VmsR, a LuxR-Type Regulator, Contributes to Virulence, Cell Motility, Extracellular Polysaccharide Production and Biofilm Formation in Xanthomonas oryzae pv. oryzicola
Abstract
1. Introduction
2. Results
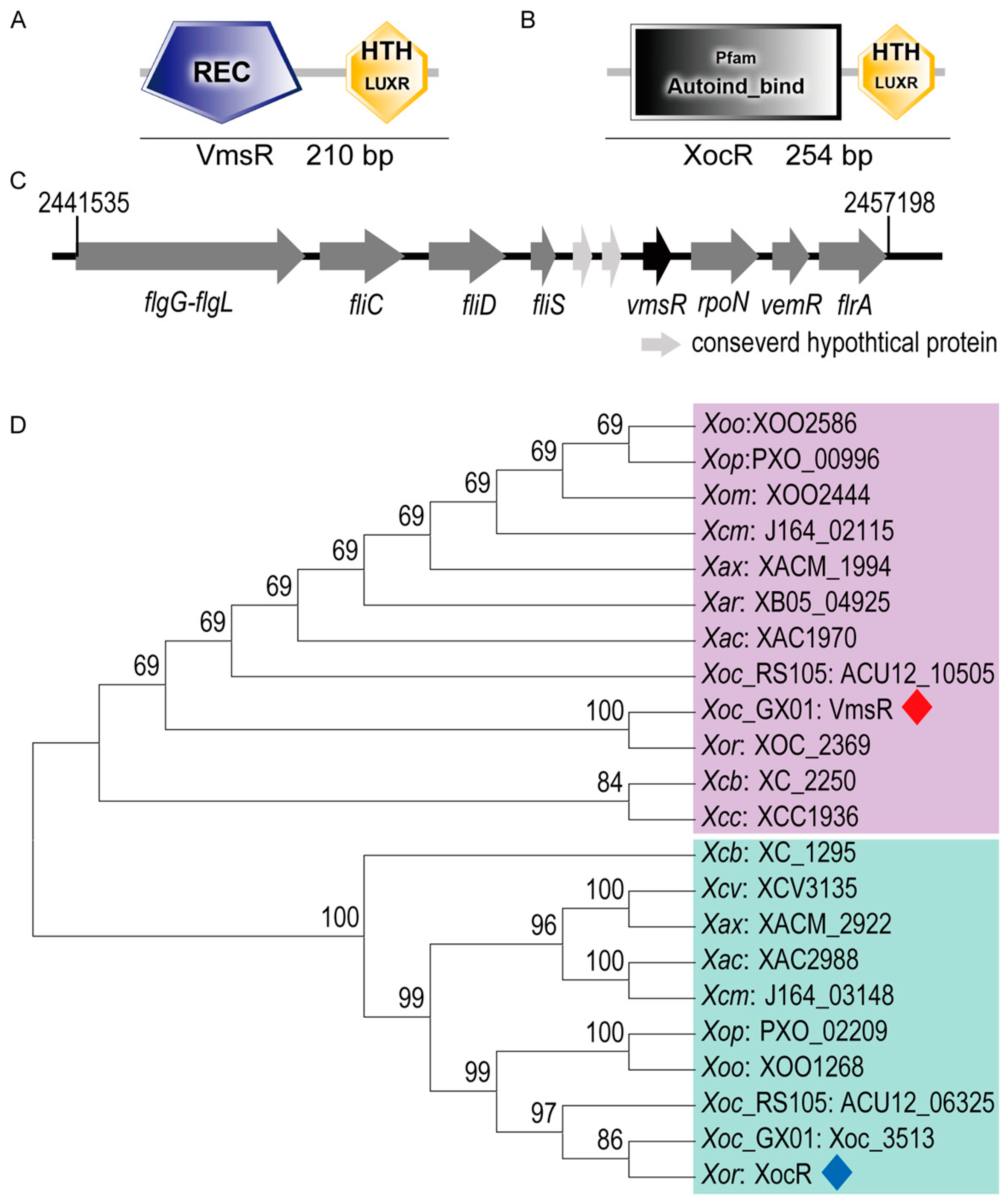
2.1. Identification of VmsR, a Putative LuxR-Type Regulator of Xoc, and Generation of vmsR Mutant and Its Complementary Strain
2.2. VmsR Positively Affects the Virulence of Xoc GX01
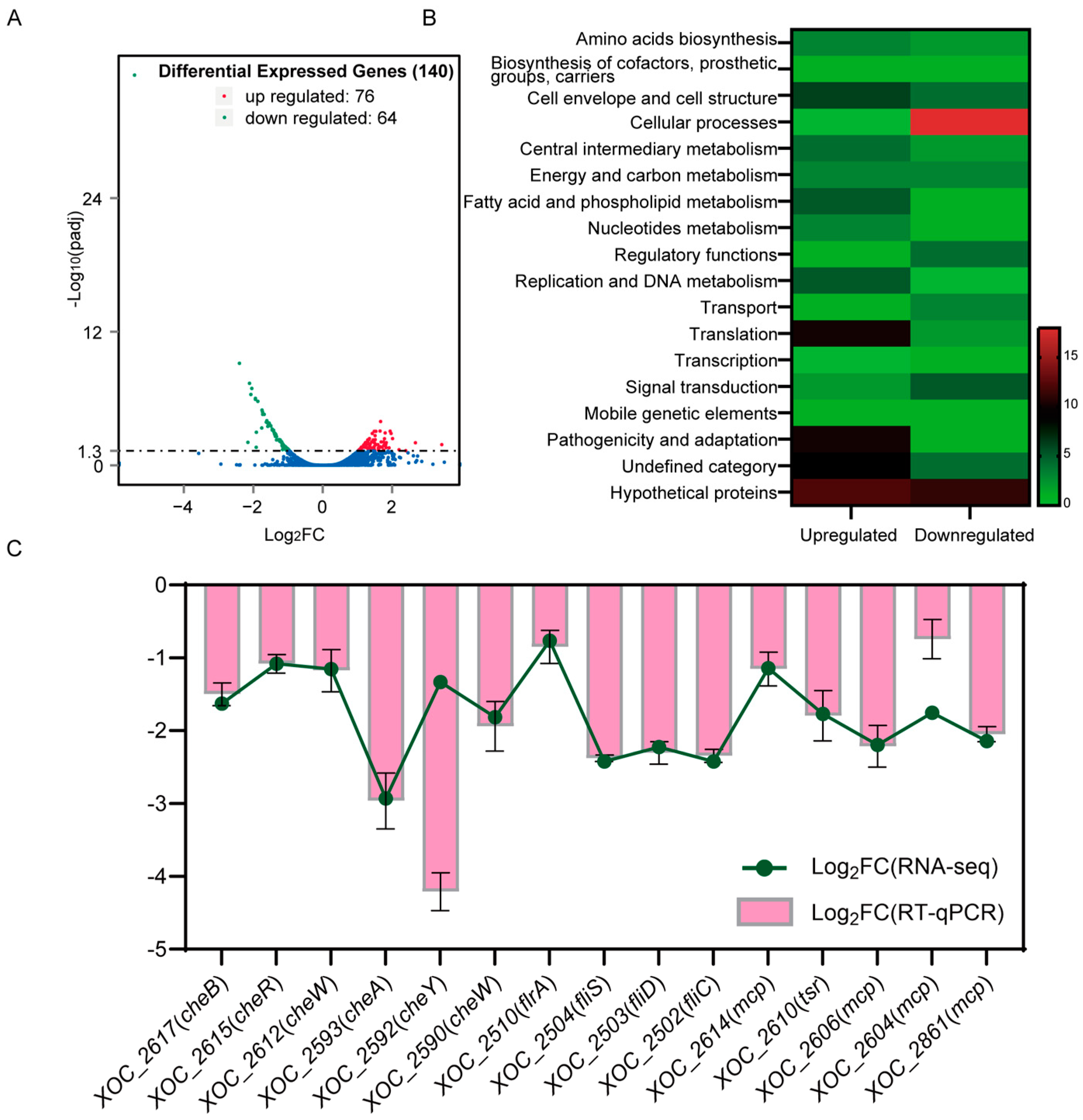
2.3. VmsR Is an Important Regulator Involved in Various Cellular Processes of Xoc
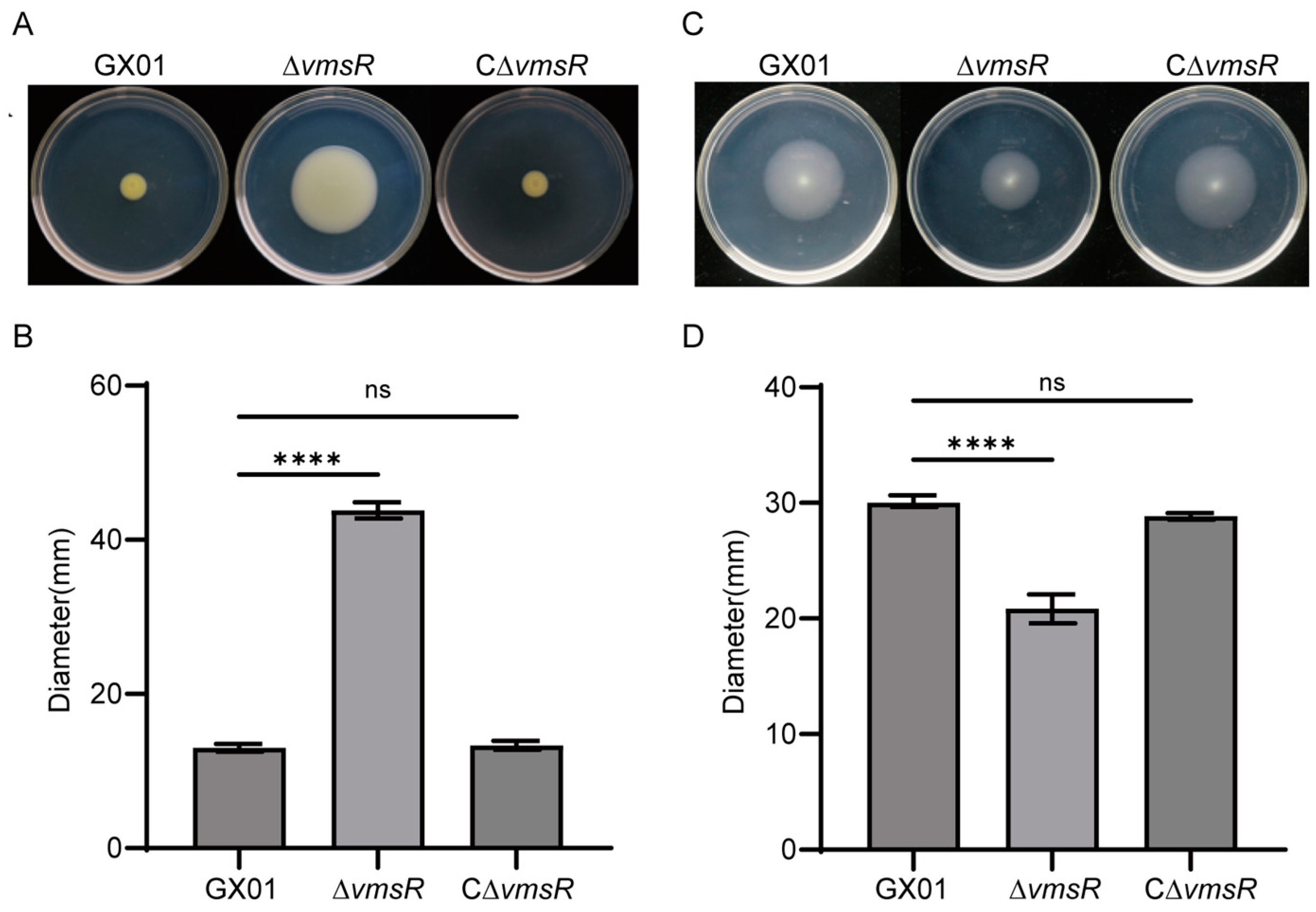
2.4. Loss of vmsR in Xoc Enhanced the Swarming Motility but Impaired the Swimming Motility
2.5. VmsR Specifically Binds to the fliC and fliS Promoter In Vitro and Facilitates Their Expression
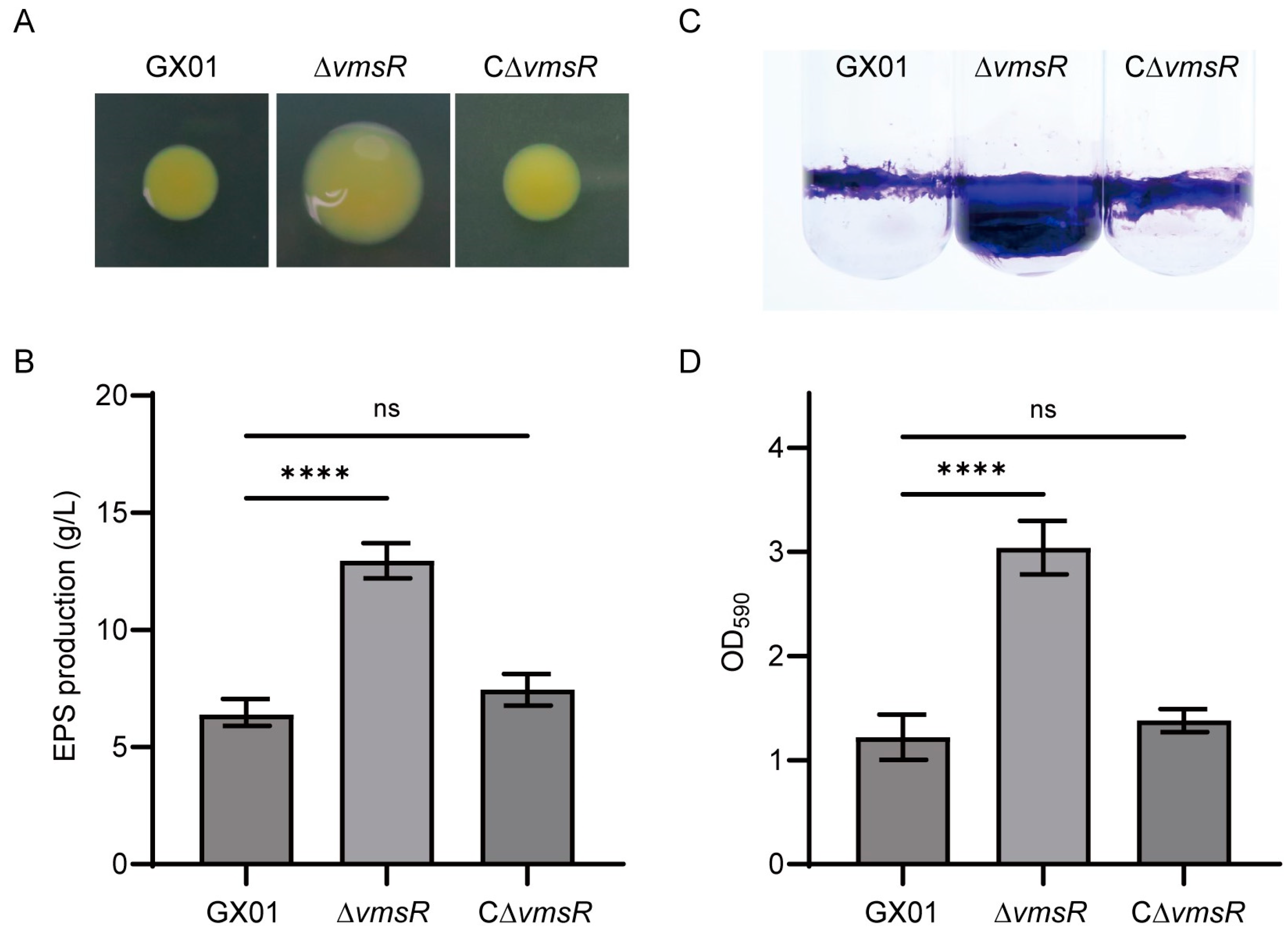
2.6. EPS Production and Biofilm Formation of the ∆vmsR Strain Were Increased
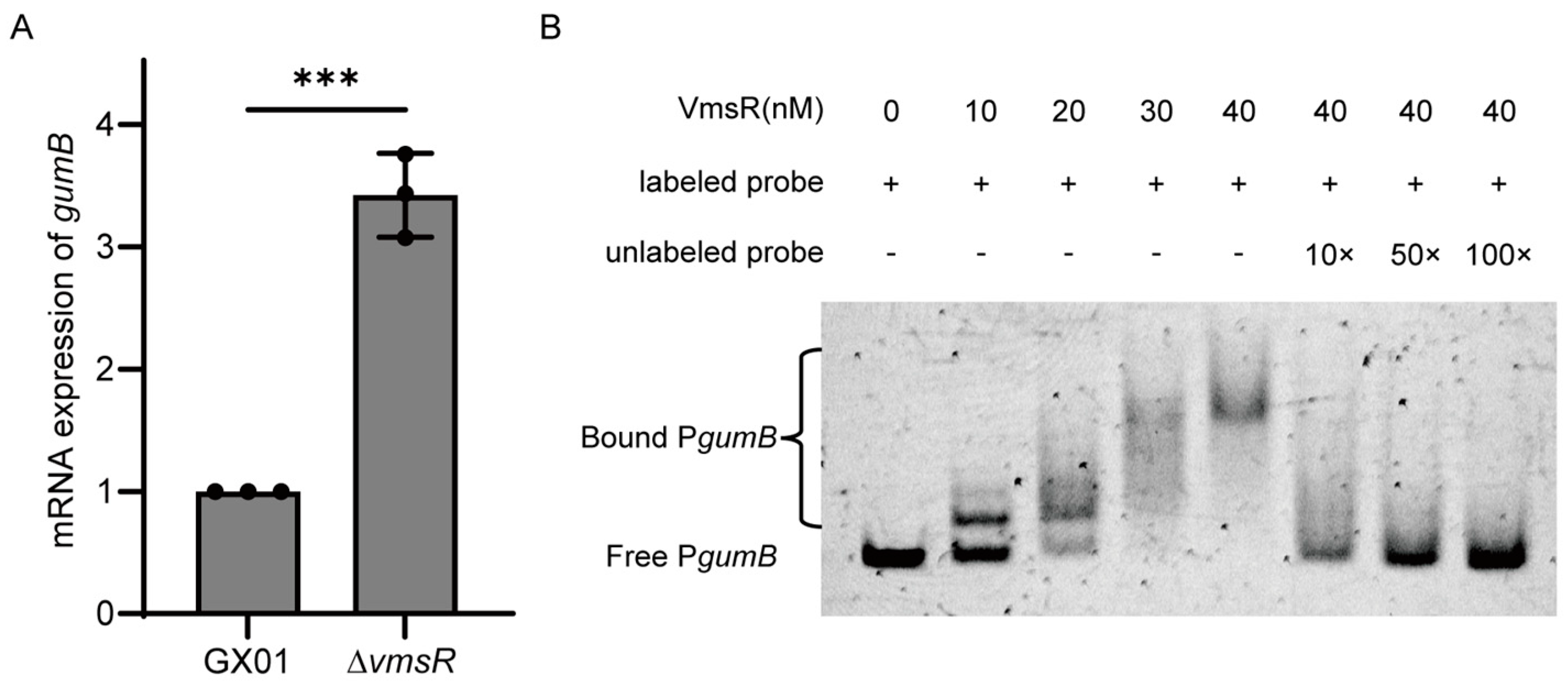
2.7. VmsR Specifically Binds to the gumB Promoter In Vitro and Inhibits Its Expression
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Media and Growth Conditions
4.2. Construction of the vmsR Deletion Mutant and Its Complementary Strain
4.3. Virulence Assay
4.4. Assay for Swimming and Swarming Motilities
4.5. Determination of Biofilm Formation and EPS Production
4.6. Transcriptome Analysis
4.7. RNA Isolation and Quantitative Reverse Transcription PCR Analysis of Gene Expression
4.8. In Vitro Electrophoretic Mobility Shift Assay (EMSA)
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NiÑO-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Y.; Zakria, M.; Zou, L.F.; Xiong, L.; Li, Z.; Ji, G.H.; Chen, G.Y. Genetic diversity of transcriptional activator-like effector genes in Chinese isolates of Xanthomonas oryzae pv. oryzicola. Phytopathology 2014, 104, 672–682. [Google Scholar] [CrossRef][Green Version]
- Yang, W.F.; Liu, Y.; Chen, L.; Gao, T.C.; Hu, B.S.; Zhang, D.F.; Liu, F.Q. Zinc uptake regulator (zur) gene involved in zinc homeostasis and virulence of Xanthomonas oryzae pv. oryzae in rice. Curr. Microbiol. 2007, 54, 307–314. [Google Scholar] [CrossRef]
- Lee, S.W.; Jeong, K.S.; Han, S.W.; Lee, S.E.; Phee, B.K.; Hahn, T.R.; Ronald, P. The Xanthomonas oryzae pv. oryzae PhoPQ two-component system is required for AvrXA21 activity, hrpG expression and virulence. J. Bacteriol. 2008, 190, 2183–2197. [Google Scholar] [CrossRef]
