Oxidized Phospholipids Regulate Tenocyte Function via Induction of Amphiregulin in Dendritic Cells
Abstract
:1. Introduction
2. Results
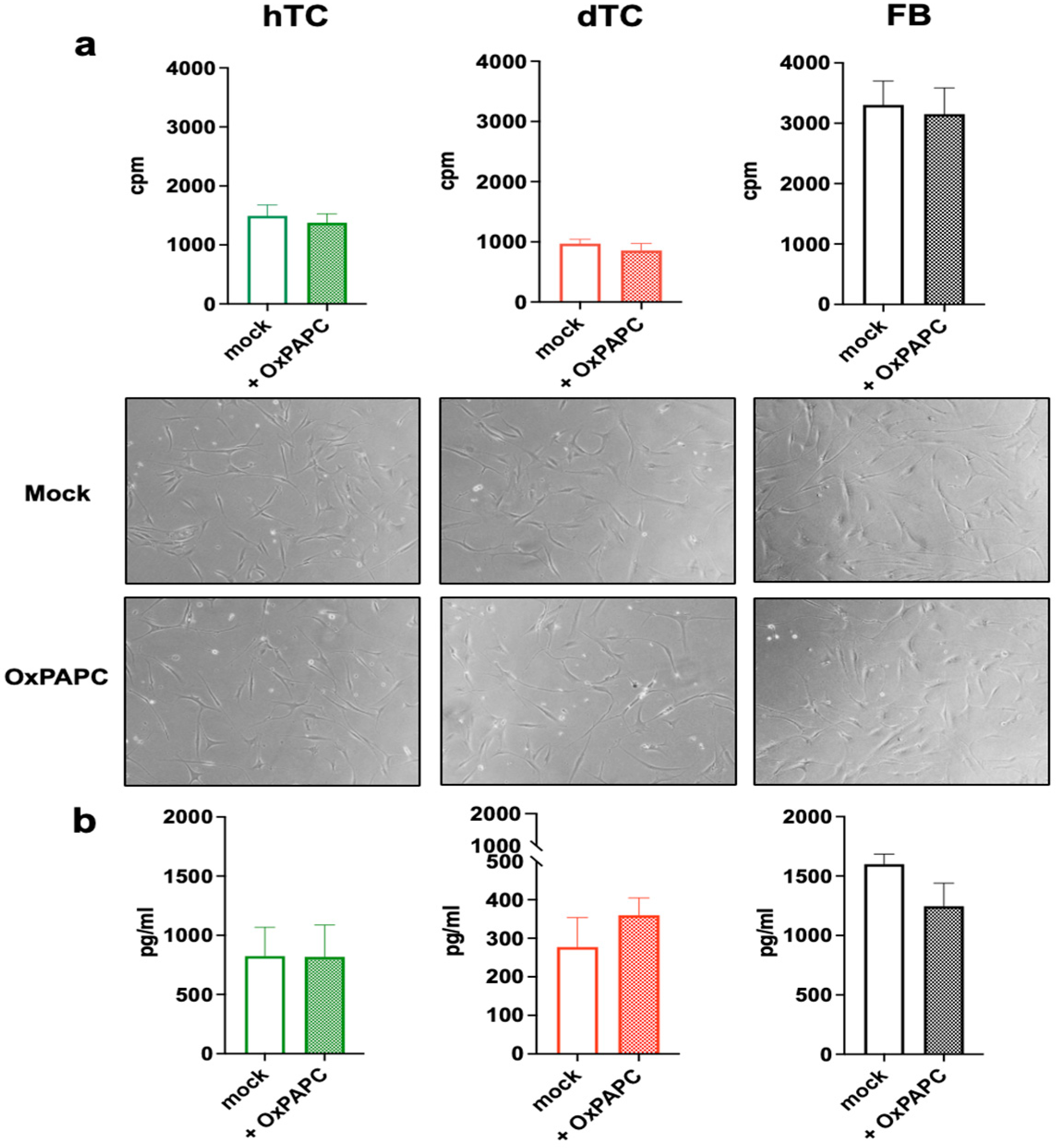
2.1. Treatment with OxPAPC Does Not Alter Proliferation or Collagen Production in Human Tenocytes
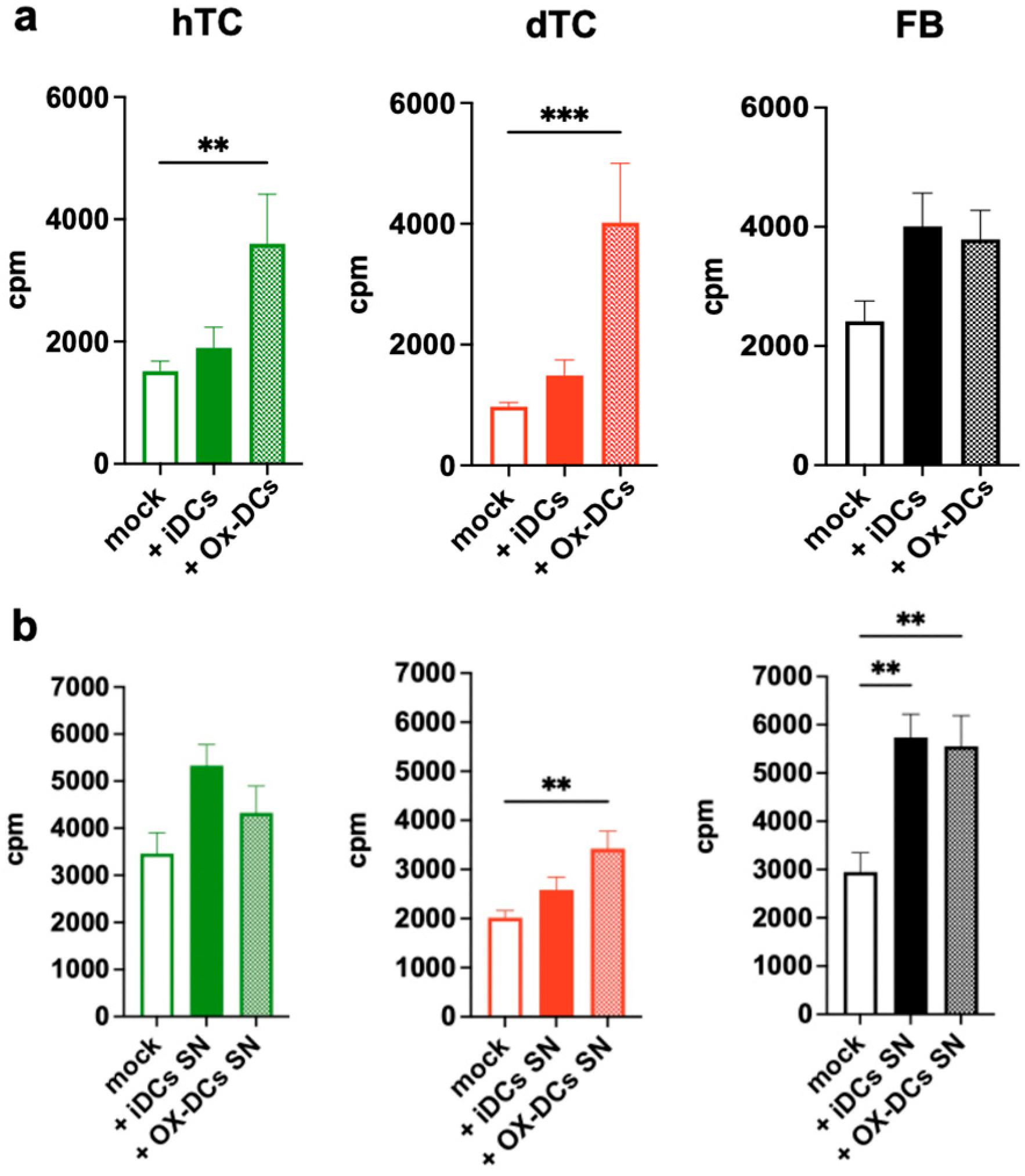
2.2. OxPAPC-Treated Dendritic Cells (Ox-DCs) Stimulate the Proliferation of dTCs, hTCs, and FBs
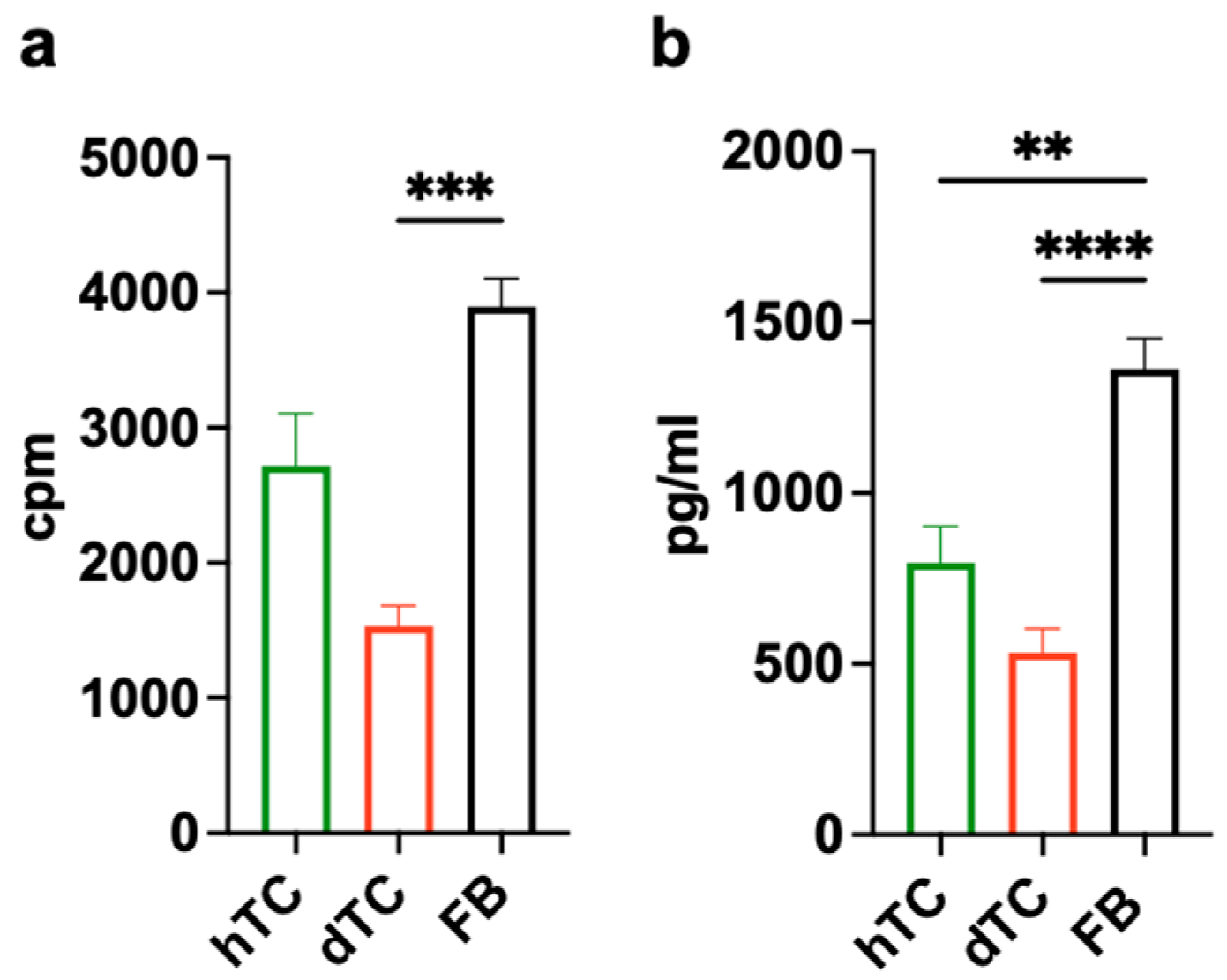
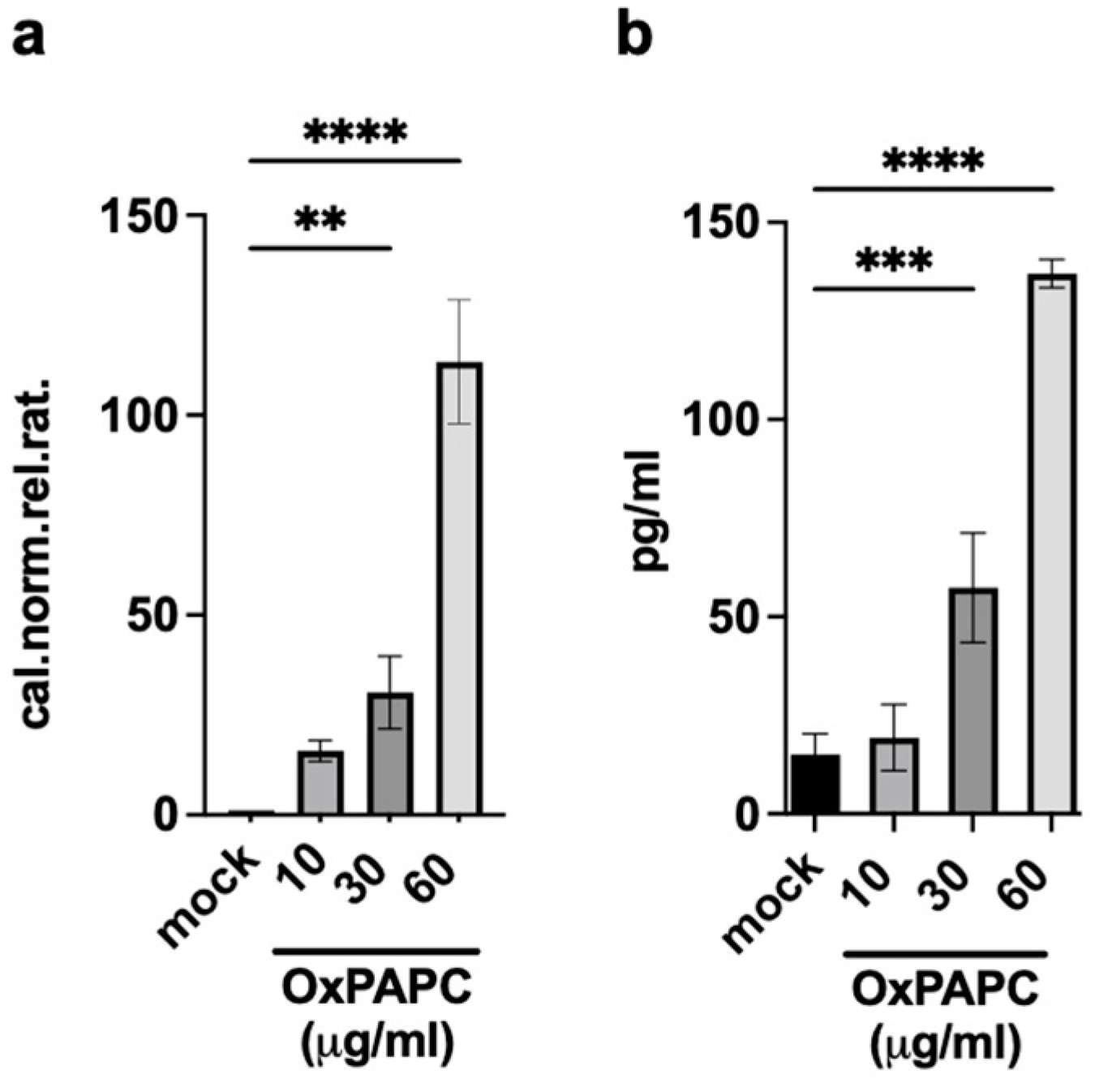
2.3. OxPAPC-Treated Dendritic Cells (Ox-DCs) Produce and Release Amphiregulin (AREG)
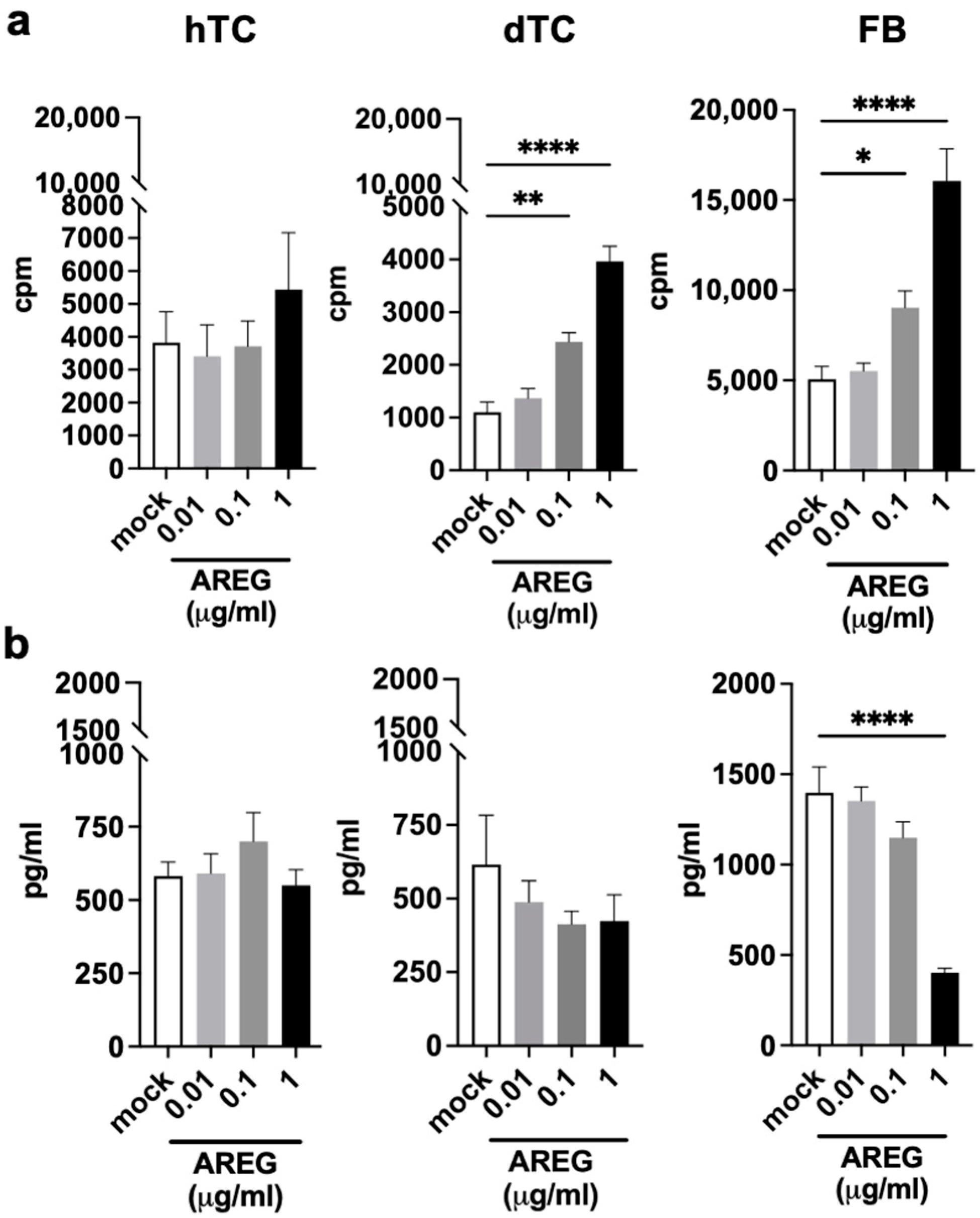
2.4. AREG Is a Growth Factor for dTCs and FBs
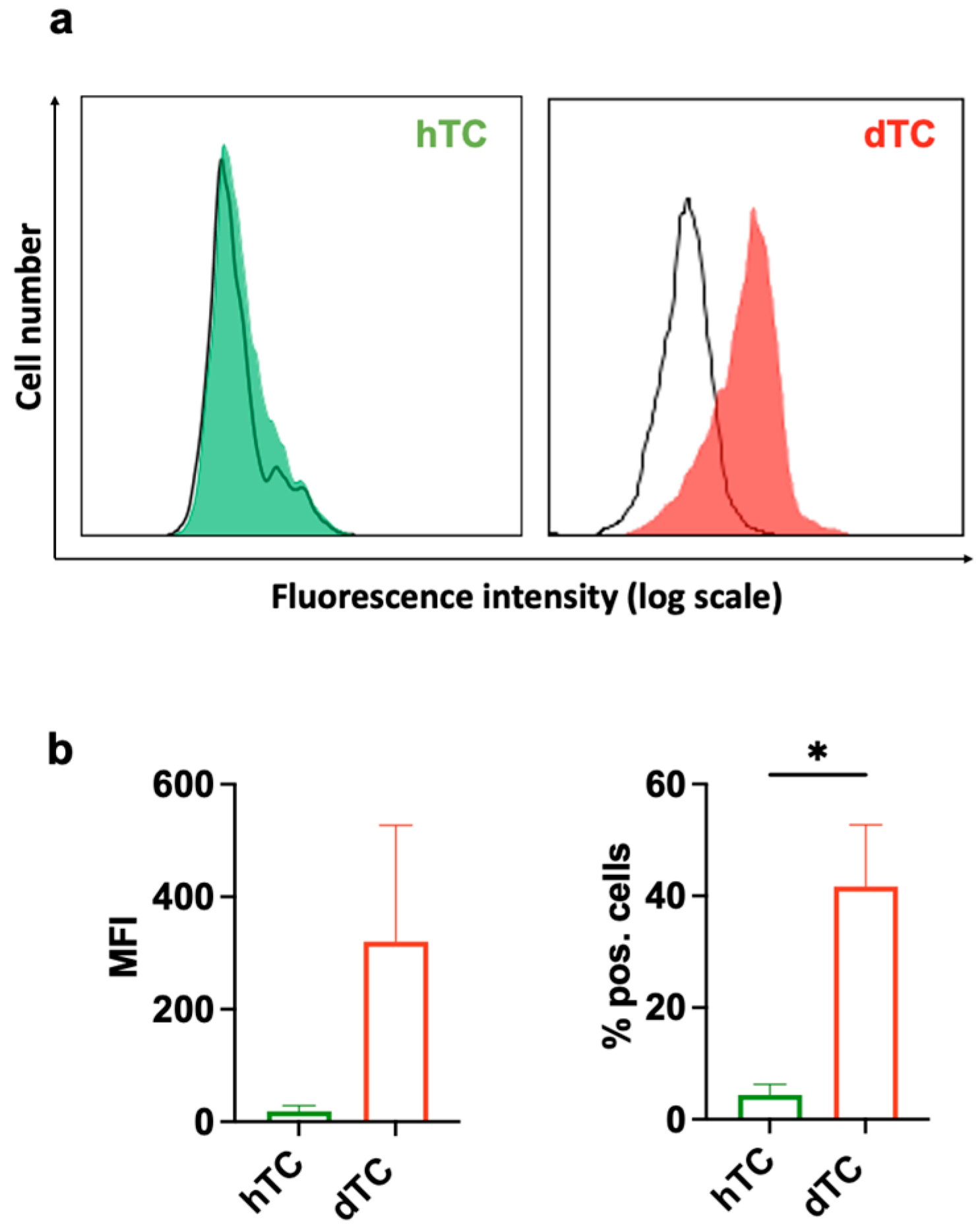
2.5. dTCs Expresses EGFR
3. Discussion
4. Materials and Methods
4.1. Isolation of PBMCs and Generation of Monocyte-Derived Dendritic Cells
4.2. Tenocytes and Fibroblasts
4.3. Cell Proliferation Assay
4.4. Collagen Production Analysis
4.5. RNA Isolation, cDNA Preparation, and Real-Time RT-PCR
4.6. Determination of AREG Production
4.7. Flow Cytometry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2023, 24, 641. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1beta Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Gregorio, A.D.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef]
- Stolk, M.; Klatte-Schulz, F.; Schmock, A.; Minkwitz, S.; Wildemann, B.; Seifert, M. New Insights into Tenocyte-Immune Cell Interplay in an in Vitro Model of Inflammation. Sci. Rep. 2017, 7, 9801. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Mottola, R.; Resta, G.; Gnasso, R.; Palermi, S.; Corrado, B.; Sirico, F.; Ruosi, C.; Aicale, R. Achilles Tendinopathy Pathogenesis and Management: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 6681. