Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride
Abstract
:1. Introduction
2. Preparation Methods of g-C3N4
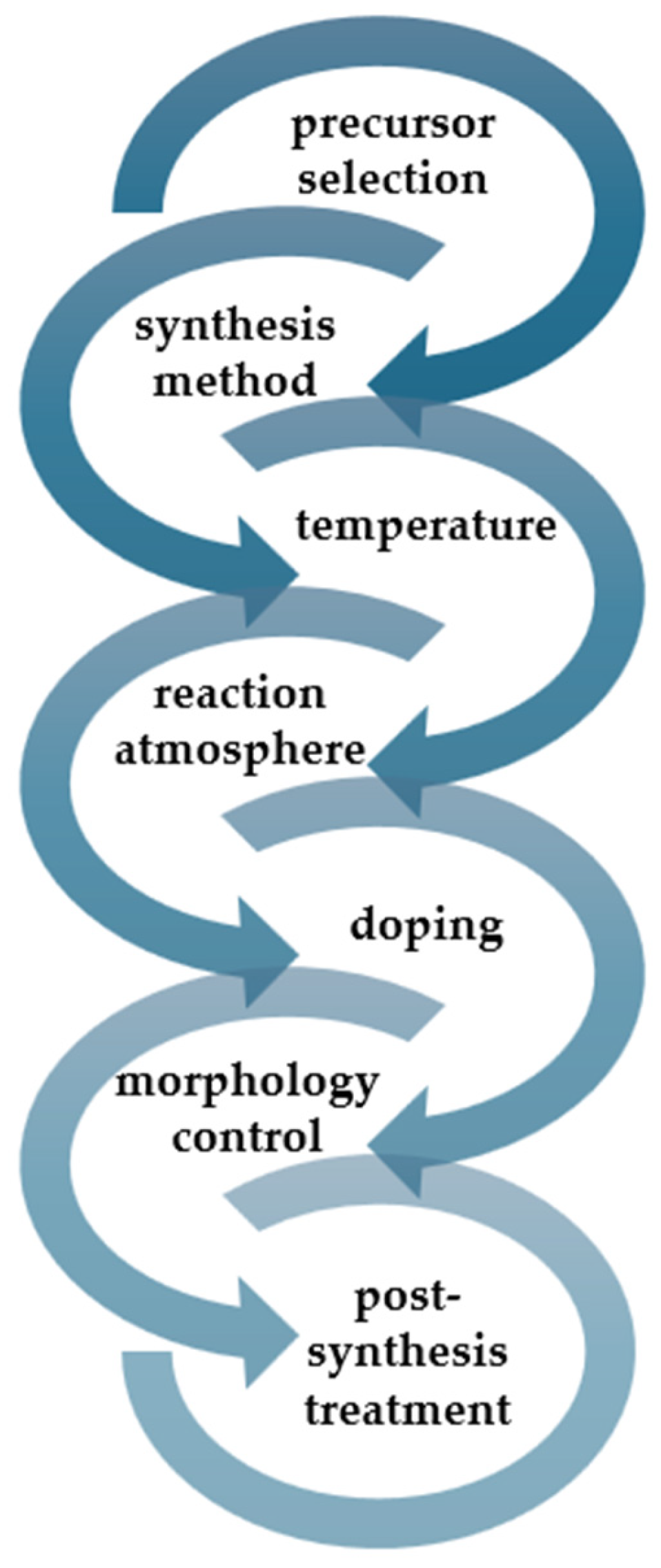
2.1. General Conditions and Precursors of g-C3N4 Green Synthesis
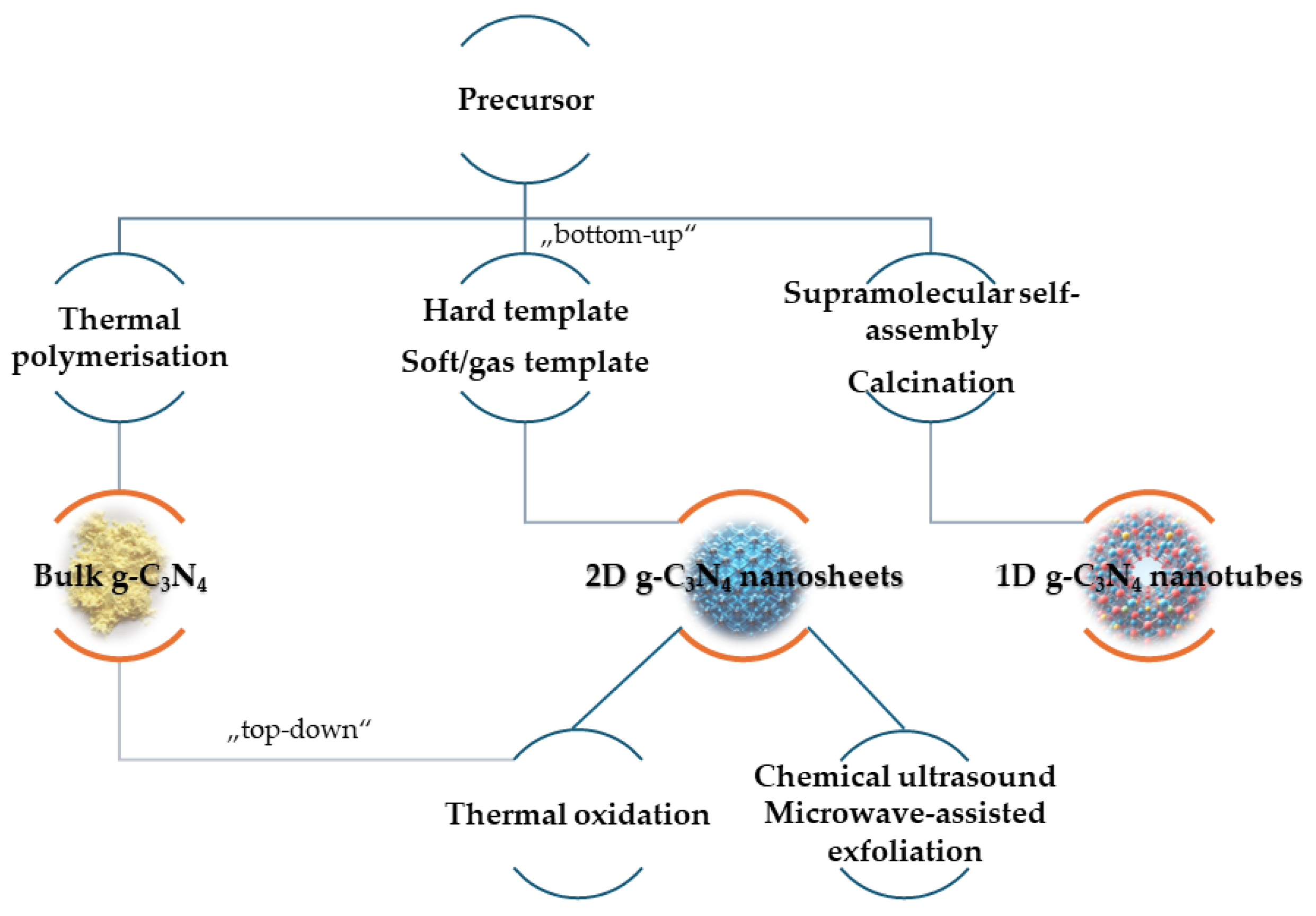
2.2. Preparation of g-C3N4 Nanomaterials
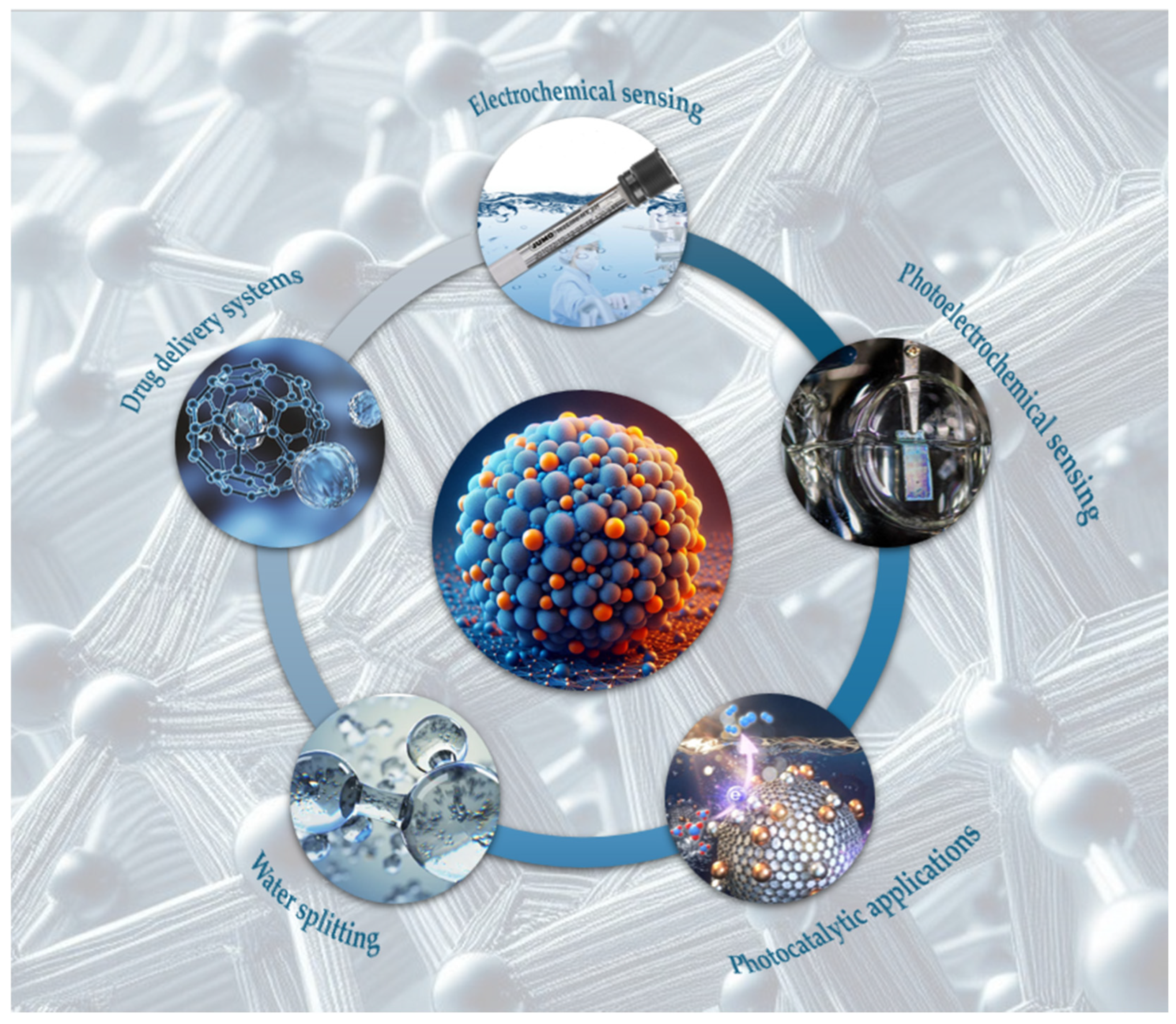
3. Applications of g-C3N4
3.1. Electrochemical Sensing
3.2. Photoelectrochemical Sensing
3.3. Photocatalytic Applications
3.4. Watter Splitting
3.5. Drug Delivery Systems and Phototherapy
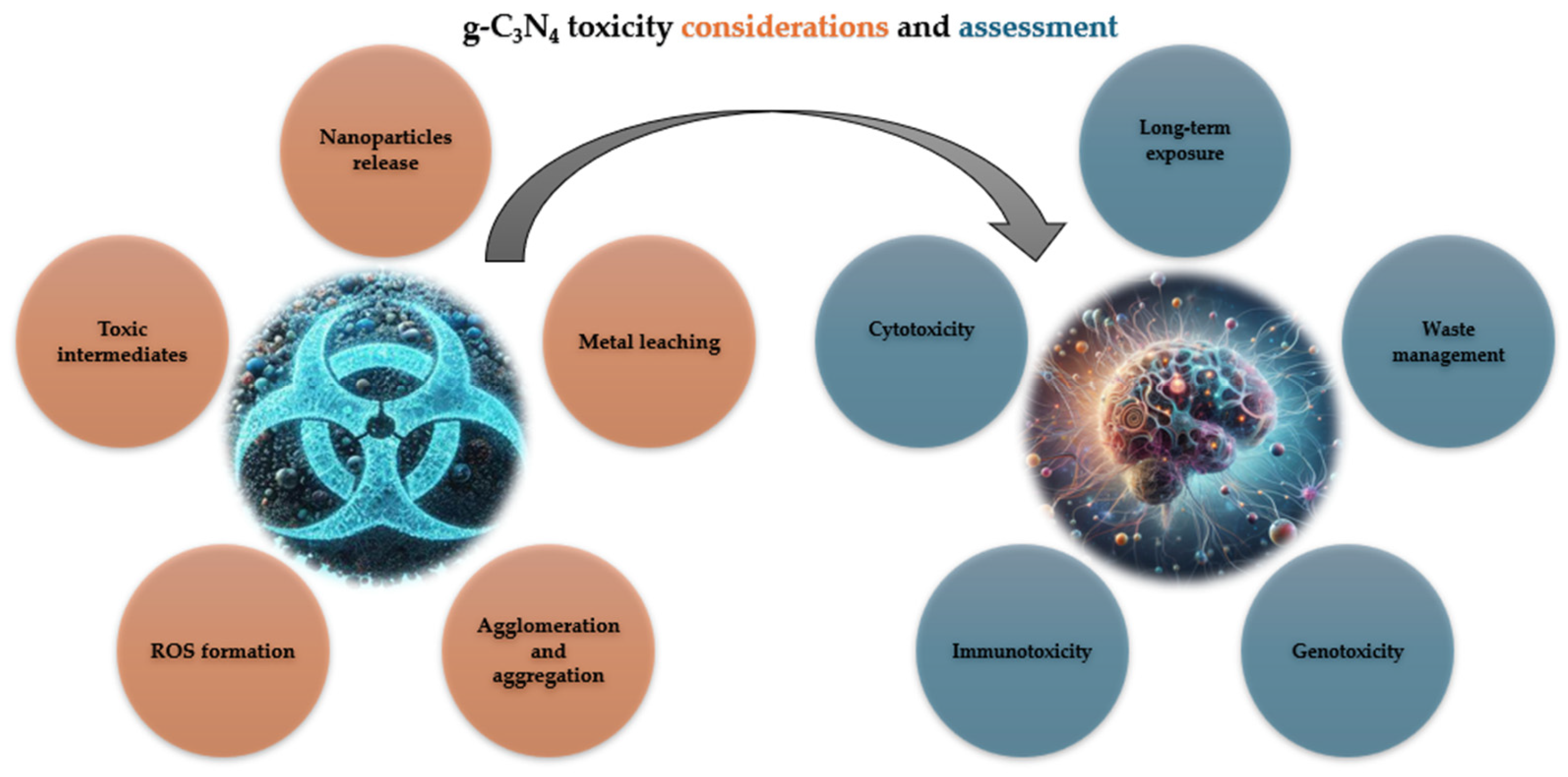
4. Toxicity Considerations of g-C3N4
5. Assessment of g-C3N4 Toxicity
5.1. In Vitro Cytotoxicity Assessment on Human Cells
| Material Type | Cell Line | Methods | Concentration | Results | Ref. |
|---|---|---|---|---|---|
| g-C3N4 nanosheets | HeLa | MTT assay | 600 μg/mL | No cytotoxic effect even at a high concentration of g-C3N4. | [77] |
| Bulk g-C3N4 | A549 | MTT assay | 5–100 μg/mL | Dose-dependent decrease in cell viability. | [78] |
| g-C3N4 nanosheets | Better biocompatibility compared to bulk material. | ||||
| t-C3N4 | MTT, WST-8 assay | 25–500 μg/mL | t-C3N4 exhibited higher toxicity compared to h-C3N4. | [80] | |
| h-C3N4 | |||||
| Bulk g-C3N4 | Saos-2 | MTT assay, fluorescence microscopy | 12.5–200 μg/mL | 48-h IC50 = 104.0 ± 8.5 μg/mL. | [79] |
| g-C3N4 nanosheets | 48-h IC50 = 27.0 ± 4.2 μg/mL. | ||||
| Bulk g-C3N4 | HFF | 48-h IC50 | No cytotoxic effect. | ||
| g-C3N4 nanosheets | |||||
| COOH-rich g-C3N4 nanosheets | MCF-7 | CCK-8 assay, fluorescence microscopy | up to 400 μg/mL | No cytotoxic effect. | [81] |
| NH3-rich g-C3N4 nanosheets | |||||
| g-C3N4 nanosheets | HaCaT | MTS assay, optical microscopy | 3.125–500 μg/mL | No cytotoxic effect. | [82] |
| Ni-doped g-C3N4 nanosheets | 24-h IC50 = 53.93 μg/mL. | ||||
| Cu-doped g-C3N4 nanosheets | 24-h IC50 = 157.00 μg/mL. | ||||
| Cu-Ni-doped g-C3N4 nanosheets | 24-h IC50 = 40.10 μg/mL. | ||||
| g-C3N4 nanosheets | PC12 | MTT assay | 1–100 μg/mL | No significant cytotoxic effect under dark or white LED irradiation conditions. | [83] |
| Metal (Fe, Cu, Zn)-doped g-C3N4 nanosheets | |||||
| Cu-doped g-C3N4 nanosheets combined with upconversion nanoparticles | 4T1 | MTT assay | 15.63–500 μg/mL | High cytotoxicity toward tumour 4T1 cells after NIR laser irradiation. | [84] |
| L929 | No obvious cytotoxic effect. |
5.2. In Vitro Cytotoxicity Assessment on Animal Cells
5.3. Ecotoxicity Tests on Microorganisms, Fishes, and Plant Seeds
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Wang, S.; Wang, L.; Cong, H.; Wang, R.; Yang, J.; Li, X.; Zhao, Y.; Wang, H. A review: G-C3N4 as a new membrane material. J. Environ. Chem. Eng. 2022, 10, 108189. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of g-C3N4. Small 2023, 19, 2300109. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2024, 2, 265–287. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yan, Y.; Zheng, S.; Xue, H.; Pang, H. Graphitic carbon nitride based materials for electrochemical energy storage. J. Mater. Chem. A 2019, 7, 901–924. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and biomedical applications of graphitic carbon nitride quantum dots. J. Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ohfuji, H.; Shinmei, T.; Irifune, T. Experimental study on the stability of graphitic C3N4 under high pressure and high temperature. Diam. Relat. Mater. 2011, 20, 819–825. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step synthesis of highly reactive gC3N4. J. Mater. Sci. Mater. Electron. 2022, 33, 9116–9125. [Google Scholar] [CrossRef]
- Hu, C.; Chu, Y.C.; Wang, M.S.; Wu, X.H. Rapid synthesis of g-C3N4 spheres using microwave-assisted solvothermal method for enhanced photocatalytic activity. J. Photochem. Photobiol. A Chem. 2017, 348, 8–17. [Google Scholar] [CrossRef]
