, A. High-performance biomass-derived activated porous biocarbons for combined pre- and post-combustion CO2 capture. ACS Sustain. Chem. Eng. 2019, 7, 7412–7420. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W.; Wang, C. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, T.C.; Yuan, S.; Liang, B. N-doped porous carbon derived from rGO-Incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. J. Power Sources 2020, 472, 228610. [Google Scholar] [CrossRef]
- Dong, X.; Jin, H.; Wang, R.; Zhang, J.; Feng, X.; Yan, C.; Chen, S.; Wang, S.; Wang, J.; Lu, J. High volumetric capacitance, ultralong life supercapacitors enabled by waxberry-derived hierarchical porous carbon materials. Adv. Energy Mater. 2018, 8, 1702695. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.; Xiao, J.; Cen, W.; Yuan, S. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.J.; Yilmaz, M.; Zhang, T.C.; Wang, Y.; Yuan, S.J. MgFe2O4-loaded N-doped biochar derived from waste cooked rice for efficient low-temperature desulfurization of H2S. Fuel 2023, 339, 127385. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiao, S.-Z. N-doping goes sp-hybridized. Nat. Chem. 2018, 10, 900–902. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; He, G.; Zhang, T.C.; Wang, Y.; Yuan, S. Direct electrocatalytic reduction of As(III) on CuSn alloy electrode: A green and sustainable strategy to recover elemental arsenic from arsenic wastewater. Ind. Eng. Chem. Res. 2024, 63, 8509–8523. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-based porous carbon for supercapacitor: The influence of preparation processes on structure and performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of modified biochars derived from bamboopyrolysis and their utilization for target component (furfural) adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Liao, Y.; Shang, Z.; Ju, G.; Wang, D.; Yang, Q.; Wang, Y.; Yuan, S. Biomass derived N-doped porous carbon made from reed straw for an enhanced supercapacitor. Molecules 2023, 28, 4633. [Google Scholar] [CrossRef] [PubMed]
- Haripriya, M.; Manimekala, T.; Dharmalingam, G.; Minakshi, M.; Sivasubramanian, R. Asymmetric supercapacitors based on ZnCo2O4 nanohexagons and orange peel derived activated carbon electrodes. Chem. Asian. J. 2024, 19, e202400202. [Google Scholar] [CrossRef]
- Lu, B.; Xiao, Z.; Zhu, H.; Xiao, W.; Wu, W.; Wang, D. Enhanced capacitive properties of commercial activated carbon by re-activation in molten carbonates. J. Power Sources 2015, 298, 74–82. [Google Scholar] [CrossRef]
- Uppugalla, S.; Srinivasan, P. High-performance supercapacitor coin cell: Polyaniline and nitrogen, sulfur-doped activated carbon electrodes in aqueous electrolyte. J. Solid State Electrochem. 2019, 23, 295–306. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel di-functional material for enhancing CO2 capture and super-capacitance. J. Mater. Sci. Technol. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Li, H.; Wu, Z.; Chen, Q.; Zhu, L.; Li, C.; Zhu, X.; Sun, Y. Magneto-electrodeposition of 3D cross-linked NiCo-LDH for flexible high-performance supercapacitors. Small Methods 2022, 6, 2101320. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, H.; Guo, F.; Tian, B.; Du, S.; Dong, Y.; Qian, L. Preparation of highly porous activated carbons from peanut shells as low-cost electrode materials for supercapacitors. J. Power Sources 2021, 34, 102180. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Rabiei Baboukani, A.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-performance red phosphorus-sulfurized polyacrylonitrile composite by electrostatic spray deposition for lithium-ion batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Lima, R.M.A.P.; dos Reis, G.S.; Lassi, U.; Lima, E.C.; Dotto, G.L.; de Oliveira, H.P. Sustainable supercapacitors based on polypyrrole-doped activated biochar from wood waste electrodes. C-J. Carbon Res. 2023, 9, 59. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Wei, G.; Li, H.; Wang, G.; Li, T.; Wang, J.; Jiang, Y.; Bao, L.; Zhang, Y. Construction of monolayer Ti3C2Tx MXene on nickel foam under high electrostatic fields for high-performance supercapacitors. Nanomaterials 2024, 14, 887. [Google Scholar] [CrossRef]
- Le Van, K.; Luong Thi Thu, T. Preparation of pore-size controllable activated carbon from rice husk using dual activating agent and Its application in supercapacitor. J. Chem. 2019, 2019, 4329609. [Google Scholar] [CrossRef]