- Bender, C.L.; Alarcón-Chaidez, F.; Gross, D.C. Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [CrossRef]
- Chan, J.W.Y.F.; Goodwin, P.H. The molecular genetics of virulence of Xanthomonas campestris. Biotechnol. Adv. 1999, 17, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef]
- Patankar, A.V.; Gonzalez, J.E. An orphan LuxR homolog of Sinorhizobium meliloti affects stress adaptation and competition for nodulation. Appl. Environ. Microbiol. 2009, 75, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Correia-Neves, M.; Moradas-Ferreira, P.; Mendes, M.V. A walk into the LuxR regulators of Actinobacteria: Phylogenomic distribution and functional diversity. PLoS ONE 2012, 7, e46758. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Myers, M.P.; Venturi, V. The inter-kingdom solo OryR regulator of Xanthomonas oryzae is important for motility. Mol. Plant Pathol. 2013, 14, 211–221. [Google Scholar] [CrossRef]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef]
- Fuqua, C.; Winans, S.C.; Greenberg, E.P. Census and consensus in bacterial ecosystems: The LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 1996, 50, 727–751. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [CrossRef]
- Fuqua, C.; Winans, S.C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 1996, 178, 435–440. [Google Scholar] [CrossRef]
- Qian, W.; Han, Z.J.; He, C.Z. Two-component signal transduction systems of Xanthomonas spp.: A lesson from genomics. Mol. Plant-Microbe Interact. 2008, 21, 151–161. [Google Scholar] [CrossRef]
- Ninfa, A.J.; Magasanik, B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc. Natl. Acad. Sci. USA 1986, 83, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Liu, Y.N.; Barber, C.E.; Dow, J.M.; Wootton, J.C.; Daniels, M.J. Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol. Gen. Genet. MGG 1991, 226, 409–417. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhang, Y.P.; Li, J.L.; Wang, N.A. Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant-Microbe Interact. 2012, 25, 165–179. [Google Scholar] [CrossRef]
