Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Phenotypic and Physiological Responses of C. humilis Plants under Drought Stress
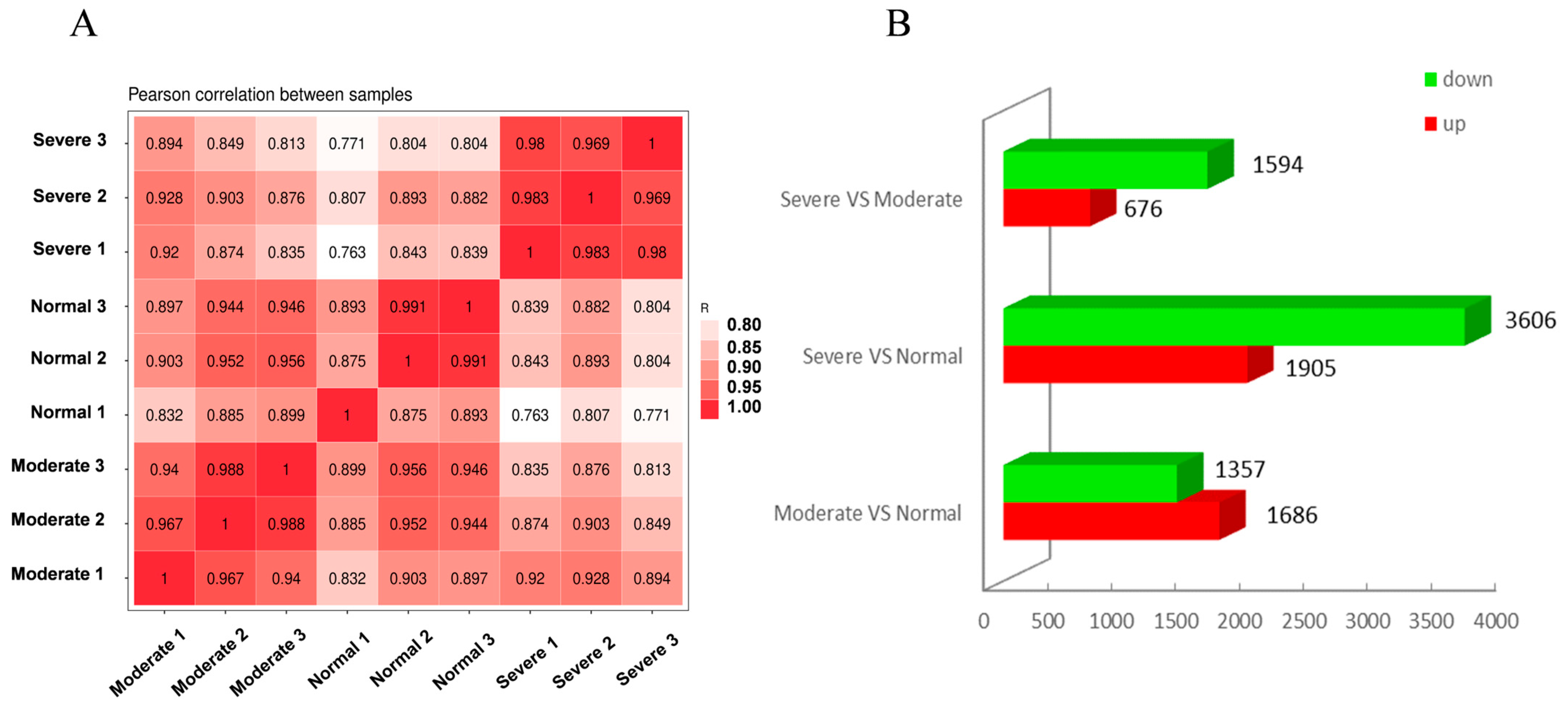
2.2. Transcriptome Analysis under Different Drought Levels
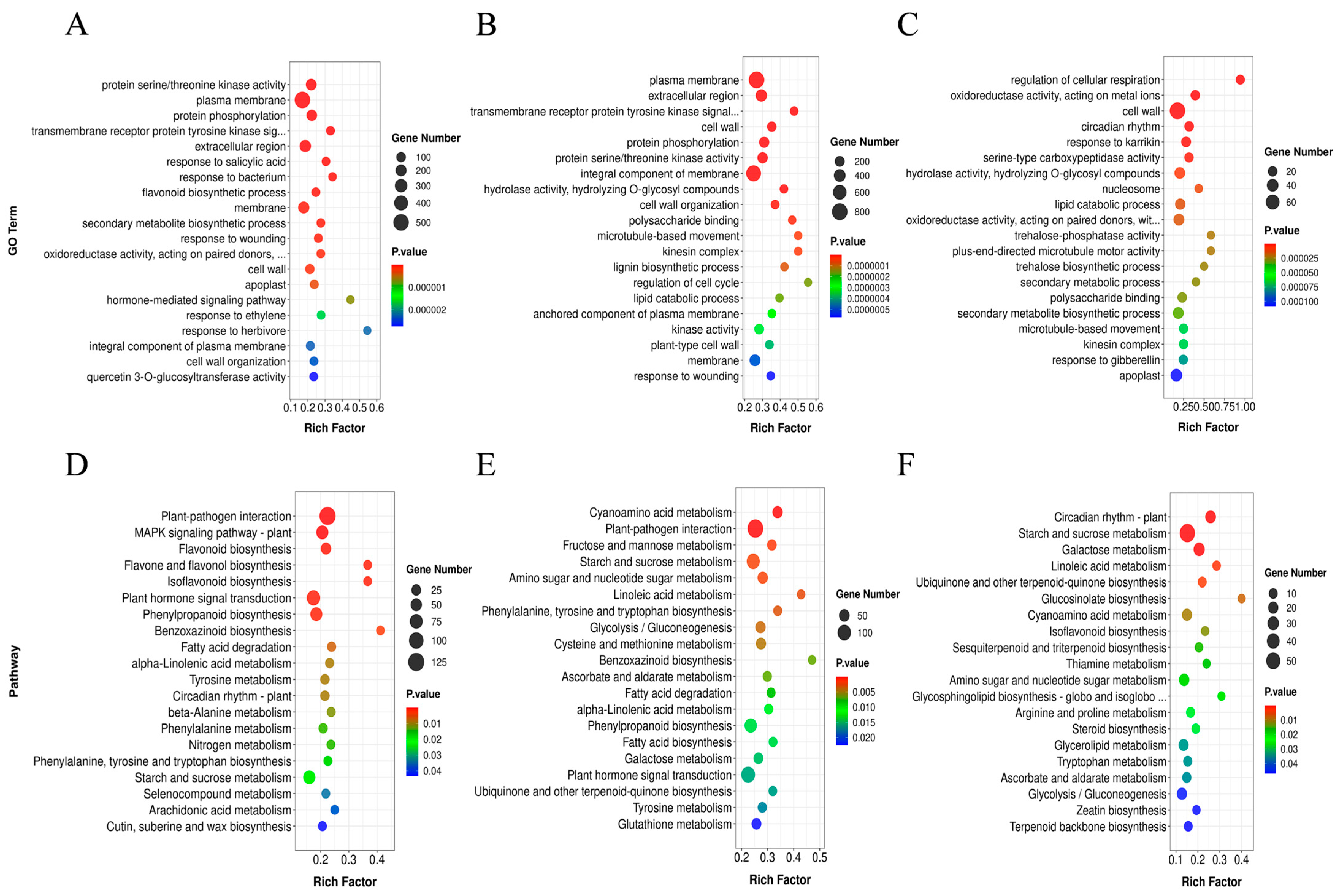
2.3. The Functional Analysis of DEGs under Different Drought Levels
2.4. Variations in Transcription-Factor Gene Expression under Drought Stress
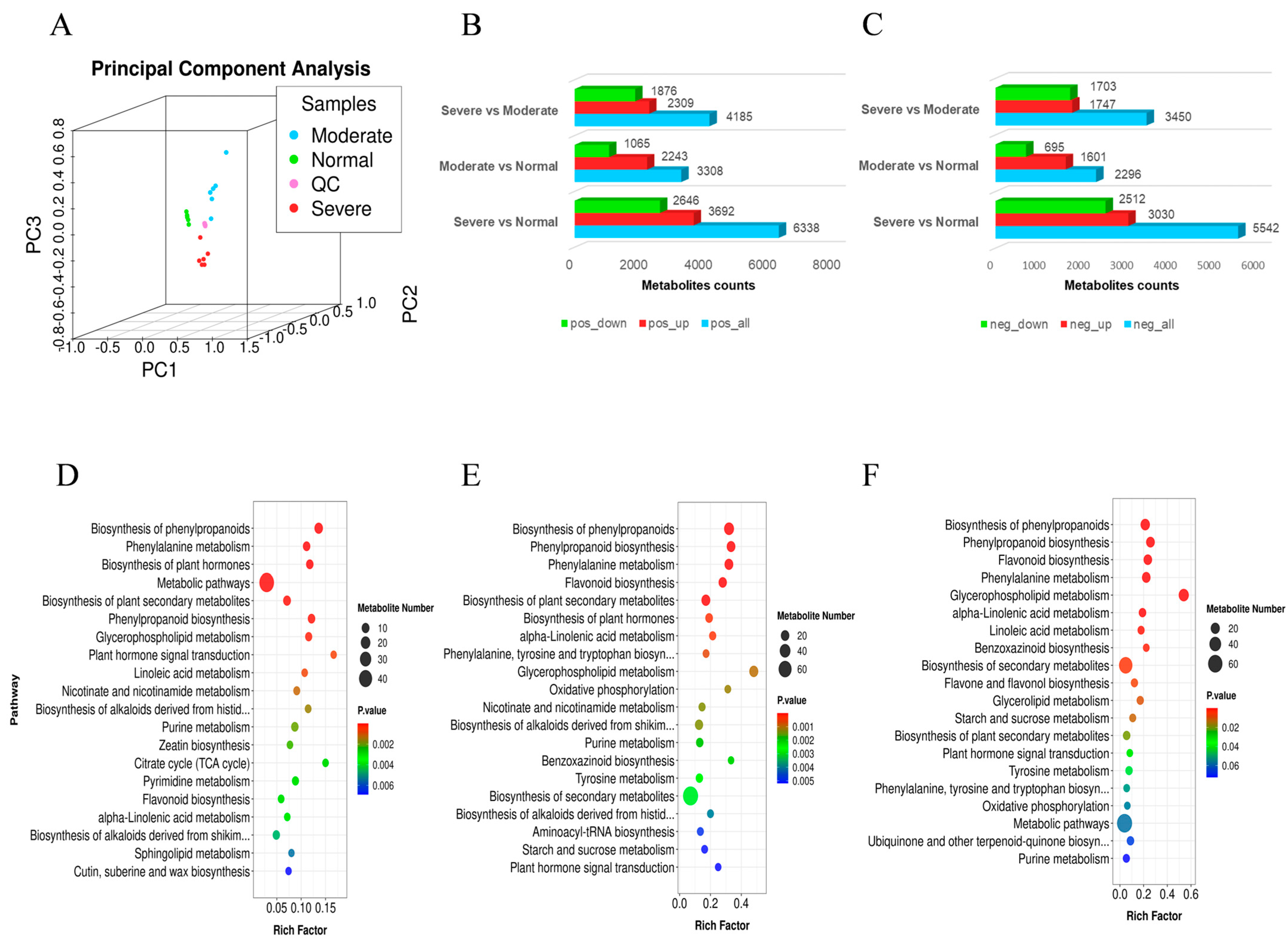
2.5. The Metabolome Analysis of C. humilis under Drought Stress
2.6. Association Analysis of Transcriptome and Metabolome Data
2.7. RNA-Seq Expression-Level Validation by qRT-PCR
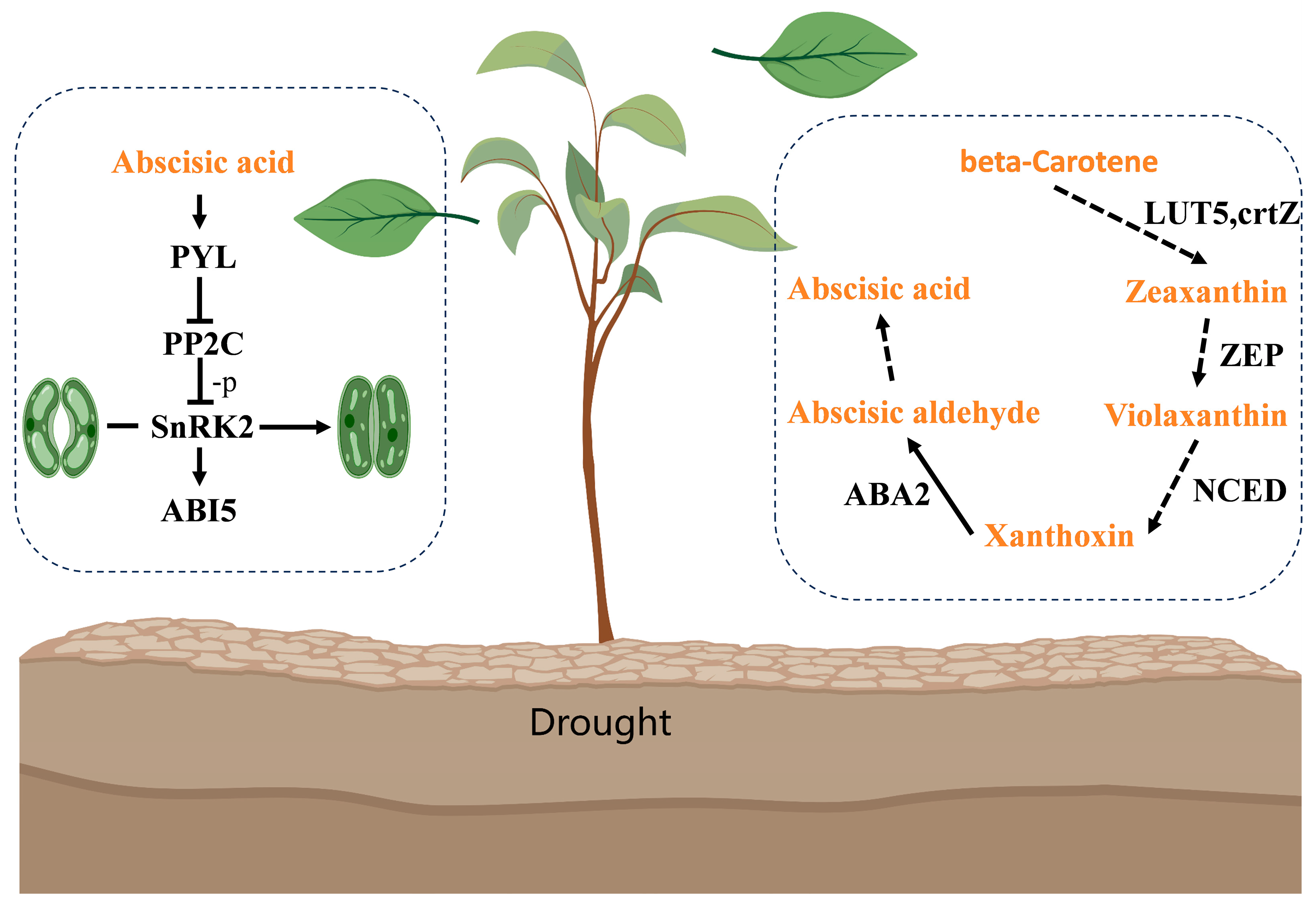
2.8. The Integrated Analysis of Genes and Metabolites Related to ABA in Plants under Drought Stress
3. Discussion
3.1. Metabolites Were Required for Drought Priming-Induced Drought Tolerance
3.2. Key Genes Regulating the Response of C. humilis to Drought Stress
3.3. Endogenous ABA Induced by Drought Priming Contributed to Increased Tolerance to Drought Stress
4. Materials and Methods
4.1. Plant Materials and Drought Treatments
4.2. Measurement of Physiological Indices of Drought Stress
4.3. RNA-Seq and Function Annotation
4.4. DEG Analysis
4.5. Metabolomic Analysis
4.6. Quantitative Real-Time Polymerase Chain-Reaction Verification
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, X.P.; Aryal, N.; Du, J.M.; Du, J.J. Oil content and fatty acid composition of the kernels of 31 genotypes of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.]. J. Hortic. Sci. Biotechnol. 2015, 90, 525–529. [Google Scholar] [CrossRef]
- Song, X.S.; Shang, Z.W.; Yin, Z.P.; Ren, J.; Sun, M.C.; Ma, X.L. Mechanism of xanthophyll-cycle-mediated photoprotection in Cerasus humilis seedlings under water stress and subsequent recovery. Photosynthetica 2011, 49, 523–530. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Zhang, H.; Li, W.; Wang, Z.; Li, H.; Tong, Q.; Qiao, G.; Liu, Y.; Tian, Y.; et al. The genome of Prunus humilis provides new insights to drought adaption and population diversity. DNA Res. 2022, 29, dsac021. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chu, L. Resource evaluation of typical energy plants and possible functional zone planning in China. Biomass Bioenergy 2008, 32, 283–288. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.N.; Zhang, Q.Y.; Song, X.S. Drought Tolerance Is Correlated with the Activity of Antioxidant Enzymes in Cerasus humilis Seedlings. Biomed. Res. Int. 2016, 2016, 9851095. [Google Scholar] [CrossRef]
- Sun, L.N.; Wang, F.; Wang, J.W.; Sun, L.J.; Gao, W.R.; Song, X.S. Overexpression of the ChVDE gene, encoding a violaxanthin de-epoxidase, improves tolerance to drought and salt stress in transgenic Arabidopsis. 3 Biotech 2019, 9, 197. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.W.; Sun, L.J.; Song, X.S. The molecular cloning and functional characterization of ChNAC1, a NAC transcription factor in Cerasus humilis. Plant Growth Regul. 2019, 89, 331–343. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; Sk, Z.A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Xiong, C.; Zhou, H.; Li, H.; Ruan, C. Yellowhorn Xso-miR5149-XsGTL1 enhances water-use efficiency and drought tolerance by regulating leaf morphology and stomatal density. Int. J. Biol. Macromol. 2023, 237, 124060. [Google Scholar] [CrossRef]
- Tong, S.; Chen, N.; Wang, D.; Ai, F.; Liu, B.; Ren, L.; Chen, Y.; Zhang, J.; Lou, S.; Liu, H.; et al. The U-box E3 ubiquitin ligase PalPUB79 positively regulates ABA-dependent drought tolerance via ubiquitination of PalWRKY77 in Populus. Plant Biotechnol. J. 2021, 19, 2561–2575. [Google Scholar] [CrossRef] [PubMed]
- Rachappanavar, V.; Padiyal, A.; Sharma, J.K.; Gupta, S.K. Plant hormone-mediated stress regulation responses in fruit crops—A review. Sci. Hortic. 2022, 304, 111302. [Google Scholar] [CrossRef]