- Singh, G.; Bahadur, R.; Ruban, A.M.; Davidraj, J.M.; Su, D.; Vinu, A. Synthesis of functionalized nanoporous biocarbons with high surface area for CO2 capture and supercapacitor applications. Green Chem. 2021, 23, 5571–5583. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Chen, H.; Zhang, C.; Zhang, Y.; Gu, C.; Xu, X.; Li, Q. Synthesis of biomass porous carbon materials from bean sprouts for hydrogen evolution reaction electrocatalysis and supercapacitor electrode. Int. J. Hydrogen Energy 2021, 46, 18887–18897. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Acharya, J.; Park, M. Preparation, characterization, and electrochemical performances of activated carbon derived from the flower of Bauhinia variegata L for supercapacitor applications. Diam. Relat. Mater. 2023, 136, 110040. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Sun, Q.; Wang, P.; Li, Y. Durian shell-derived N, O, P-doped activated porous carbon materials and their electrochemical performance in supercapacitor. J. Mater. Sci. 2020, 55, 10142–10154. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Ayala-Cortés, A.; Longoria, A.; Pacheco-Catalán, D.E.; Okoye, P.U.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Cuentas-Gallegos, A.K. Activated carbons obtained by environmentally friendly activation using solar energy for their use in neutral electrolyte supercapacitors. J. Energy Storage 2022, 52, 104888. [Google Scholar] [CrossRef]
- Song, X.; Ma, X.; Li, Y.; Ding, L.; Jiang, R. Tea waste derived microporous active carbon with enhanced double-layer supercapacitor behaviors. Appl. Surf. Sci. 2019, 487, 189–197. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, J.; Yılmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-doped porous biochar derived from cornstalk for high performance CO2 adsorption and electrochemical energy storage. Sep. Purif. Technol. 2022, 299, 121719. [Google Scholar] [CrossRef]
- Ukkakimapan, P.; Sattayarut, V.; Wanchaem, T.; Yordsri, V.; Phonyiem, M.; Ichikawa, S.; Obata, M.; Fujishige, M.; Takeuchi, K.; Wongwiriyapan, W.; et al. Preparation of activated carbon via acidic dehydration of durian husk for supercapacitor applications. Diam. Relat. Mater. 2020, 107, 107906. [Google Scholar] [CrossRef]
- Veerakumar, P.; Maiyalagan, T.; Raj, B.G.S.; Guruprasad, K.; Jiang, Z.; Lin, K.-C. Paper flower-derived porous carbons with high-capacitance by chemical and physical activation for sustainable applications. Arab. J. Chem. 2020, 13, 2995–3007. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Pinheiro Lima, R.M.A.; Larsson, S.H.; Subramaniyam, C.M.; Dinh, V.M.; Thyrel, M.; de Oliveira, H.P. Flexible supercapacitors of biomass-based activated carbon-polypyrrole on eggshell membranes. J. Environ. Chem. Eng. 2021, 9, 106155. [Google Scholar] [CrossRef]
- Jia, P.; Wang, Z.; Wang, X.; Qin, K.; Gao, J.; Sun, J.; Xia, G.; Dong, T.; Gong, Y.; Yu, Z.; et al. Nanoporous carbon materials derived from Zanthoxylum Bungeanum peel and seed for electrochemical supercapacitors. Nanomaterials 2024, 14, 836. [Google Scholar] [CrossRef]

| Carbon Precursor | Activating Agent | Electrolyte Used | Highest Specific Capacitance (F/g) | Ref. |
|---|---|---|---|---|
| Peanut shells | KOH | 6 M KOH | 208.3 | This work |
| Reed straw | KOH | 6 M KOH | 202.8 | [46] |
| Casein | C6H5K3O7 | 3 M KOH | 177 | [58] |
| Bean sprouts | / | 1 M KOH | 203.8 | [59] |
| Koiralo flower | KOH | 6M KOH | 165 | [60] |
| Durian shells | (NH4)2HPO4 | 1 M H2SO4 | 184 | [61] |
| Agave angustifolia leaves | K2CO3 | 1 M Na2SO4 | 200 | [62] |
| Tea | KOH | 1 M KOH | 167 | [63] |
| Cornstalk | K2CO3 | 6 M KOH | 203.5 | [64] |
| Durian husk | NaOH | 1 M TEABF4. | 145 | [65] |
| Paper flower | ZnCl2 | 1 M H2SO4 | 118 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, Q.; Liu, J.; Li, J.; Liu, W.; Wang, Y.; Yuan, S. Nitrogen-Doped Porous Carbons Derived from Peanut Shells as Efficient Electrodes for High-Performance Supercapacitors. Int. J. Mol. Sci. 2024, 25, 7583. https://doi.org/10.3390/ijms25147583
Liu S, Zhang Q, Liu J, Li J, Liu W, Wang Y, Yuan S. Nitrogen-Doped Porous Carbons Derived from Peanut Shells as Efficient Electrodes for High-Performance Supercapacitors. International Journal of Molecular Sciences. 2024; 25(14):7583. https://doi.org/10.3390/ijms25147583
Chicago/Turabian StyleLiu, Shibo, Qishan Zhang, Jiani Liu, Jiarui Li, Wenjia Liu, Yuan Wang, and Shaojun Yuan. 2024. "Nitrogen-Doped Porous Carbons Derived from Peanut Shells as Efficient Electrodes for High-Performance Supercapacitors" International Journal of Molecular Sciences 25, no. 14: 7583. https://doi.org/10.3390/ijms25147583







