Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood
Abstract
:1. Introduction
2. Results
2.1. Participants
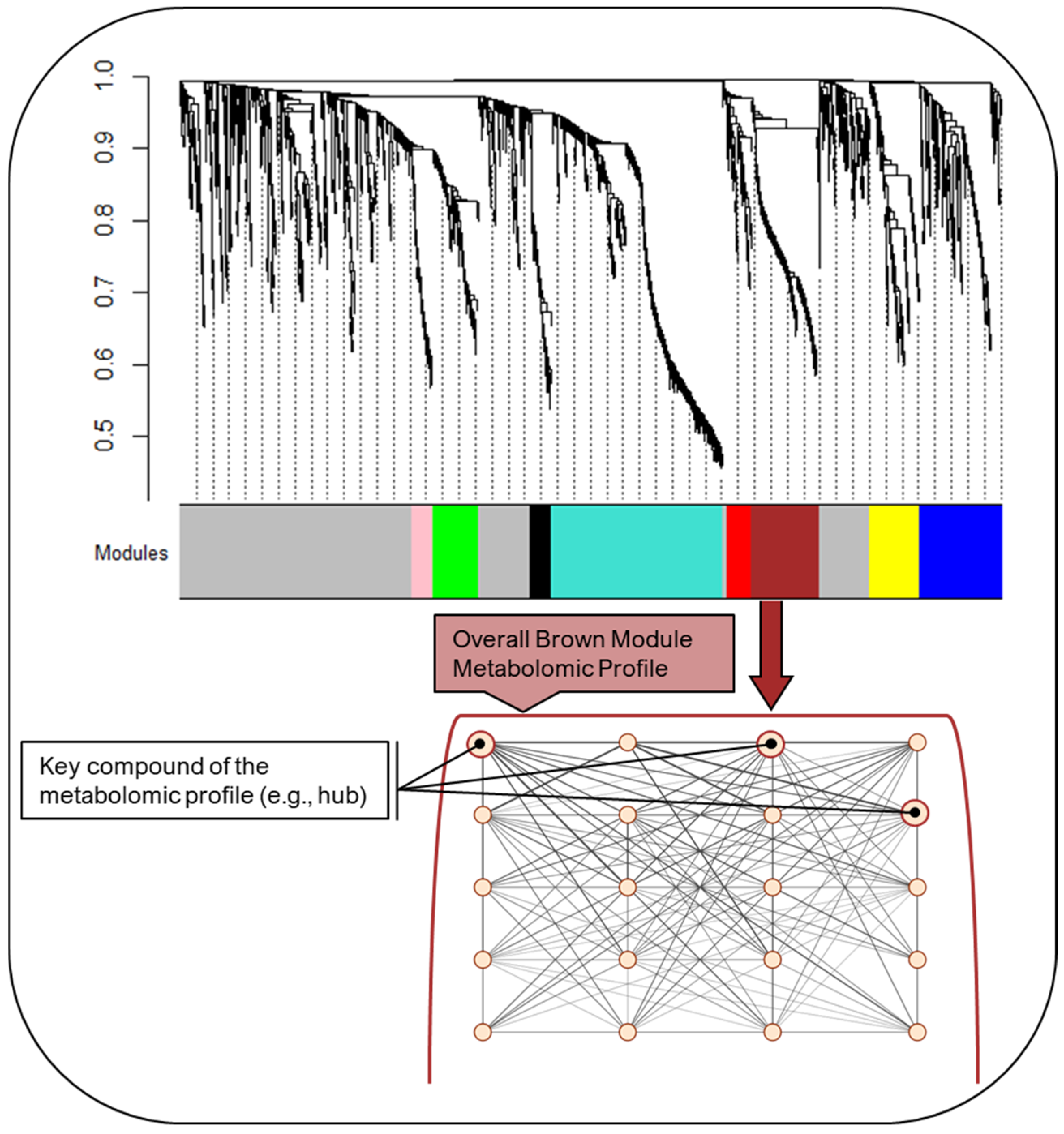
2.2. Maternal Mid-Pregnancy Metabolomic Profiling Modules
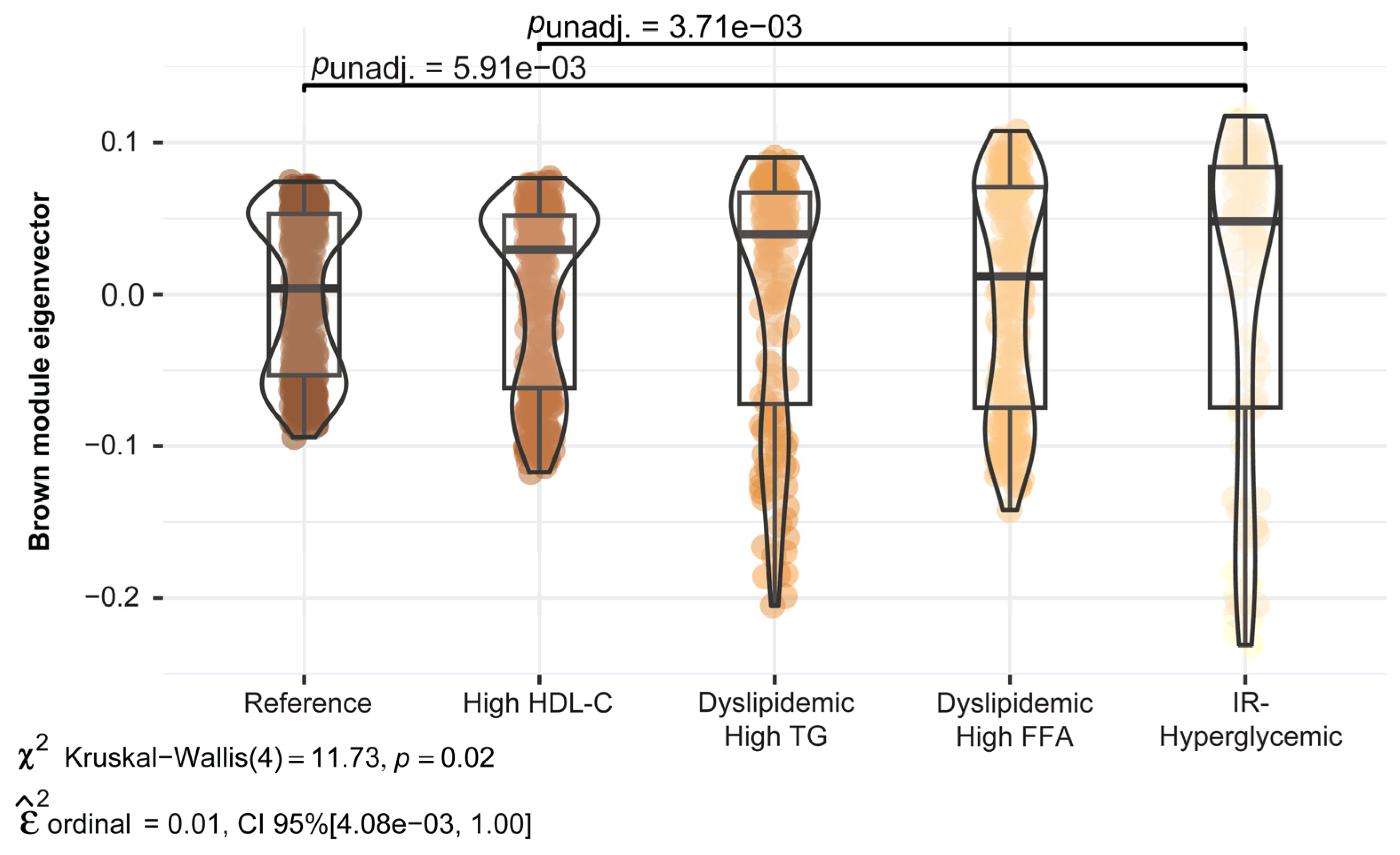
2.3. Sensitivity Analysis
3. Discussion
Study Strengths and Limitations
4. Materials and Methods
4.1. Setting and Participants
4.2. Sociodemographic, Lifestyle, and Perinatal Characteristics Data
4.3. Metabolic Subgroups Based on Conventional Metabolic Biomarkers: Laboratory Methods, Subgroup Derivation, and Analytic Approach
4.4. Untargeted Metabolomic Profiling: Laboratory Methods, Profile Derivation, and Analytical Approach
4.5. Sensitivity Analysis
4.6. Missing Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, E.C.; Kechris, K.; Cohen, C.C.; Michelotti, G.; Dabelea, D.; Perng, W. Metabolomic Profiles in Childhood and Adolescence Are Associated with Fetal Overnutrition. Metabolites 2022, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Allard, C.; Battista, M.-C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.-F. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Hivert, M.F.; Udler, M.S. Defining Heterogeneity Among Women with Gestational Diabetes Mellitus. Diabetes 2020, 69, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Begum, S.I.; Vieira, M.C.; Seed, P.; Lawlor, D.L.; Sattar, N.; Nelson, S.M.; Welsh, P.; Pasupathy, D.; Poston, L.; et al. Metabolic phenotyping by treatment modality in obese women with gestational diabetes suggests diverse pathophysiology: An exploratory study. PLoS ONE 2020, 15, e0230658. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Pasupathy, D.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; Briley, A.L.; Seed, P.T.; Welsh, P.; Poston, L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 2017, 60, 1903–1912. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Powe, C.E.; Lowe, W.L., Jr.; White, S.L.; Scholtens, D.M.; Yang, J.; Zhu, Y.; Zhang, C.; Hivert, M.F.; Kwak, S.H.; et al. Refining the diagnosis of gestational diabetes mellitus: A systematic review and meta-analysis. Commun. Med. 2023, 3, 185. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Oken, E.; Dabelea, D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019, 62, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, A.; Gregory, E. Increases in Prepregnancy Obesity: United States, 2016–2019; NCHS Data Brief, No 392; National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Gregory, E.C.; Ely, D.M. Trends and Characteristics in Gestational Diabetes: United States, 2016–2020. Natl. Vital Stat. Rep. 2022, 71, 1–15. [Google Scholar]