- Liu, X.; Gao, T.; Liu, C.; Mao, K.; Gong, X.; Li, C.; Ma, F. Fruit crops combating drought: Physiological responses and regulatory pathways. Plant Physiol. 2023, 192, 1768–1784. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Liu, Y.; Yu, Y.; Li, H.; Wang, X.; Pang, Y.; Cao, H.; Sun, Q. Molecular and physiological mechanisms of strigolactones-mediated drought stress in crab apple (Malus hupehensis Rehd.) seedlings. Sci. Hortic. 2023, 311, 111800. [Google Scholar] [CrossRef]
- Xie, Y.; Bao, C.; Chen, P.; Cao, F.; Liu, X.; Geng, D.; Li, Z.; Li, X.; Hou, N.; Zhi, F.; et al. Abscisic acid homeostasis is mediated by feedback regulation of MdMYB88 and MdMYB124. J. Exp. Bot. 2020, 72, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, W.; Jing, P.; Hou, X.; Fan, X. Overexpression of VyDOF8, a Chinese wild grapevine transcription factor gene, enhances drought tolerance in transgenic tobacco. Environ. Exp. Bot. 2021, 190, 104592. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, N.; Li, L.; Qin, G.; Ren, K.; Wang, H.; Deng, J.; Ding, D. Corrigendum: Tetraploidy in Citrus wilsonii enhances drought tolerance via synergistic regulation of photosynthesis, phosphorylation, and hormonal changes. Front. Plant Sci. 2023, 14, 875011. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Terry, L.A. Effect of deficit irrigation and methyl jasmonate application on the composition of strawberry (Fragaria x ananassa) fruit and leaves. Sci. Hortic. 2016, 199, 63–70. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.; Li, Y.; Zhang, J.; Zhu, Y.; Rodriguez, P.L.; Xu, W. Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H(+)-ATPase 2. Sci. Adv. 2021, 7, eabd4113. [Google Scholar] [CrossRef] [PubMed]
- Korwin Krukowski, P.; Colanero, S.; Sutti, A.; Martignago, D.; Conti, L. How Changes in ABA Accumulation and Signaling Influence Tomato Drought Responses and Reproductive Development. Int. J. Plant Biol. 2023, 14, 162–176. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA—Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Shi, W.-S.; Liu, Y.; Gao, X.-M.; Hu, B.; Sun, H.-R.; Li, X.-Y.; Yang, Y.; Li, X.-F.; Liu, Z.