- Xu, T.; Hur, J.; Niu, P.; Wang, S.; Lee, S.; Chun, S.E.; Li, L. Synthesis of crystalline g-C3N4 with rock/molten salts for efficient photocatalysis and piezocatalysis. Green Energy Environ. 2024, 9, 890–898. [Google Scholar] [CrossRef]
- Yadav, R.M.; Kumar, R.; Aliyan, A.; Dobal, P.S.; Biradar, S.; Vajtai, R.; Singh, D.P.; Martí, A.A.; Ajayan, P.M. Facile synthesis of highly fluorescent free-standing films comprising graphitic carbon nitride (g-C3N4) nanolayers. New J. Chem. 2020, 44, 2644–2651. [Google Scholar] [CrossRef]
- Umapathi, R.; Raju, C.V.; Ghoreishian, S.M.; Rani, G.M.; Kumar, K.; Oh, M.H.; Park, J.P.; Huh, Y.S. Recent advances in the use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord. Chem. Rev. 2022, 470, 214708. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Eshaghi, M.M.; Shamsabadipour, A.; Ghotekar, S.; Rahdar, A.; Ferreira, L.F.R. Graphitic carbon nitride (g-C3N4) synthesis methods, surface functionalization, and drug delivery applications: A review. J. Drug Deliv. Sci. Technol. 2023, 79, 104001. [Google Scholar] [CrossRef]
- Nihal; Sharma, R.; Kaur, N.; Sharma, M.; Choudhary, B.C.; Goswamy, J.K. Transition metal (Ni, Pd and Pt)-embedded graphitic carbon nitride (gCN) monolayer as an acetone sensor: A computational and experimental study. J. Mater. Sci. Mater. Electron. 2023, 34, 1005. [Google Scholar] [CrossRef]
- Molaei, M.J. Graphitic carbon nitride (g-C3N4) synthesis and heterostructures, principles, mechanisms, and recent advances: A critical review. Int. J. Hydrogen Energy 2023, 48, 32708–32728. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Omarov, S.O.; Lobinsky, A.A.; Nevedomskiy, V.N.; Popkov, V.I. Steam exfoliation of graphitic carbon nitride as efficient route toward metal-free electrode materials for hydrogen production. Int. J. Hydrogen Energy 2023, 48, 27671–27678. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Silva, C.G.; Faria, J.L.; Silva, A.M. The effect of precursor selection on the microwave-assisted synthesis of graphitic carbon nitride. Catal. Today 2023, 424, 113868. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Schnepp, Z. Soft and hard templating of graphitic carbon nitride. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 14081–14092. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2evolution activity. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B 2019, 256, 117854. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
- Vinoth, S.; Devi, K.S.; Pandikumar, A. A comprehensive review on graphitic carbon nitride-based electrochemical and biosensors for environmental and healthcare applications. Trends Analyt. Chem. 2021, 140, 116274v. [Google Scholar] [CrossRef]
- Abebe, H.A.; Diro, A.; Kitte, S.A. Voltammetric determination of tryptophan at graphitic carbon nitride modified carbon paste electrode. Heliyon 2023, 9, e21033. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, M.; Malode, S.J.; Alqarni, S.A.; Shetti, N.P. Graphitic carbon nitride (g–C3N4)-based electrochemical sensors for the determination of antiviral drug acyclovir. Mater. Chem. Phys. 2024, 312, 128650. [Google Scholar] [CrossRef]
- Sharif Manesh, S.; Masrournia, M. Carbon nitride nanoparticles modified carbon paste electrodes as potentiometric sensors for determination of nickel (II) and chromium (III) ions in tap water samples. J. Iran. Chem. Soc. 2021, 18, 1219–1229. [Google Scholar] [CrossRef]
- Singh, S.; Naithani, A.; Kandari, K.; Roy, S.; Sain, S.; Roy, S.S.; Wadhwa, S.; Tauseef, S.M.; Mathur, A. Oxygenated graphitic carbon nitride based electro-chemical sensor for dibenzofuran detection. Diam. Relat. Mater. 2023, 139, 110276. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kebede, T.G.; Ntsendwana, B.; Nxumalo, E.N. Fe-MOF derived graphitic carbon nitride nanocomposites as novel electrode materials for the electrochemical sensing of 2, 4-dichlorophenol in wastewater. Synth. Met. 2023, 299, 117452. [Google Scholar] [CrossRef]