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue Plan for Achilles: Therapeutics Steering the Fate and Functions of Stem Cells in Tendon Wound Healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Citro, V.; Clerici, M.; Boccaccini, A.R.; Della Porta, G.; Maffulli, N.; Forsyth, N.R. Tendon Tissue Engineering: An Overview of Biologics to Promote Tendon Healing and Repair. J. Tissue Eng. 2023, 14, 20417314231196275. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; El Khatib, M.; Prencipe, G.; Citeroni, M.R.; Faydaver, M.; Mauro, A.; Berardinelli, P.; Cerveró-Varona, A.; Haidar-Montes, A.A.; Turriani, M.; et al. Tendon Immune Regeneration: Insights on the Synergetic Role of Stem and Immune Cells during Tendon Regeneration. Cells 2022, 11, 434. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057. [Google Scholar] [CrossRef] [PubMed]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. In Situ Investigation of the Oxidation of a Phospholipid Monolayer by Reactive Oxygen Species. Biophys. J. 2023, 122, 2007–2022. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.-L.; Binder, C.J.; Stöckl, J. Generation and Biological Activities of Oxidized Phospholipids. Antioxid. Redox Signal 2010, 12, 1009–1059. [Google Scholar] [CrossRef]
- Fu, P.; Birukov, K.G. Oxidized Phospholipids in Control of Inflammation and Endothelial Barrier. Transl. Res. 2009, 153, 166. [Google Scholar] [CrossRef]
- Blüml, S.; Kirchberger, S.; Bochkov, V.N.; Krönke, G.; Stuhlmeier, K.; Majdic, O.; Zlabinger, G.J.; Knapp, W.; Binder, B.R.; Stöckl, J.; et al. Oxidized Phospholipids Negatively Regulate Dendritic Cell Maturation Induced by TLRs and CD40. J. Immunol. 2005, 175, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Yang, J.; Wei, Y.; Wei, X. The Role of Oxidized Phospholipids in the Development of Disease. Mol. Asp. Med. 2020, 76, 100909. [Google Scholar] [CrossRef]