-B.; et al. MdPP2C24/37, Protein Phosphatase Type 2Cs from Apple, Interact with MdPYL2/12 to Negatively Regulate ABA Signaling in Transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 14375. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.F.; Li, Y.T.; Zheng, L.; Lu, Z.W.; Zhou, Y.B.; Chen, J.; Chen, M.; Zhang, J.P.; Sun, G.Z.; et al. Mitogen-activated protein kinase TaMPK3 suppresses ABA response by destabilising TaPYL4 receptor in wheat. New Phytol. 2022, 236, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; An, J.P.; Gao, N.; Wang, X.; Chen, X.X.; Wang, X.F.; Zhang, S.; You, C.X. MdTCP46 interacts with MdABI5 to negatively regulate ABA signalling and drought response in apple. Plant Cell Environ. 2022, 45, 3233–3248. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Wang, Z.; Ren, Z.; Wang, C.H.; Wang, P.; Zhu, J.K.; Li, X.; Duan, C.G. NUCLEAR PORE ANCHOR and EARLY in SHORT DAYS 4 negatively regulate abscisic acid signaling by inhibiting Snf1-related protein kinase2 activity and stability in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 2060–2074. [Google Scholar] [CrossRef]
- Shi, X.; Tian, Q.; Deng, P.; Zhang, W.; Jing, W. The rice aldehyde oxidase OsAO3 gene regulates plant growth, grain yield, and drought tolerance by participating in ABA biosynthesis. Biochem. Biophys. Res. Commun. 2021, 548, 189–195. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Yang, S.; Mao, D.; Wang, F.; Chen, L.; Liang, M. SiNCED1, a 9-cis-epoxycarotenoid dioxygenase gene in Setaria italica, is involved in drought tolerance and seed germination in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1121809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, G.; Jiang, J.; Li, Y.; Liu, X.; Li, J.; Sun, J.; Wang, Q.; Liu, D.; Luo, Z.; et al. Chromosome-scale Cerasus humilis genome assembly reveals gene family evolution and possible genomic basis of calcium accumulation in fruits. Sci. Hortic. 2022, 299, 111012. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Sun, H. Targeted and untargeted metabolomics reveals deep analysis of drought stress responses in needles and roots of Pinus taeda seedlings. Front. Plant Sci. 2022, 13, 1031466. [Google Scholar] [CrossRef]