- Niaz, A.; Arain, M.B.; Soylak, M. Sensitive determination of iodide at graphitic carbon nitride-chitosan composite modified screen-printed electrode in urine and salt using cathodic stripping voltammetry. Microchem. J. 2024, 200, 110430. [Google Scholar] [CrossRef]
- Ahmad, K.; Raza, W.; Alsulmi, A.; Kim, H. Fabrication of electrochemical sensor for metronidazole using MoS2/graphite-like carbon nitride composite modified glassy carbon electrode. Diam. Relat. Mater. 2023, 138, 110178. [Google Scholar] [CrossRef]
- Dasi, A.; Asadpour-Zeynali, K.; Saeb, E. Preparation of a fast and simple electrochemical sensor of bismuth telluride decorated on graphitic carbon nitride nanosheets for determination of epinephrine in biological samples. Synth. Met. 2024, 304, 117589. [Google Scholar] [CrossRef]
- Koventhan, C.; Shanmugam, R.; Chen, S.M. Development of highly sensitive electrochemical sensor for antipsychotic drug perphenazine using perovskite structured lanthanum cobalt oxide nanoparticles wrapped graphitic carbon nitride nanocomposites. Electrochim. Acta 2023, 467, 143096. [Google Scholar] [CrossRef]
- Svitkova, V.; Palchetti, I. Functional polymers in photoelectrochemical biosensing. Bioelectrochemistry 2020, 136, 107590. [Google Scholar] [CrossRef] [PubMed]
- Svitková, V.; Konderíková, K.; Nemčeková, K. Photoelectrochemical aptasensors for detection of viruses. Monatsh. Chem. 2022, 153, 963. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Han, D.; Yang, H.; Hong, Q.; Fang, Y.; Zhou, Z.; Shen, Y.; Liu, S.; Huang, C.; et al. Carbon nitride-based heterojunction photoelectrodes with modulable charge-transfer pathways toward selective biosensing. Anal. Chem. 2023, 95, 13716–13724. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Xiao, Q.; Li, M.; Xu, Y.; Qiu, X. Sensitive detection of p-nitrotoluene based on a copper cluster modified carbon nitride nanosheets photoelectrochemical sensor. Appl. Catal. A Gen. 2023, 649, 118964. [Google Scholar] [CrossRef]
- Yan, P.; Jin, Y.; Xu, L.; Mo, Z.; Qian, J.; Chen, F.; Yuan, J.; Xu, H.; Li, H. Enhanced photoelectrochemical aptasensing triggered by nitrogen deficiency and cyano group simultaneously engineered 2D carbon nitride for sensitively monitoring atrazine. Biosens. Bioelectron. 2022, 206, 114144. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wang, J.; Zhuang, Q.; Wu, S.; Yu, Y.; Ding, K. An electrochemiluminescence biosensor based on Graphitic carbon nitride luminescence quenching for detection of AFB1. Food Chem. 2023, 404, 134183. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Varodi, C.; Coros, M.; Kacso, I.; Radu, T.; Cozar, B.I.; Mirel, V.; Pruneanu, S. Investigation of L-tryptophan electrochemical oxidation with a graphene-modified electrode. Biosensors 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Naghian, E.; Marzi Khosrowshahi, E.; Sohouli, E.; Pazoki-Toroudi, H.R.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. Electrochemical oxidation and determination of antiviral drug acyclovir by modified carbon paste electrode with magnetic CdO nanoparticles. Front. Chem. 2020, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Masrournia, M. A novel modified carbon paste electrode for the determination of chromium(III) in water. J. Anal. Chem. 2018, 73, 824–831. [Google Scholar] [CrossRef]
- Gupta, A.K.; Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Aravindan, S.; Singh, D.; Mathur, A.; Kumar, R. Label-Free Electrochemical Detection of Dibenzofuran Using MnO2 Nanofibres. IEEE Sens. J. 2020, 20, 12537–12542. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Hong Nhung, V.T.; Thu, V.T.; Ngoc Nga, D.T.; Pham Truong, T.N.; Giang, H.T.; Hai Yen, P.T.; Phong, P.H.; Vu, T.A.; Thu Ha, V.T. An electrochemical sensor based on copper-based metal–organic framework-reduced graphene oxide composites for determination of 2,4-dichlorophenol in water. RSC Adv. 2020, 10, 42212–42220. [Google Scholar] [CrossRef]