- Salomon, R.G. Structural Identification and Cardiovascular Activities of Oxidized Phospholipids. Circ. Res. 2012, 11, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Zupkovitz, G.; Kirchberger, S.; Seyerl, M.; Bochkov, V.N.; Stuhlmeier, K.; Majdic, O.; Zlabinger, G.J.; Seiser, C.; Stöckl, J. Epigenetic Regulation of Dendritic Cell Differentiation and Function by Oxidized Phospholipids. Blood 2009, 114, 5481–5489. [Google Scholar] [CrossRef]
- Tan, Y.; Li, J.-X.; Xiang, X.-D.; Lü, J.-L. Oxidized Low-Density Lipoprotein, OXPAPC, Corrects Defects in Maturation and Cytokine Secretion of Peripheral Blood Dendritic Cells from Sepsis Patients. Int. J. Clin. Exp. Med. 2014, 7, 2067–2073. [Google Scholar]
- Johnstone, S.R.; Ross, J.; Rizzo, M.J.; Straub, A.C.; Lampe, P.D.; Leitinger, N.; Isakson, B.E. Oxidized Phospholipid Species Promote in Vivo Differential Cx43 Phosphorylation and Vascular Smooth Muscle Cell Proliferation. Am. J. Pathol. 2009, 175, 916–924. [Google Scholar] [CrossRef]
- Cherepanova, O.A.; Pidkovka, N.A.; Sarmento, O.F.; Yoshida, T.; Gan, Q.; Adiguzel, E.; Bendeck, M.P.; Berliner, J.; Leitinger, N.; Owens, G.K. Oxidized Phospholipids Induce Type VIII Collagen Expression and Vascular Smooth Muscle Cell Migration. Circ. Res. 2009, 104, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ellis, I.M.; Schnabel, L.V.; Berglund, A.K. Defining the Profile: Characterizing Cytokines in Tendon Injury to Improve Clinical Therapy. J. Immunol. Regen. Med. 2022, 16, 2468–4988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Zhou, K.; Wu, D.; Yao, X.; Heng, B.C.; Zhou, J.; Liu, H.; Ouyang, H. Interplay of Forces and the Immune Response for Functional Tendon Regeneration. Front. Cell Dev. Biol. 2021, 9, 657621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, X.; Lu, J.; Hernigou, P.; Jin, F. The Role of Macrophage Polarization in Tendon Healing and Therapeutic Strategies: Insights from Animal Models. Front. Bioeng. Biotechnol. 2024, 12, 1366398. [Google Scholar] [CrossRef]