- Ferrandino, A.; Pagliarani, C.; Pérez-Álvarez, E.P. Secondary metabolites in grapevine: Crosstalk of transcriptional, metabolic and hormonal signals controlling stress defence responses in berries and vegetative organs. Front. Plant Sci. 2023, 14, 1124298. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B.; Winkler, J.R. Hole hopping through tyrosine/tryptophan chains protects proteins from oxidative damage. Proc. Natl. Acad. Sci. USA 2015, 112, 10920–10925. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef]
- Nguyen, V.N.T.; Moon, S.; Jung, K.-H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J. Plant Physiol. 2014, 171, 1276–1288. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Wu, X.; Wang, J.; Li, H.; Zhang, R. Transcriptomic and physiological responses of contrasting maize genotypes to drought stress. Front. Plant Sci. 2022, 13, 928897. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Li, F.; Li, D.; Dong, Y.; Fan, Y. Bioinformatics Analysis of WRKY Family Genes in Erianthus fulvus Ness. Genes 2022, 13, 2102. [Google Scholar] [CrossRef]
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Liu, A.; Yang, Y.; Diouf, D.; You, J.; Zhang, X. Ectopic expression of the sesame MYB transcription factor SiMYB305 promotes root growth and modulates ABA-mediated tolerance to drought and salt stresses in Arabidopsis. AoB Plants 2020, 12, plz081. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Z.; Su, L.; Gong, L.; Xin, H. Vitis Myb14 confer cold and drought tolerance by activating lipid transfer protein genes expression and reactive oxygen species scavenge. Gene 2024, 890, 147792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pei, Y.; Zhu, F.; He, Q.; Zhou, Y.; Ma, B.; Chen, X.; Guo, J.; Khan, A.; Jahangir, M.; et al. CaSnRK2.4-mediated phosphorylation of CaNAC035 regulates abscisic