- Khunseeraksa, V.; Kongkaew, S.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Electrochemical sensor for the quantification of iodide in urine of pregnant women. Mikrochim. Acta 2020, 187, 591. [Google Scholar] [CrossRef]
- Zokhtareh, R.; Rahimnejad, M.; Najafpour-Darzi, G.; Karimi-Maleh, H. A novel sensing platform for electrochemical detection of metronidazole antibiotic based on green-synthesized magnetic Fe3O4 nanoparticles. Environ. Res. 2023, 216, 114643. [Google Scholar] [CrossRef]
- Soosaimanickam, C.; Sakthivel, A.; Murugavel, K.; Alwarappan, S. Zeolite imidazolate framework-based platform for the electrochemical detection of epinephrine. J. Electrochem. Soc. 2023, 170, 107504. [Google Scholar] [CrossRef]
- Heli, H.; Sattarahmady, N.; Zare, S.N. Electrooxidation and determination of perphenazine on a graphene oxide nanosheet-modified electrode. RSC Adv. 2015, 5, 21005–21011. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.H.; Zhang, H.; Guo, X.M. Construction of electrochemical and photoelectrochemical sensing platform based on porphyrinic metal-organic frameworks for determination of ascorbic acid. Nanomaterials 2022, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liang, G.; Zhang, C.; Fan, L.; Yan, W.; Guo, Y.; Shuang, S.; Bi, Y.; Li, F.; Dong, C. Visible-light-driven photoelectrochemical sensing platform based on BiOI nanoflowers/TiO2 nanotubes for detection of atrazine in environmental samples. J. Hazard. Mater. 2021, 409, 124894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiong, C.; Li, J.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.M. High-performance electrochemiluminescence sensors based on ultra-stable perovskite quantum dots@ZIF-8 composites for aflatoxin B1 monitoring in corn samples. Food Chem. 2023, 410, 135325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, H.; Li, X.; Fan, J.; Xiang, Q. Carbon–graphitic carbon nitride hybrids for heterogeneous photocatalysis. Small 2021, 17, 2005231. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cheng, M.; Lai, C.; Li, L.; Yang, X.; Du, L.; Zhang, G.; Wang, G.; Yang, L. Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis. Coord. Chem. Rev. 2023, 474, 214846. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Wang, Z.; Wu, J.; Lu, H.; Yuan, L.; Zhu, Q.; Zeng, X. Renewable ultrathin carbon nitride nanosheets and its practical utilization for photocatalytic decarboxylation free radical coupling reaction. Chem. Eng. J. 2023, 466, 142990. [Google Scholar] [CrossRef]
- Yuan, X.; Xie, R.; Zhang, Q.; Sun, L.; Long, X.; Xia, D. Oxygen functionalized graphitic carbon nitride as an efficient metal-free ozonation catalyst for atrazine removal: Performance and mechanism. Sep. Purif. Technol. 2019, 211, 823–831. [Google Scholar] [CrossRef]
- Fernandes, E.; Mazierski, P.; Miodyńska, M.; Klimczuk, T.; Zaleska-Medynska, A.; Oliveira, J.; Matos, A.M.; Martins, R.C.; Gomes, J. Emerging contaminants and pathogenic microorganisms elimination in secondary effluent by graphitic carbon nitride photocatalytic ozonation processes. Catal. Today 2024, 432, 114624. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, J.; Qian, Y.; Chen, X.; Han, Y.; Zeng, X.; Qiu, B.; Zhu, Q. Order-Disorder Engineering of Carbon Nitride for Photocatalytic H2O2 Generation Coupled with Pollutant Removal. ACS Appl. Mater. Interfaces 2024, 16, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Hota, P.; Das, A.; Maiti, D.K. A short review on generation of green fuel hydrogen through water splitting. Int. J. Hydrogen Energy 2023, 48, 523–541. [Google Scholar] [CrossRef]
- Mohan, A.A.; Sandhyarani, N. Carbon nanostructures for energy generation and storage. In Applications of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–94. [Google Scholar] [CrossRef]