- Wengelnik, K.; VandenAckerveken, G.; Bonas, U. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant-Microbe Interact. 1996, 9, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Noël, L.; Thieme, F.; Nennstiel, D.; Bonas, U. cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 2001, 41, 1271–1281. [Google Scholar] [CrossRef]
- Henrichsen, J. Bacterial surface translocation: A survey and a classification. Bacteriol. Rev. 1972, 36, 478–503. [Google Scholar] [CrossRef]
- Jarrell, K.F.; McBride, M.J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 2008, 6, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef]
- Partridge, J.D.; Harshey, R.M. Swarming: Flexible Roaming Plans. J. Bacteriol. 2013, 195, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bree, A.C.; Liu, J.; Patrick, J.E.; Chien, P.; Kearns, D.B. Adaptor-mediated Lon proteolysis restricts Bacillus subtilis hyperflagellation. Proc. Natl. Acad. Sci. USA 2015, 112, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Harshey, R.M.; Partridge, J.D. Shelter in a swarm. J. Mol. Biol. 2015, 427, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Tasteyre, A.; Barc, M.-C.; Collignon, A.; Boureau, H.; Karjalainen, T. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 2001, 69, 7937–7940. [Google Scholar] [CrossRef]
- Dingle Tanis, C.; Mulvey George, L.; Armstrong Glen, D. Mutagenic analysis of the Clostridium difficile flagellar proteins, FliC and FliD, and their contribution to virulence in hamsters. Infect. Immun. 2011, 79, 4061–4067. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011, 19, 449–455. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Futur. Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.N.; Li, Y.M.; Carpenter, S.C.D.; Dan, X.; Li, T.J.; Wu, Q.Z.; Wang, L.; Jiang, W.; Huang, S.; Tang, J.L.; et al. Complete genome resource of Xanthomonas oryzae pv. oryzicola GX01 isolated in South China. Mol. Plant-Microbe Interact. 2022, 35, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, Y.; Qian, G.; Liu, F. XocR, a LuxR solo required for virulence in Xanthomonas oryzae pv. oryzicola. Front. Cell. Infect. Microbiol. 