- Bles, N.; Pietrantonio, L.D.; Boeynaems, J.-M.; Communi, D. ATP Confers Tumorigenic Properties to Dendritic Cells by Inducing Amphiregulin Secretion. Blood 2010, 116, 3219–3226. [Google Scholar] [CrossRef]
- Minutti, C.M.; Modak, R.V.; Macdonald, F.; Kopf, M.; Henderson, N.C.; Zaiss, D.M.; Li, F.; Smyth, D.J.; Dorward, D.A.; Blair, N.; et al. A Macrophage-Pericyte Axis Directs Tissue Restoration via Amphiregulin-Induced Transforming Growth Factor Beta Activation. Immunity 2019, 50, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Sun, E.G.; Choi, J.N.; Kim, D.H.; Kim, J.H.; Ryu, K.H.; Shim, H.J.; Hwang, J.E.; Bae, W.K.; Kim, H.R.; et al. Fibroblast Growth Factor Receptor 4 Increases Epidermal Growth Factor Receptor (EGFR) Signaling by Inducing Amphiregulin Expression and Attenuates Response to EGFR Inhibitors in Colon Cancer. Cancer Sci. 2020, 111, 3268–3278. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Kagan, J.C. Innate Immune Detection of Lipid Oxidation as a Threat Assessment Strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef]
- Serbulea, V.; Deweese, D.; Leitinger, N. The Effect of Oxidized Phospholipids on Phenotypic Polarization and Function of Macrophages. Free Radic. Biol. Med. 2017, 111, 156–168. [Google Scholar] [CrossRef]
- Gioia, M.D.; Zanoni, I. Dooming Phagocyte Responses: Inflammatory Effects of Endogenous Oxidized Phospholipids. Front. Endocrinol. 2021, 12, 6268842. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Stuart, P.; Swindell, W.; Tsoi, L.; Li, B.; Gandarillas, A.; Lambert, S.; Johnston, A.; Nair, R.; Elder, J. The EGF Receptor Ligand Amphiregulin Controls Cell Division via FoxM1. Oncogene 2016, 35, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Platen, C.; Oppermann, L.; Doughty, C.; Ludwig, A.; Babendreyer, A.; Orlikowsky, T.W. EGF-Receptor against Amphiregulin (AREG) Influences Costimulatory Molecules on Monocytes and T Cells and Modulates T-Cell Responses. J. Immunol. Res. 2023, 202, 8883045. [Google Scholar] [CrossRef]