- Wang, T.H.; Nguyen, T.K.A.; Doong, R.A. Phosphorene nanosheet decorated graphitic carbon nitride nanofiber for photoelectrochemically enhanced hydrogen evolution from water splitting. J. Taiwan Inst. Chem. Eng. 2022, 141, 104577. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Y.; Yu, X.; Yin, Y.; Tian, G. Engineering high-coordinated cerium single-atom sites on carbon nitride nanosheets for efficient photocatalytic amine oxidation and water splitting into hydrogen. Chem. Eng. J. 2023, 462, 142084. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Díez, A.M.; Silva, C.G.; Faria, J.L.; Sanromán, M.Á.; Silva, A.M.; Pazos, M. Tuning graphitic carbon nitride (g-C3N4) electrocatalysts for efficient oxygen evolution reaction (OER). Fuel 2024, 360, 130575. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, J.; Lin, H.; Yin, Z.; Li, J.; Lu, M.; Guo, L.; Xi, P.; Tang, Y.; Yan, C.H. Optimized metal chalcogenides for boosting water splitting. Adv. Sci. 2020, 7, 1903070. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The application of perovskite materials in solar water splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Luo, D.; Shi, T.; Li, Q.H.; Xu, Q.; Strømme, M.; Zhang, Q.F.; Xu, C. Green, general and low-cost synthesis of porous organic polymers in sub-kilogram scale for catalysis and CO2 capture. Angew. Chem. Int. Ed. 2023, 62, e202305225. [Google Scholar] [CrossRef] [PubMed]
- Perveen, M.; Nazir, S.; Arshad, A.W.; Khan, M.I.; Shamim, M.; Ayub, K.; Khan, M.A.; Igbal, J. Therapeutic potential of graphitic carbon nitride as a drug delivery system for cisplatin (anticancer drug): A DFT approach. Biophys. Chem. 2020, 267, 106461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Guo, H.-L.; Xie, A.-J.; Shen, Y.-H.; Zhu, M.-Z. 4-in-1 Fe3O4/g-C3N4@PPy-DOX nanocomposites: Magnetic targeting guided trimode combinatorial chemotherapy/PDT/PTT for cancer. J. Inorg. Biochem. 2021, 215, 111329. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Kim, J.-O. Optimization of photocatalytic performance of a gC3N4–TiO2 nanocomposite for phenol degradation in visible light. Mat. Chem. Phys. 2021, 261, 124246. [Google Scholar] [CrossRef]
- Che, S.; Zhang, L.; Wang, T.; Su, D.; Wang, C. Graphitic Carbon Nitride-Based Photocatalysts for Biological Applications. Adv. Sustain. Syst. 2021, 6, 2100294. [Google Scholar] [CrossRef]
- Lin, L.-S.; Cong, Z.-X.; Li, J.; Ke, K.-M.; Guo, S.-S.; Yang, H.-H.; Chen, G.-N. Graphitic-phase C3N4 nanosheets as efficient photosensitizers and pH-responsive drug nanocarriers for cancer imaging and therapy. J. Mater. Chem. B 2014, 2, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhou, S.; Deng, L.; Shi, Z.; Jiang, H.; Zhou, S. Enhanced photocatalytic degradation of sulfadiazine via g-C3N4/carbon dots nanosheets under nanoconfinement: Synthesis, Biocompatibility and Mechanism. J. Environ. Chem. Eng. 2020, 8, 104612. [Google Scholar] [CrossRef]
- Davardoostmanesh, M.; Ahmadzadeh, H.; Goharshadi, E.K.; Meshkini, A.; Sistanipour, E. Graphitic carbon nitride nanosheets prepared by electrophoretic size fractionation as an anticancer agent against human bone carcinoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110803. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Latiff, N.M.; Mazánek, V.; Rosli, F.R.; Chia, H.L.; Sofer, Z.; Pumera, M. Triazine- and heptazine-based carbon nitrides: Toxicity. ACS Appl. Nano Mater. 2018, 1, 4442–4449. [Google Scholar] [CrossRef]
- Huang, Q.; Hao, L.; Zhou, R.; Zhu, B.; Zhao, H.; Cai, X. Synthesis, characterization, and biological study of carboxyl- and amino-rich g-C3N4 nanosheets by different processing routes. J. Biomed. Nanotechnol. 2018, 14, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Pieta, I.S.; Gieroba, B.; Kalisz, G.; Pieta, P.; Nowakowski, R.; Naushad, M.; Rathi, A.; Gawande, M.B.; Sroka-Bartnicka, A.; Zboril, R. Developing benign Ni/g-C3N4 catalysts for CO2 hydrogenation: Activity and toxicity study. Ind. Eng. Chem. Res. 2022, 61, 10496–10510. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Lee, B.I.; Ko, J.W.; Park, C.B. Photoactive g-C3N4 nanosheets for light-induced suppression of Alzheimer’s β-amyloid aggregation and toxicity. Adv. Healthc. Mater. 2016, 5, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yang, M.; He, F.; Zhang, X.; Gao, Y.; An, B.; Ding, H.; Gai, S.; Yang, P. Multiple therapeutic mechanisms of pyrrolic N-rich g-C3N4 nanosheets with enzyme-like function in the tumor microenvironment. J. Colloid Interface Sci. 2023, 650, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.V.; Fascineli, M.L.; Silva, I.C.R.; Rodrigues, M.O.; Chaker, J.A.; Grisolia, C.K.; Moya, S.E.; Campos, A.F.C.; Sousa, M.H. Carbon nitride nanosheets magnetically decorated with Fe3O4 nanoparticles by homogeneous precipitation: Adsorption-photocatalytic performance and acute toxicity assessment. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100549. [Google Scholar] [CrossRef]
- Berhanu, S.; Gebremariam, H.; Chufamo, S. The g-C3N4@CdO/ZnO ternary composite: Photocatalysis, thermodynamics and acute toxicity studies. Heliyon 2022, 8, e11612. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moniem, S.M.; El-Liethy, M.A.; Ibrahim, H.S.; Ali, M.E.M. Innovative green/non-toxic Bi2S3@g-C3N4 nanosheets for dark antimicrobial activity and photocatalytic depollution: Turnover assessment. Ecotoxicol. Environ. Saf. 2021, 226, 112808. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Cai, Z.; Ma, C.; White, J.C.; Cao, Y.; Chang, Z.; Xu, X.; Han, L.; Jia, W.; Zhao, J.; et al. Root exposure of graphitic carbon nitride (g-C3N4) modulates metabolite profile and endophytic bacterial community to alleviate cadmium- and arsenate-induced phytotoxicity to rice (Oryza sativa L.). ACS Nano 2023, 17, 19724–19739. [Google Scholar] [CrossRef] [PubMed]
| Synthesis Type | Result | Efficiency and Purity |
|---|---|---|
| Thermal polymerisation | Produces bulk g-C3N4 with high crystallinity and decent photocatalytic properties. However, this method often results in a limited surface area and poor dispersion in solutions. | High purity with moderate efficiency due to limited surface area and electron–hole recombination issues. |
| Solvothermal and hydrothermal methods | Creates g-C3N4 with various morphologies, such as nanosheets or nanospheres, and can introduce porosity. | Higher surface area and enhanced photocatalytic activity compared to bulk g-C3N4. Purity depends on the solvent and reaction conditions but is generally good. |
| Template-assisted synthesis | Produces porous or structured g-C3N4 with high surface areas and tailored morphologies. | High surface area leads to improved catalytic performance. Template removal can impact purity if not performed thoroughly. |
| Chemical vapour deposition (CVD) | Yields thin films of g-C3N4 with controlled thickness and high uniformity. | Very high purity and good control over material properties, leading to efficient catalytic and electronic applications. |
| Molten salt synthesis | Produces g-C3N4 with a high degree of crystallinity and large surface area. | High efficiency due to better dispersion and surface properties. Purity is generally high but depends on the post-synthesis washing to remove residual salts. |
| Doping and composite formation | Enhances electronic properties, photocatalytic activity, and stability. Creates composites with synergistic properties. | Improved efficiency due to enhanced charge separation and increased active sites. Purity can be impacted by the choice of dopants and composite materials. |
| Exfoliation techniques | Produces ultrathin g-C3N4 nanosheets with high surface areas and excellent catalytic properties. | High efficiency due to increased active sites and better light absorption. Purity is generally high if exfoliation is performed without introducing impurities. |
| Analyte | g-C3N4-Based Sensing Platform | Ref. | LOD (μM) | Comparison Material | LOD (μM) | Ref. |