- Hernandez, T.L.; Friedman, J.E.; Barbour, L.A. Insulin Resistance in Pregnancy: Implications for Mother and Offspring. In Insulin Resistance: Childhood Precursors of Adult Disease; Zeitler, P.S., Nadeau, K.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 67–94. [Google Scholar]
- Barbour, L.A.; Hernandez, T.L. Maternal Non-glycemic Contributors to Fetal Growth in Obesity and Gestational Diabetes: Spotlight on Lipids. Curr. Diabetes Rep. 2018, 18, 37. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Brinton, J.T.; Glueck, D.H.; Shapiro, A.L.; Harrod, C.S.; Lynch, A.M.; Siega-Riz, A.; Dabelea, D. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am. J. Clin. Nutr. 2015, 101, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Kechris, K.; Jansson, T.; Dabelea, D.; Perng, W. Novel Metabolic Subtypes in Pregnant Women and Risk of Early Childhood Obesity in Offspring. JAMA Netw. Open 2023, 6, e237030. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.F.; Perng, W.; Watkins, S.M.; Newgard, C.S.; Kenny, L.C.; Kristal, B.S.; Patti, M.E.; Isganaitis, E.; DeMeo, D.L.; Oken, E.; et al. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. J. Dev. Orig. Health Dis. 2015, 6, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, W.E.; Buhimschi, C.S.; Brown, T.L.; Zhao, G.; Summerfield, T.L.; Buhimschi, I.A. Transcriptomics-Based Subphenotyping of the Human Placenta Enabled by Weighted Correlation Network Analysis in Early-Onset Preeclampsia with and without Fetal Growth Restriction. Hypertension 2023, 80, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, B.; Liu, Y.; Aung, M.T.; Rosario-Pabón, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D.; Garmire, L.X. Maternal plasma lipids are involved in the pathogenesis of preterm birth. Gigascience 2022, 11, giac004. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Doyon, M.; Arguin, M.; Perron, P.; Bouchard, L.; Hivert, M.-F. A prospective study of maternal adiposity and glycemic traits across pregnancy and mid-childhood metabolomic profiles. Int. J. Obes. 2021, 45, 860–869. [Google Scholar] [CrossRef]