acid synthesis in pepper (Capsicum annuum L.) responding to cold stress. Plant J. 2023, 117, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, N.; He, H.; Tan, Q.; Wen, B.; Zhang, R.; Fu, X.; Xiao, W.; Chen, X.; Li, D.; et al. Prunus persica transcription factor PpNAC56 enhances heat resistance in transgenic tomatoes. Plant Physiol. Biochem. 2022, 182, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, J.; Guo, B.; Li, X.; Yang, T.; Yang, H.; Li, N.; Wang, B.; Yu, Q. A group III WRKY transcription factor, SlWRKY52, positively regulates drought tolerance in tomato. Environ. Exp. Bot. 2023, 215, 105513. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Z.; Wang, S.; Feng, S.; Wang, R.; Zhu, C.; Zhong, L.; Cheng, Y.; Bao, M.; Zhang, F. DcWRKY33 promotes petal senescence in carnation (Dianthus caryophyllus L.) by activating genes involved in the biosynthesis of ethylene and abscisic acid and accumulation of reactive oxygen species. Plant J. 2023, 113, 698–715. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Li, W.; Yao, A.; Niu, C.; Hou, R.; Liu, W.; Wang, Y.; Zhang, L.; Han, D. Overexpression of Malus baccata WRKY40 (MbWRKY40) enhances stress tolerance in Arabidopsis subjected to cold and drought. Plant Stress. 2023, 10, 100209. [Google Scholar] [CrossRef]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Farre, G.; Naqvi, S.; Breitenbach, J.; Sanahuja, G.; Bai, C.; Sandmann, G.; Capell, T.; Christou, P.; Zhu, C. Cloning and functional characterization of the maize carotenoid isomerase and β-carotene hydroxylase genes and their regulation during endosperm maturation. Transgenic Res. 2010, 19, 1053–1068. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Brychkova, G.; de Oliveira, C.L.; Gomes, L.A.A.; de Souza Gomes, M.; Fort, A.; Esteves-Ferreira, A.A.; Sulpice, R.; McKeown, P.C.; Spillane, C. Regulation of Carotenoid Biosynthesis and Degradation in Lettuce (Lactuca sativa L.) from Seedlings to Harvest. Int. J. Mol. Sci. 2023, 24, 10310. [Google Scholar] [CrossRef] [PubMed]