- Yusuf Zanna, M.; Rahaman Yasmin, A.; Rahman Omar, A.; Suri Arshad, S.; Razak Mariatulqabtiah, A.; Hamid Nur-Fazila, S.; Isa Nur Mahiza, M. Molecular Sciences Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
- Liu, K. Dendritic Cells. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 741–749. [Google Scholar]
- Mao, X.; Yao, L.; Li, M.; Zhang, X.; Weng, B.; Zhu, W.; Ni, R.; Chen, K.; Yi, L.; Zhao, J.; et al. Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization. Cells 2022, 11, 2770. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Clements, A.E.B.; Kink, J.A.; Choi, U.; Baer, G.S.; Halanski, M.A.; Hematti, P.; Vanderby, R. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 2019, 37, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The Role of the Macrophage in Tendinopathy and Tendon Healing. J. Orthop. Res. 2020, 38, 1666–1675. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert. Rev. Mol. Med. 2011, 13, 23. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. 2005, 87, 187–202. [Google Scholar]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719. [Google Scholar] [CrossRef]
- Sugg, K.B.; Lubardic, J.; Gumucio, J.P.; Mendias, C.L. Changes in Macrophage Phenotype and Induction of Epithelial-to-Mesenchymal Transition Genes Following Acute Achilles Tenotomy and Repair. J. Orthop. Res. 2014, 32, 944–951. [Google Scholar] [CrossRef] [PubMed]
- De La Durantaye, M.; Piette, A.B.; Van Rooijen, N.; Frenette, J. Macrophage Depletion Reduces Cell Proliferation and Extracellular Matrix Accumulation but Increases the Ultimate Tensile Strength of Injured Achilles Tendons. J. Orthop. Res. 2014, 32, 279–285. [Google Scholar] [CrossRef]