- Scholtens, D.M.; Bain, J.R.; Reisetter, A.C.; Muehlbauer, M.J.; Nodzenski, M.; Stevens, R.D.; Ilkayeva, O.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Metabolic Networks and Metabolites Underlie Associations Between Maternal Glucose During Pregnancy and Newborn Size at Birth. Diabetes 2016, 65, 2039–2050. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, L.; Lv, Y.; Miao, Z.; Wang, Y.; Yan, J.; Li, J.; Li, C.; Ding, H. LC-MS/MS based untargeted lipidomics uncovers lipid signatures of late-onset preeclampsia. Biochimie 2023, 208, 46–55. [Google Scholar] [CrossRef]
- Zhang, L.; Bi, S.; Liang, Y.; Huang, L.; Li, Y.; Huang, M.; Huang, B.; Deng, W.; Liang, J.; Gu, S.; et al. Integrated Metabolomic and Lipidomic Analysis in the Placenta of Preeclampsia. Front. Physiol. 2022, 13, 807583. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shih, A.Z.L.; Woo, Y.C.; Fong, C.H.Y.; Leung, O.Y.; Janus, E.; Cheung, B.M.Y.; Lam, K.S.L. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A 15-Year Prospective Study in Chinese. PLoS ONE 2016, 11, e0163424. [Google Scholar] [CrossRef]
- Melmed, S.; Auchus, R.J.; Goldfine, A.B.; Koenig, R.; Rosen, C.J.; Williams, R.H. Williams textbook of endocrinology. In Textbook of Endocrinology, 14th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Ghasemi, A.; Tohidi, M.; Derakhshan, A.; Hasheminia, M.; Azizi, F.; Hadaegh, F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015, 52, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Leiva Sisnieguez, C.E.; Balbín, E.; Dulbecco, C.A.; Aizpurúa, M.; Marillet, A.G.; Reaven, G.M. Relation Among the Plasma Triglyceride/High-Density Lipoprotein Cholesterol Concentration Ratio, Insulin Resistance, and Associated Cardio-Metabolic Risk Factors in Men and Women. Am. J. Cardiol. 2012, 109, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; Farabi, S.S.; Friedman, J.E.; Hirsch, N.M.; Reece, M.S.; Van Pelt, R.E.; Hernandez, T.L. Postprandial Triglycerides Predict Newborn Fat More Strongly than Glucose in Women with Obesity in Early Pregnancy. Obesity 2018, 26, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Slotkowski, R.; VanOrmer, M.; Akbar, A.; Hahka, T.; Thompson, M.; Rapoza, R.; Ulu, A.; Thoene, M.; Lyden, E.; Mukherjee, M.; et al. Bioactive metabolites of OMEGA-6 and OMEGA-3 fatty acids are associated with inflammatory cytokine concentrations in