|---|---|---|---|---|---|---|
| Tryptophan | g-C3N4-modified CPE | [29] | 0.085 | Graphene-modified CGE | 0.3 | [44] |
| Acyclovir | g-C3N4-modified CPE | [30] | 3 × 10−3 | Magnetic CdO NPs-modified GCE | 0.3 | [45] |
| Heavy metals (Cr, Ni) | g-C3N4, MWCNT-modified CPE | [31] | 6.7 × 10−3 and 0.012 | Zeolite and chlorinated MWCNT-modified CPE | 0.06 (Cr) | [46] |
| Dibenzofuran | Oxygenated g-C3N4-modified SPE | [32] | 1.58 × 10−6 | Silver electrode modified with MnO2 nanofibers | 1.2 × 10−3 | [47] |
| 2,4-dichlorophenol | Fe-MOF/g-C3N4-modified SPE | [33] | 1.2 × 10−3 | Cu-MOF/rGO composite-modified GCE | 0.083 | [48] |
| Iodide ions (I−) | g-C3N4/chitosan-modified SPE | [34] | 0.01 | Silver oxide microparticles PAA/PVA-modified GCE | 0.3 | [49] |
| Metronidazole | MoS2/g-C3N4-modified GCE | [35] | 0.09 | Graphene nanosheets/Fe3O4 modified-GCE | 0.23 × 10−3 | [50] |
| Epinephrine | Bi2Te3 /g-C3N4-modified GCE | [36] | 0.71 | Zeolite imidazole framework on GCE | 2.1 | [51] |
| Perphenazine | LaCoO3/g-C3N4-modified GCE | [37] | 4.3 × 10−3 | Graphene oxide nanosheets on GCE | 38.4 | [52] |
| Ascorbic acid | FTO glass/g-C3N4/BiOI PEC platform | [40] | 3.3 | Cu-porphyrin MOF | 0.023 | [53] |
| P-nitroroluene | g-C3N4 nanosheets/Cu(2%) PEC platform | [41] | 0.13 | - | - | - |
| Atrazine | g-C3N4 nanosheets doped with cyano groups and N deficiencies on ITO glass PEC aptasensor | [42] | 3.33 × 10−11 | BiOI nanoflowers/TiO2 nanotubes PEC platform | 0.5 × 10−6 | [54] |
| Aflatoxin B1 | g-C3N4 + aptamer on GCE | [43] | 0.005 ng/mL | Methylamine perovskite quantum dots, encapsulated by ZIF-8 MOF on GCE | 3.5 fg/mL | [55] |
| Material Type | Bandgap (eV) | Stability | Cost and Scalability | Environmental Impact | Other Drawbacks |
|---|---|---|---|---|---|
| g-C3N4 | 2.7—easily excited by visible light. | Thermally and chemically stable. | Cheap and abundant precursors, relatively simple synthesis, effective for large-scale applications. | Environmentally compatible. | Prone to rapid electron–hole recombination, functionalisation is necessary to overcome drawbacks. |
| Metal oxides | Several catalysts such as TiO2, SnO2, ZnO, and NiO are limited by larger bandgaps (>3.2)—not able to adsorb visible light. | Extremely stable. | Limited availability and high cost of several noble metals (Pt, Ru, Ir, Pd). Difficult for large-scale production. | Can be environmentally hazardous due to the potential release of toxic heavy metals. | Prone to deactivation due to adsorption of impurities or changes in pH. In nanoparticle form, prone to agglomeration. |
| Metal chalcogenides | Typically a much narrower bandgap (1.2–2.4). | Susceptible to photocorrosion and environmental degradation. | Several metals are expensive and low in abundance. Difficult for large-scale production. | Can be environmentally hazardous due to the potential release of toxic heavy metals. | Limited activity, low conductivity, low synthesis yield. |
| Perovskite materials | Adjustable, depending on the composition. | Prone to degradation from oxygen, moisture, UV exposure, and high temperatures (above 80). | Low cost of materials, faces challenges in large-scale processing. | Some perovskites contain lead. Additionally, toxic solvents are employed in processing. | Low catalytic performance when used alone. |
| Porous organic polymers (POPs) | Adjustable, depending on composition. | Generally stable. | Inexpensive precursors, scalable. Estimated production cost lower than 10 USD/kg. | Generally nontoxic, energy-efficient, and recyclable, though case-specific. | Relatively large pore size (>1 nm), which may be a limiting factor in some applications. |
| Risk | Impact |
|---|---|
| Toxic intermediates | As phenol undergoes degradation, various intermediate products are formed. Some of these intermediates may exhibit toxicity to humans and aquatic organisms, potentially posing risks to environmental and human health. |