2015, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Zhang, L.; Jiang, B.L.; Zhang, Z.C.; Xu, R.Q.; Tang, D.J.; Qin, J.; Jiang, W.; Zhang, X.; Liao, J.; et al. Comparative and functional genomics reveals genetic diversity and determinants of host specificity among reference strains and a large collection of Chinese isolates of the phytopathogen Xanthomonas campestris pv. campestris. Genome Biol. 2007, 8, R218. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Jia, Y.T.; Ren, S.X.; He, Y.Q.; Feng, J.X.; Lu, L.F.; Sun, Q.H.; Ying, G.; Tang, D.J.; Tang, H.; et al. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 2005, 15, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Lehti, T.A.; Bauchart, P.; Dobrindt, U.; Korhonen, L.K.; Westerlund-Wikström, B. The fimbriae activator MatA switches off motility in Escherichia coli by repression of the flagellar master operon flhDC. Microbiology 2012, 158, 1444–1455. [Google Scholar] [CrossRef]
- Cellini, A.; Buriani, G.; Correia, C.; Fiorentini, L.; Vandelle, E.; Polverari, A.; Santos, C.; Vanneste, J.L.; Spinelli, F. Host-specific signal perception by PsaR2 LuxR solo induces Pseudomonas syringae pv. actinidiae virulence traits. Microbiol. Res. 2022, 260, 127048. [Google Scholar] [CrossRef]
- Guan, W.; Wang, T.L.; Huang, Q.; Tian, E.Y.; Liu, B.; Yang, Y.W.; Zhao, T.C. A LuxR-type regulator, AcrR, regulates flagellar assembly and contributes to virulence, motility, biofilm formation, and growth ability of Acidovorax citrulli. Mol. Plant Pathol. 2020, 21, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef]
- Liu, A.; Mi, Z.H.; Zheng, X.Y.; Yu, Y.; Su, H.N.; Chen, X.L.; Xie, B.B.; Zhou, B.C.; Zhang, Y.Z.; Qin, Q.L. Exopolysaccharides play a role in the swarming of the benthic bacterium Pseudoalteromonas sp. SM9913. Front. Microbiol. 2016, 7, 473. [Google Scholar] [CrossRef]
- Dye, K.J.; Yang, Z.M. Analysis of vegetative biofilms with microtiter plates. Front. Microbiol. 2022, 13, 894562. [Google Scholar] [CrossRef] [PubMed]
- Vorhölter, F.-J.; Schneiker, S.; Goesmann, A.; Krause, L.; Bekel, T.; Kaiser, O.; Linke, B.; Patschkowski, T.; Rückert, C.; Schmid, J.; et al. The genome of Xanthomonas campestris pv. campestris B100 and its use for the reconstruction of metabolic pathways involved in xanthan biosynthesis. J. Biotechnol. 2008, 134, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sourjik, V.; Muschler, P.; Scharf, B.; Schmitt, R. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J. Bacteriol. 