maternal and infant plasma at the time of delivery. Clin. Nutr. ESPEN 2024, 60, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, P.; Narayanan, V.; Zhu, D.; Medhora, M.; Jacobs, E.R.; Chandramohan, Y.; Selvaraj, V.; Dhanasekaran, A. Prophylactic supplementation of 20-HETE ameliorates hypoxia/reoxygenation injury in pulmonary vascular endothelial cells by inhibiting apoptosis. Acta Histochem. 2020, 122, 151461. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Chapter Two—Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. In Advances in Pharmacology; Hardwick, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 35–84. [Google Scholar]
- Huang, Y.; Gao, S.; Chen, J.; Albrecht, E.; Zhao, R.; Yang, X. Maternal butyrate supplementation induces insulin resistance associated with enhanced intramuscular fat deposition in the offspring. Oncotarget 2017, 8, 13073–13084. [Google Scholar] [CrossRef]
- Mathew, O.P.; Ranganna, K.; Yatsu, F.M. Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells. Biomed Pharmacother 2010, 64, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Corcoran, T.B.; Mas, E.; Durand, T.; Galano, J.M.; Roberts, L.J.; Paech, M.; Muchatuta, N.A.; Phillips, M.; Mori, T.A. Is there a role for isofurans and neuroprostanes in pre-eclampsia and normal pregnancy? Antioxid Redox Signal 2012, 16, 165–169. [Google Scholar] [CrossRef]
- Gallardo, J.M.; Gómez-López, J.; Medina-Bravo, P.; Juárez-Sánchez, F.; Contreras-Ramos, A.; Galicia-Esquivel, M.; Sánchez-Urbina, R.; Klünder-Klünder, M. Maternal obesity increases oxidative stress in the newborn. Obesity 2015, 23, 1650–1654. [Google Scholar] [CrossRef]
- Loy, S.L.; Sirajudeen, K.N.; Hamid Jan, J.M. The effects of prenatal oxidative stress levels on infant adiposity development during the first year of life. J. Dev. Orig. Health Dis. 2014, 5, 142–151. [Google Scholar] [CrossRef]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.M.; Keil, A.P.; van ‘t Erve, T.J.; Deterding, L.J.; Williams, J.G.; Lih, F.B.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Longitudinal profiles of plasma eicosanoids during pregnancy and size for gestational age at delivery: A nested case-control study. PLoS Med. 2020, 17, e1003271. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy. Antioxidants 2023, 12, 994. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, Z. Serum acylcarnitines levels as a potential predictor for gestational diabetes: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1217237. [Google Scholar] [CrossRef]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Harrod, C.S.; Fingerlin, T.E.; Chasan-Taber, L.; Reynolds, R.M.; Glueck, D.H.; Dabelea, D. Exposure to prenatal smoking and early-life body composition: The healthy start study. Obesity 2015, 23, 234–241. [Google Scholar] [CrossRef]
- Flanagin, A.; Frey, T.; Christiansen, S.L.; AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef]
- Crume, T.L.; Shapiro, A.L.; Brinton, J.T.; Glueck, D.H.; Martinez, M.; Kohn, M.; Harrod, C.; Friedman, J.E.; Dabelea, D. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: The healthy start study. J. Clin. Endocrinol. Metab. 2015, 100, 1672–1680. [Google Scholar] [CrossRef]
- Francis, E.C.; Dabelea, D.; Ringham, B.M.; Sauder, K.A.; Perng, W. Maternal blood glucose level and offspring glucose-insulin homeostasis: What is the role of offspring adiposity? Diabetologia 2021, 64, 83. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. National Institutes of Health consensus development conference statement: Diagnosing gestational diabetes mellitus, March 4–6, 2013. Obstet Gynecol. 2013, 122 Pt 1, 358–369. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Perez De Souza, L.; Alseekh, S.; Brotman, Y.; Fernie, A.R. Network-based strategies in metabolomics data analysis and interpretation: From molecular networking to biological interpretation. Expert Rev. Proteom. 2020, 17, 243–255. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Horvath, S.; Geschwind, D.H. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 12698–12703. [Google Scholar] [CrossRef]
- Langfelder, P.; Luo, R.; Oldham, M.C.; Horvath, S. Is my network module preserved and reproducible? PLoS Comput. Biol. 2011, 7, e1001057. [Google Scholar] [CrossRef]

| Full Sample | Reference | High HDL-C | Dyslipidemic–High TG | Dyslipidemic–High FFA | IR–Hyperglycemic | ||
|---|---|---|---|---|---|---|---|
| Maternal Characteristics: | (n = 1065) | (n = 360) | (n = 289) | (n = 149) | (n = 180) | (n = 87) | p-Value |
| Age, years; mean ± SD | 28.1 ± 6.2 | 27.9 ± 6.5 | 29.1 ± 5.7 | 28.6 ± 5.9 | 27.1 ± 6.3 | 26.2 ± 6.3 | <0.001 |
| race/ethnicity (%, n) | <0.001 | ||||||
| Hispanic | 23.4 (249) | 59.4 (214) | 65.1 (188) | 55.7 (83) | 43.3 (78) | 32.2 (28) | |