- Gao, N.; Yin, J.; Sang Yoon, G.; Mi, Q.-S.; Yu, F.-S.X. Dendritic Cell-Epithelium Interplay Is a Determinant Factor for Corneal Epithelial Wound Repair. Am. J. Pathol. 2011, 179, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, J.; Meller, S.; Conrad, C.; Di Nardo, A.; Homey, B.; Lauerma, A.; Arai, N.; Gallo, R.L.; DiGiovanni, J.; Gilliet, M. Plasmacytoid Dendritic Cells Sense Skin Injury and Promote Wound Healing through Type i Interferons. J. Exp. Med. 2010, 207, 2921–2930. [Google Scholar] [CrossRef]
- D’Arpa, N.; Accardo-Palumbo, A.; Amato, G.; D’Amelio, L.; Pileri, D.; Cataldo, V.; Mogavero, R.; Lombardo, C.; Napoli, B.; Conte, F. Circulating Dendritic Cells Following Burn. Burns 2009, 35, 513–518. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Vinish, M.; Cui, W.; Stafford, E.; Bae, L.; Hawkins, H.; Cox, R.; Toliver-Kinsky, T. Dendritic Cells Modulate Burn Wound Healing by Enhancing Early Proliferation. Wound Repair Regen. 2016, 24, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Henn, D.; Zhao, D.; Sivaraj, D.; Trotsyuk, A.; Bonham, C.A.; Fischer, K.S.; Kehl, T.; Fehlmann, T.; Greco, A.H.; Kussie, H.C.; et al. Cas9-Mediated Knockout of Ndrg2 Enhances the Regenerative Potential of Dendritic Cells for Wound Healing. Nat. Commun. 2023, 14, 4729. [Google Scholar] [CrossRef] [PubMed]
- Seyerl, M.; Blüml, S.; Kirchberger, S.; Bochkov, V.N.; Oskolkova, O.; Majdic, O.; Stöckl, J. Oxidized Phospholipids Induce Anergy in Human Peripheral Blood T Cells. Eur. J. Immunol. 2008, 38, 778–787. [Google Scholar] [CrossRef]