2000, 182, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Wang, T.L.; Huang, Q.; Zhao, M.; Tian, E.Y.; Liu, Y.F.; Liu, B.; Yang, Y.W.; Zhao, T.C. Transcriptomic and functional analyses reveal roles of AclR, a luxR-type global regular, in regulating motility and virulence of Acidovorax citrulli. Mol. Plant-Microbe Interact. 2021, 34, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed]
- Steffens, T.; Vorhölter, F.J.; Teckentrup, J.; Hublik, G.; Walhorn, V.; Anselmetti, D.; Pühler, A.; Niehaus, K.; Ortseifen, V. Two flagellar mutants of Xanthomonas campestris are characterized by enhanced xanthan production and higher xanthan viscosity. J. Biotechnol. 2022, 347, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef]
- Song, H.C.; Kang, Y.H.; Zhang, D.X.; Chen, L.; Qian, A.D.; Shan, X.F.; Li, Y. Great effect of porin(aha) in bacterial adhesion and virulence regulation in Aeromonas veronii. Microb. Pathog. 2019, 126, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Auvray, F.; Thomas, J.; Fraser, G.M.; Hughes, C. Flagellin polymerisation control by a cytosolic export chaperone. J. Mol. Biol. 2001, 308, 221–229. [Google Scholar] [CrossRef]
- Minamino, T.; Kinoshita, M. Structure, assembly, and function of flagella responsible for bacterial locomotion. EcoSal Plus 2023, 11, eesp-0011-2023. [Google Scholar] [CrossRef]
- Xu, S.; Peng, Z.; Cui, B.; Wang, T.; Song, Y.; Zhang, L.; Wei, G.; Wang, Y.; Shen, X. FliS modulates FlgM activity by acting as a non-canonical chaperone to control late flagellar gene expression, motility and biofilm formation in Yersinia pseudotuberculosis. Environ. Microbiol. 2014, 16, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, D.; Harikrishnan, A.; Jyoti, K.; Ali, M.I.; Jeevaratnam, K. A compound isolated from Alpinia officinarum Hance. inhibits swarming motility of Pseudomonas aeruginosa and down regulates virulence genes. J. Appl. Microbiol. 2020, 128, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Lewenza, S.; Marr, A.K.; Hancock, R.E.W. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J. Bacteriol. 2007, 189, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Bains, M.; Brazas, M.D.; Hancock, R.E.W. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 2008, 190, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechere, J.C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.H.; Huang, L.; Liu, G.F.; Leng, M.; Lu, G.T. PilG and PilH antagonistically control flagellum-dependent and pili-dependent motility in the phytopathogen Xanthomonas campestris pv. campestris. BMC Microbiol. 2020, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Fujishige, N.A.; Kapadia, N.N.; De Hoff, P.L.; Hirsch, A.M. Investigations of biofilm formation. FEMS Microbiol. Ecol. 2006, 56, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rangaraj, N.; Sonti, R.V. Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol. Plant-Microbe Interact. 2009, 22, 73–85. [Google Scholar] [CrossRef]
- Rigano, L.A.; Siciliano, F.; Enrique, R.; Sendin, L.; Filippone, P.; Torres, P.S.; Qüesta, J.; Dow, J.M.; Castagnaro, A.P.; Vojnov, A.A.; et al. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant-Microbe Interact. 2007, 20, 1222–1230. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wei, C.; Jiang, W.D.; Wang, L.; Li, C.R.; Wang, Y.Y.; Dow, J.M.; Sun, W.X. The HD-GYP domain protein RpfG of Xanthomonas oryzae pv. oryzicola regulates synthesis of extracellular polysaccharides that contribute to biofilm formation and virulence on rice. PLoS ONE 2013, 8, e59428. [Google Scholar] [CrossRef]
- An, S.W.; Wu, J.E.; Zhang, L.H. Modulation of Pseudomonas aeruginosa biofilm dispersal by a cyclic-di-GMP phosphodiesterase with a putative hypoxia-sensing domain. Appl. Environ. Microb. 2010, 76, 8160–8173. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Kolter, R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 2004, 186, 4457–4465. [Google Scholar] [CrossRef] [PubMed]
- Kuchma, S.L.; Brothers, K.M.; Merritt, J.H.; Liberati, N.T.; Ausubel, F.M.; O’Toole, G.A. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 2007, 189, 8165–8178. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and Type I Pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Houry, A.; Briandet, R.; Aymerich, S.; Gohar, M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology 2010, 156, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Prigent-Combaret, C.; Prensier, G.; Le Thi, T.T.; Vidal, O.; Lejeune, P.; Dorel, C. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: Role of flagella, curli and colanic acid. Environ. Microbiol. 2000, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Haagensen, J.A.J.; Schembri, M.A.; Zechner, E.L.; Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 2003, 48, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Laganenka, L.; López María, E.; Colin, R.; Sourjik, V. Flagellum-mediated mechanosensing and RflP control motility state of pathogenic Escherichia coli. mBio 2020, 11, e02269-19. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Antani, J.D.; Lele, P.P.; Chen, J.; Nan, B.Y.; Kuhn, M.J.; Persat, A.; Bru, J.L.; Hoyland-Kroghsbo, N.M.; Siryaporn, A.; et al. Roadmap on emerging concepts in the physical biology of bacterial biofilms: From surface sensing to community formation. Phys. Biol. 2021, 18, 051501. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef] [PubMed]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Peng, J.L.; Liu, Q.Q.; Chang, Z.; Huang, Y.X.; Tang, J.L.; Lu, G.T. Xanthomonas campestris VemR enhances the transcription of the T3SS key regulator HrpX via physical interaction with HrpG. Mol. Plant Pathol. 2023, 24, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Chaban, B.; Hughes, H.V.; Beeby, M. The flagellum in bacterial pathogens: For motility and a whole lot more. Semin. Cell Dev. Biol. 2015, 46, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Katzen, F.; Ferreiro, D.U.; Oddo, C.G.; Ielmini, M.V.; Becker, A.; Pühler, A.; Ielpi, L. Xanthomonas campestris pv. campestris gum mutants: Effects on xanthan biosynthesis and plant virulence. J. Bacteriol. 1998, 180, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Vojnov, A.A.; Slater, H.; Daniels, M.J.; Dow, J.M. Expression of the gum operon directing xanthan biosynthesis in Xanthomonas campestris and its regulation in planta. Mol. Plant-Microbe Interact. 2001, 14, 768–774. [Google Scholar] [CrossRef][Green Version]
- Chen, H.T.; Yu, C.Q.; Wu, H.; Li, G.Q.; Li, C.R.; Hong, W.; Yang, X.Y.; Wang, H.; You, X.F. Recent advances in histidine kinase-targeted antimicrobial agents. Front. Chem. 2022, 10, 866392. [Google Scholar] [CrossRef]