| Non-Hispanic Black | 15.1 (161) | 19.4 (70) | 11.8 (34) | 4.7 (7) | 18.9 (34) | 18.4 (16) | |
| Non-Hispanic White | 55.5 (591) | 16.1 (58) | 17.3 (50) | 30.9 (46) | 33.3 (60) | 40.2 (35) | |
| Non-Hispanic Other a | 6.0 (64) | 5.0 (18) | 5.9 (17) | 8.7 (13) | 4.4 (8) | 9.2 (8) | |
| Education (%, n) | <0.001 | ||||||
| High school or less | 30.8 (328) | 30.3 (109) | 18.0 (52) | 37.6 (56) | 36.7 (66) | 51.7 (45) | |
| Some college/assoc. degree | 22.4 (238) | 17.2 (62) | 23.5 (68) | 25.5 (38) | 26.1 (47) | 26.4 (23) | |
| College graduate | 23.9 (255) | 23.3 (84) | 28.7 (83) | 22.8 (34) | 22.2 (40) | 16.1 (14) | |
| Graduate degree | 22.9 (244) | 29.2 (105) | 29.8 (86) | 14.1 (21) | 15.0 (27) | 5.8 (5) | |
| Nulliparous (%, n) | 48.5 (516) | 47.5 (171) | 57.1 (165) | 40.3 (60) | 47.2 (85) | 40.2 (35) | 0.002 |
| Smoked during pregnancy (%, n) | 8.1 (87) | 9.2 (33) | 3.5 (10) | 13.4 (20) | 8.3 (15) | 10.3 (9) | 0.002 |
| Pre-pregnancy BMI; mean ± SD | 25.5 ± 6.0 | 23.8 ± 4.5 | 23.8 ± 4.4 | 27.1 ± 5.2 | 26.5 ± 6.6 | 33.1 ± 8.5 | <0.001 |
| Pre-pregnancy BMI ≥30.0 kg/m2 (%, n) | 18.5 (196) | 10.3 (37) | 7.6 (22) | 28.2 (42) | 24.7 (44) | 58.6 (51) | <0.001 |
| Gestational diabetes mellitus (%, n) | 4.4 (43) | 1.2 (4) | 2.6 (7) | 6.0 (8) | 6.0 (10) | 17.3 (14) | <0.001 |
| Gestational weight gain (%, n) | 0.029 | ||||||
| Insufficient | 23.5 (249) | 25.4 (91) | 19.8 (57) | 25.5 (38) | 23.6 (42) | 24.1 (21) | |
| Adequate | 29.2 (309) | 31.3 (112) | 29.9 (86) | 30.2 (45) | 27.0 (48) | 20.7 (18) | |
| Excessive | 47.4 (502) | 43.3 (155) | 50.4 (145) | 44.3 (66) | 49.4 (88) | 55.2 (48) | |
| Biomarkers ~18 gestational weeks (%, n) | |||||||
| Glucose ≥ 95 mg/dL | 1.5 (16) | 0.8 (3) | 0.4 (1) | 0.7 (1) | 0.0 (0) | 12.6 (11) | <0.001 |
| Insulin ≥ 25 uIU/mL | 8.4 (88) | 0.3 (1) | 0.4 (1) | 8.2 (12) | 2.3 (4) | 81.4 (70) | <0.001 |
| HOMA-IR ≥ 2.9 | 24.5 (255) | 12.6 (44) | 10.1 (29) | 44.5 (65) | 18.3 (32) | 100.0 (85) | <0.001 |
| TGs:HDL-C ≥ 2.5 | 27.0 (283) | 9.1 (32) | 5.2 (15) | 95.9 (139) | 28.8 (51) | 52.9 (46) | <0.001 |
| TGs ≥ 150 mg/dL | 23.8 (249) | 3.7 (13) | 15.9 (46) | 90.3 (131) | 13.0 (23) | 41.4 (36) | <0.001 |
| Total-C ≥ 200 mg/dL | 31.2 (327) | 4.0 (14) | 72.7 (210) | 46.2 (67) | 11.3 (20) | 18.4 (16) | <0.001 |
| HDL-C ≤ 50 mg/dL | 20.3 (213) | 19.4 (68) | 0.0 (0) | 43.5 (63) | 27.7 (49) | 37.9 (33) | <0.001 |