| Incomplete degradation | Incomplete degradation of phenol or its intermediates could result in the accumulation of persistent organic pollutants in the environment, leading to long-term ecological impacts and potential bioaccumulation in the food chain. |
| Unwanted ROS | Uncontrolled generation of ROS during the photocatalytic process may cause oxidative stress in aquatic organisms and disrupt ecosystems, particularly in sensitive aquatic environments. |
| Release of nanoparticles | While g-C3N4 is generally considered to be biocompatible, the long-term effects of nanoparticle exposure on human health and the environment are still not fully understood. |
| Consideration | Impact |
|---|---|
| Particle size and shape | Nanoparticles of g-C3N4 may exhibit different properties and behaviours compared to bulk materials. Their small size and high surface area-to-volume ratio could increase interactions with biological systems, potentially leading to adverse effects such as cellular uptake, oxidative stress, and inflammation. |
| Chemical composition | The chemical composition of g-C3N4, including any surface functionalisation or impurities, could influence its toxicity profile. For example, surface groups or contaminants may enhance cellular uptake or trigger immune responses, leading to cytotoxic or immunotoxic effects. |
| Biological interactions | When introduced into biological systems, g-C3N4 nanoparticles may interact with cellular components such as proteins, lipids, and nucleic acids. These interactions could disrupt cellular processes, interfere with signalling pathways, or induce cellular damage, ultimately leading to cytotoxicity or genotoxicity. |
| Oxidative stress | Nanoparticles of g-C3N4 have the potential to generate ROS through photoactivation or chemical reactions. Excessive ROS production can overwhelm cellular antioxidant defences, leading to oxidative stress and cellular damage. |
| Biodegradation and clearance | The biodegradation and clearance of g-C3N4 nanoparticles from the body are critical factors in determining their long-term toxicity. If nanoparticles persist in biological tissues or accumulate in organs over time, they may elicit adverse effects such as chronic inflammation, fibrosis, or organ damage. |
| Aggregation and agglomeration | Nanoparticles of g-C3N4 may agglomerate or aggregate in biological fluids or tissues, altering their physicochemical properties and biological interactions. Aggregated nanoparticles could lead to localised toxicity, impaired cellular uptake, or obstruction of biological pathways. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drdanová, A.P.; Krajčovičová, T.E.; Gál, M.; Nemčeková, K.; Imreová, Z.; Ryba, J.; Naumowicz, M.; Homola, T.; Mackuľak, T.; Svitková, V. Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride. Int. J. Mol. Sci. 2024, 25, 7634. https://doi.org/10.3390/ijms25147634
Drdanová AP, Krajčovičová TE, Gál M, Nemčeková K, Imreová Z, Ryba J, Naumowicz M, Homola T, Mackuľak T, Svitková V. Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride. International Journal of Molecular Sciences. 2024; 25(14):7634. https://doi.org/10.3390/ijms25147634
Chicago/Turabian StyleDrdanová, Alexandra Paulína, Timea Ema Krajčovičová, Miroslav Gál, Katarína Nemčeková, Zuzana Imreová, Jozef Ryba, Monika Naumowicz, Tomáš Homola, Tomáš Mackuľak, and Veronika Svitková. 2024. "Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride" International Journal of Molecular Sciences 25, no. 14: 7634. https://doi.org/10.3390/ijms25147634
APA StyleDrdanová, A. P., Krajčovičová, T. E., Gál, M., Nemčeková, K., Imreová, Z., Ryba, J., Naumowicz, M., Homola, T., Mackuľak, T., & Svitková, V. (2024). Unveiling Versatile Applications and Toxicity Considerations of Graphitic Carbon Nitride. International Journal of Molecular Sciences, 25(14), 7634. https://doi.org/10.3390/ijms25147634