- Bochkov, V.; Gesslbauer, B.; Mauerhofer, C.; Philippova, M.; Erne, P.; Oskolkova, O. V Pleiotropic Effects of Oxidized Phospholipids. Free Radic. Biol. Med. 2017, 111, 6–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, J.-Y.; Lee, C.-M.; Cho, W.-K.; Kang, M.-J.; Koff, J.L.; Yoon, P.-O.; Chae, J.; Park, H.-O.; Elias, J.A.; et al. Amphiregulin, an Epidermal Growth Factor Receptor Ligand, Plays an Essential Role in the Pathogenesis of Transforming Growth Factor-Induced Pulmonary Fibrosis. J. Biol. Chem. 2012, 287, 41991–42000. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Morton, P.E.; Takeichi, T.; Salam, A.; Roberts, N.; Proudfoot, L.E.; Mellerio, J.E.; Aminu, K.; Wellington, C.; Patil, S.N.; et al. Epithelial Inflammation Resulting from an Inherited Loss-of-Function Mutation in EGFR. J. Investig. Dermatol. 2014, 134, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, V.; Schwerdt, G.; Schreier, B.; Kopf, M.; Mildenberger, S.; Benndorf, R.A.; Gekle, M. EGFR Activation Differentially Affects the Inflammatory Profiles of Female Human Aortic and Coronary Artery Endothelial Cells. Sci. Rep. 2023, 13, 22827. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediat. Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef] [PubMed]
- Ciardulli, M.C.; Scala, P.; Giudice, V.; Santoro, A.; Selleri, C.; Oliva, F.; Maffulli, N.; Della Porta, G. Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone. Cells 2022, 11, 2545. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Adamovic, A.; Ruttensteiner, L.; Steiner, K.; Tajpara, P.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Cytotoxicity of Lecithin-Based Nanoemulsions on Human Skin Cells and Ex Vivo Skin Permeation: Comparison to Conventional Surfactant Types. Int. J. Pharm. 2019, 566, 383–390. [Google Scholar] [CrossRef]
- Steiner, K.; Josef Schmolz, J.; Hoang, F.; Wolf, H.; Seiser, S.; Elbe-Bürger, A.; Klang, V. Surfactants for Stabilization of Dermal Emulsions and Their Skin Compatibility under UVA Irradiation: Diacyl Phospholipids and Polysorbate 80 Result in High Viability Rates of Primary Human Skin Cells. Int. J. Pharm. 2024, 653, 123903. [Google Scholar] [CrossRef]