| FFAs ≥ 472 µEq/L | 24.4 (253) | 0.3 (1) | 13.2 (38) | 27.6 (40) | 81.7 (143) | 35.6 (31) | <0.001 |
| TNF-α ≥ 1.36 pg/mL | 25.3 (266) | 27.5 (97) | 22.8 (66) | 26.2 (38) | 26.1 (46) | 21.8 (19) | 0.646 |
| Module Color Label | Module Size (# of Features) | Mean Connectivity | Pathway Enrichment |
|---|---|---|---|
| Pink | 59 | 21.2 | Pyruvate metabolism Glycine, serine, alanine and threonine metabolism |
| Black | 60 | 17.7 | Tyrosine metabolism Aspartate and asparagine metabolism Glycine, serine, alanine and threonine metabolism |
| * Red | 69 | 7.3 | Urea cycle/amino group metabolism Aspartate and asparagine metabolism |
| Green | 130 | 24.9 | Squalene and cholesterol biosynthesis Hexose phosphorylation Fatty acid metabolism |
| * Yellow | 138 | 14.5 | Bile acid biosynthesis Linoleate metabolism Fatty acid activation and metabolism De novo fatty acid biosynthesis Omega-3 fatty acid metabolism C21-steroid hormone biosynthesis and metabolism |
| * Brown | 189 | 30.5 | Androgen and estrogen biosynthesis and metabolism C21-steroid hormone biosynthesis and metabolism Cytochrome P450 enzymes D4 & E4-neuroprostanes formation Biopterin metabolism Butyrate metabolism |
| * Blue | 231 | 8.9 | Carnitine shuttle De novo fatty acid biosynthesis Vitamin A (retinol) metabolism Glycerophospholipid metabolism |
| Turquoise | 400 | 50.1 | Tryptophan metabolism Tyrosine metabolism Aspartate and asparagine metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, E.C.; Kechris, K.; Johnson, R.K.; Rawal, S.; Pathmasiri, W.; Rushing, B.R.; Du, X.; Jansson, T.; Dabelea, D.; Sumner, S.J.; et al. Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood. Int. J. Mol. Sci. 2024, 25, 7620. https://doi.org/10.3390/ijms25147620
Francis EC, Kechris K, Johnson RK, Rawal S, Pathmasiri W, Rushing BR, Du X, Jansson T, Dabelea D, Sumner SJ, et al. Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood. International Journal of Molecular Sciences. 2024; 25(14):7620. https://doi.org/10.3390/ijms25147620
Chicago/Turabian StyleFrancis, Ellen C., Katerina Kechris, Randi K. Johnson, Shristi Rawal, Wimal Pathmasiri, Blake R. Rushing, Xiuxia Du, Thomas Jansson, Dana Dabelea, Susan J. Sumner, and et al. 2024. "Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood" International Journal of Molecular Sciences 25, no. 14: 7620. https://doi.org/10.3390/ijms25147620