- Zhu, P.C.; Li, Y.M.; Yang, X.; Zou, H.F.; Zhu, X.L.; Niu, X.N.; Xu, L.H.; Jiang, W.; Huang, S.; Tang, J.L.; et al. Type VI secretion system is not required for virulence on rice but for inter-bacterial competition in Xanthomonas oryzae pv. oryzicola. Res. Microbiol. 2020, 171, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bogdanove, A. Inoculation and Virulence Assay for Bacterial Blight and Bacterial Leaf Streak of Rice. In Rice Protocols; Yang, Y., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 249–255. [Google Scholar]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Garner, M.M.; Revzin, A. The use of gel electrophoresis to detect and study nucleic acid-protein interactions. Trends Biochem. Sci. 1986, 11, 395–396. [Google Scholar] [CrossRef]
- Hellman, L.M.; Fried, M.G. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat. Protoc. 2007, 2, 1849–1861. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, X.; Wang, J.; Liao, L.; Qin, H.; Zhang, R.; Li, C.; He, Y.; Huang, S. VmsR, a LuxR-Type Regulator, Contributes to Virulence, Cell Motility, Extracellular Polysaccharide Production and Biofilm Formation in Xanthomonas oryzae pv. oryzicola. Int. J. Mol. Sci. 2024, 25, 7595. https://doi.org/10.3390/ijms25147595
Zhang Y, Zhao X, Wang J, Liao L, Qin H, Zhang R, Li C, He Y, Huang S. VmsR, a LuxR-Type Regulator, Contributes to Virulence, Cell Motility, Extracellular Polysaccharide Production and Biofilm Formation in Xanthomonas oryzae pv. oryzicola. International Journal of Molecular Sciences. 2024; 25(14):7595. https://doi.org/10.3390/ijms25147595
Chicago/Turabian StyleZhang, Yaqi, Xiyao Zhao, Jiuxiang Wang, Lindong Liao, Huajun Qin, Rongbo Zhang, Changyu Li, Yongqiang He, and Sheng Huang. 2024. "VmsR, a LuxR-Type Regulator, Contributes to Virulence, Cell Motility, Extracellular Polysaccharide Production and Biofilm Formation in Xanthomonas oryzae pv. oryzicola" International Journal of Molecular Sciences 25, no. 14: 7595. https://doi.org/10.3390/ijms25147595
APA StyleZhang, Y., Zhao, X., Wang, J., Liao, L., Qin, H., Zhang, R., Li, C., He, Y., & Huang, S. (2024). VmsR, a LuxR-Type Regulator, Contributes to Virulence, Cell Motility, Extracellular Polysaccharide Production and Biofilm Formation in Xanthomonas oryzae pv. oryzicola. International Journal of Molecular Sciences, 25(14), 7595. https://doi.org/10.3390/ijms25147595