- Majdic, O.; Ligeti, E.; Stöckl, J.; Kirchberger, S.; Oskolkova, O.; Bochkov, V.N.; Blüml, S.; Rosc, B.; Lorincz, A.; Seyerl, M. The Oxidation State of Phospholipids Controls the Oxidative Burst in Neutrophil Granulocytes. J. Immunol. Ref. 2008, 181, 4347–4353. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinnarò, V.; Kirchberger, S.; Künig, S.; Gil Cantero, S.; Ciardulli, M.C.; Della Porta, G.; Blüml, S.; Elbe-Bürger, A.; Bochkov, V.; Stöckl, J. Oxidized Phospholipids Regulate Tenocyte Function via Induction of Amphiregulin in Dendritic Cells. Int. J. Mol. Sci. 2024, 25, 7600. https://doi.org/10.3390/ijms25147600
Pinnarò V, Kirchberger S, Künig S, Gil Cantero S, Ciardulli MC, Della Porta G, Blüml S, Elbe-Bürger A, Bochkov V, Stöckl J. Oxidized Phospholipids Regulate Tenocyte Function via Induction of Amphiregulin in Dendritic Cells. International Journal of Molecular Sciences. 2024; 25(14):7600. https://doi.org/10.3390/ijms25147600
Chicago/Turabian StylePinnarò, Veronica, Stefanie Kirchberger, Sarojinidevi Künig, Sara Gil Cantero, Maria Camilla Ciardulli, Giovanna Della Porta, Stephan Blüml, Adelheid Elbe-Bürger, Valery Bochkov, and Johannes Stöckl. 2024. "Oxidized Phospholipids Regulate Tenocyte Function via Induction of Amphiregulin in Dendritic Cells" International Journal of Molecular Sciences 25, no. 14: 7600. https://doi.org/10.3390/ijms25147600
APA StylePinnarò, V., Kirchberger, S., Künig, S., Gil Cantero, S., Ciardulli, M. C., Della Porta, G., Blüml, S., Elbe-Bürger, A., Bochkov, V., & Stöckl, J. (2024). Oxidized Phospholipids Regulate Tenocyte Function via Induction of Amphiregulin in Dendritic Cells. International Journal of Molecular Sciences, 25(14), 7600. https://doi.org/10.3390/ijms25147600