- Efremov, G.I.; Ashikhmin, A.A.; Shchennikova, A.V.; Kochieva, E.Z. Comparative Characteristics of Genes 9-Cis-Epoxycarotinoid-Dioxygenase SINCED1 and SINCED2 during the Development and Ripening of Tomato Fruit. Russ. J. Plant Physiol. 2023, 70, 17. [Google Scholar] [CrossRef]
- Endo, A.; Nelson, K.M.; Thoms, K.; Abrams, S.R.; Nambara, E.; Sato, Y. Functional characterization of xanthoxin dehydrogenase in rice. J. Plant Physiol. 2014, 171, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Giorio, P.; Giorio, G.; Guadagno, C.R.; Cellini, F.; Stigliani, L.A.; D’Ambrosio, C. Carotenoid content, leaf gas-exchange, and non-photochemical quenching in transgenic tomato overexpressing the β-carotene hydroxylase 2 gene (CrtR-b2). Environ. Exp. Bot. 2012, 75, 1–8. [Google Scholar] [CrossRef]
- Basso, M.F.; Contaldi, F.; Celso, F.L.; Karalija, E.; Paz-Carrasco, L.C.; Barone, G.; Ferrante, A.; Martinelli, F. Expression profile of the NCED/CCD genes in chickpea and lentil during abiotic stress reveals a positive correlation with increased plant tolerance. Plant Sci. 2023, 336, 111817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, G.; Gu, T.; Ding, J.; Li, Y. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca. Mol. Genet. Genom. 2017, 292, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Ringer, K.L.; Davis, E.M.; Croteau, R. Monoterpene metabolism. Cloning, expression, and characterization of (-)-isopiperitenol/(-)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol. 2005, 137, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Zhao, Z.Q.; Song, D.D.; Yuan, Y.X.; Sun, H.J.; Zhao, J.F.; Chen, Y.L.; Zhang, C.G. SnRK2.6 interacts with phytochrome B and plays a negative role in red light-induced stomatal opening. Plant Signal Behav. 2021, 16, 1913307. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Yin, P.; Li, W.; Wang, L.; Yan, C.; Lin, Z.; Wu, J.Z.; Wang, J.; Yan, S.F.; Yan, N. The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol. Cell 2011, 42, 662–672. [Google Scholar] [CrossRef]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.M.; Sun, J. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Soma, F.; Takahashi, F.; Kidokoro, S.; Kameoka, H.; Suzuki, T.; Uga, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Constitutively active B2 Raf-like kinases are required for drought-responsive gene expression upstream of ABA-activated SnRK2 kinases. Proc. Natl. Acad. Sci. USA 2023, 120, e2221863120. [Google Scholar] [CrossRef]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.R.; Shinozaki, K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef]
- Dey, A.; Samanta, M.K.; Gayen, S.; Maiti, M.K. The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression. BMC Plant Biol. 2016, 16, 158. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 Confers Abscisic Acid Sensitivity and Tolerance to Drought Stress in Rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Z.; Lu, J.; Wei, X.; Qi, M.; Yin, Z.; Li, T. SlSnRK2.3 interacts with SlSUI1 to modulate high temperature tolerance via Abscisic acid (ABA) controlling stomatal movement in tomato. Plant Sci. 2022, 321, 111305. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 Attenuates Drought Resistance by Regulating JA and ABA Signaling in Rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef] [PubMed]
- Aleman, F.; Yazaki, J.; Lee, M.; Takahashi, Y.; Kim, A.Y.; Li, Z.; Kinoshita, T.; Ecker, J.R.; Schroeder, J.I. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 Transcription Factor: A putative link of ABA and JA signaling. Sci. Rep. 2016, 6, 28941. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Y.; Hu, Y.; Yin, L.; Qu, P.; Wang, P.; Mu, X.; Zhang, S.; Xie, P.; Cheng, C.; et al. Comparison of Bioactive Compounds and Antioxidant Activities in Differentially Pigmented Cerasus humilis Fruits. Molecules 2023, 28, 6272. [Google Scholar] [CrossRef]
- Su, L.; Dai, Z.; Li, S.; Xin, H. A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed]


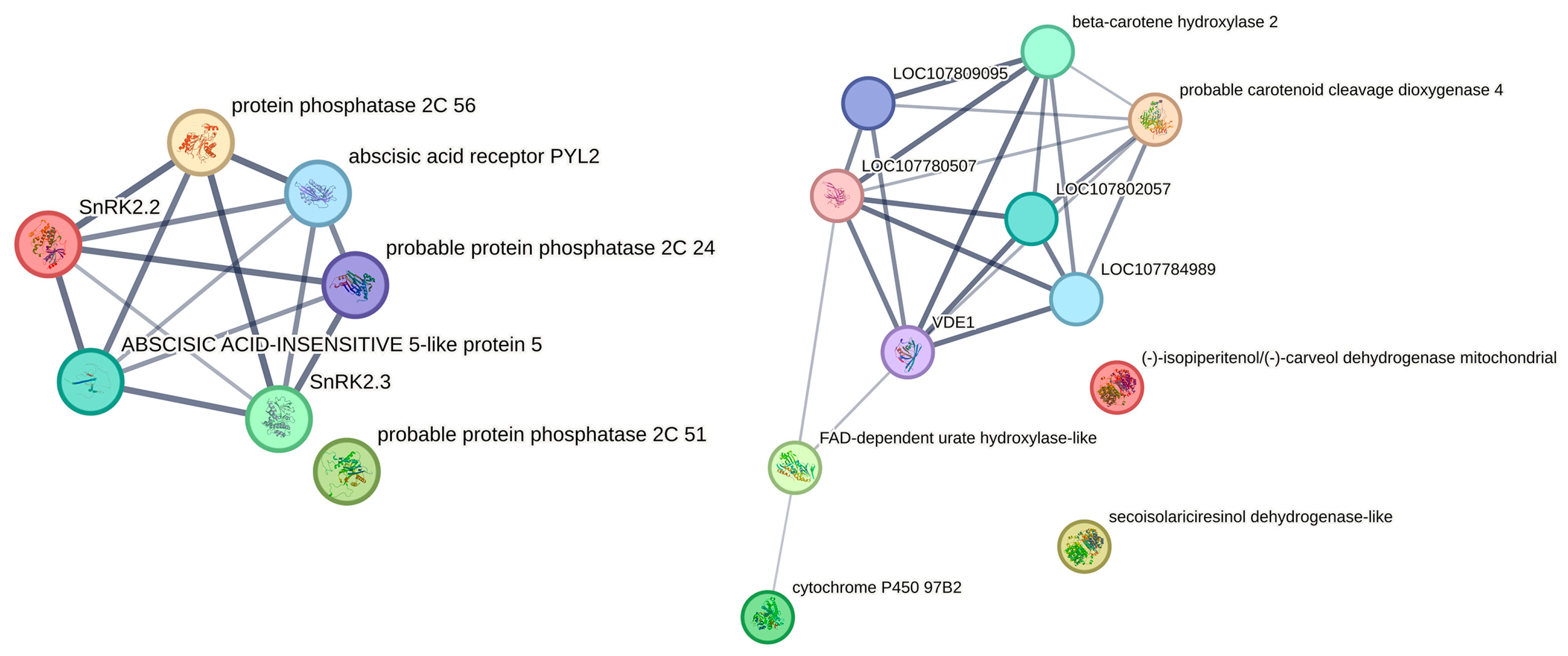
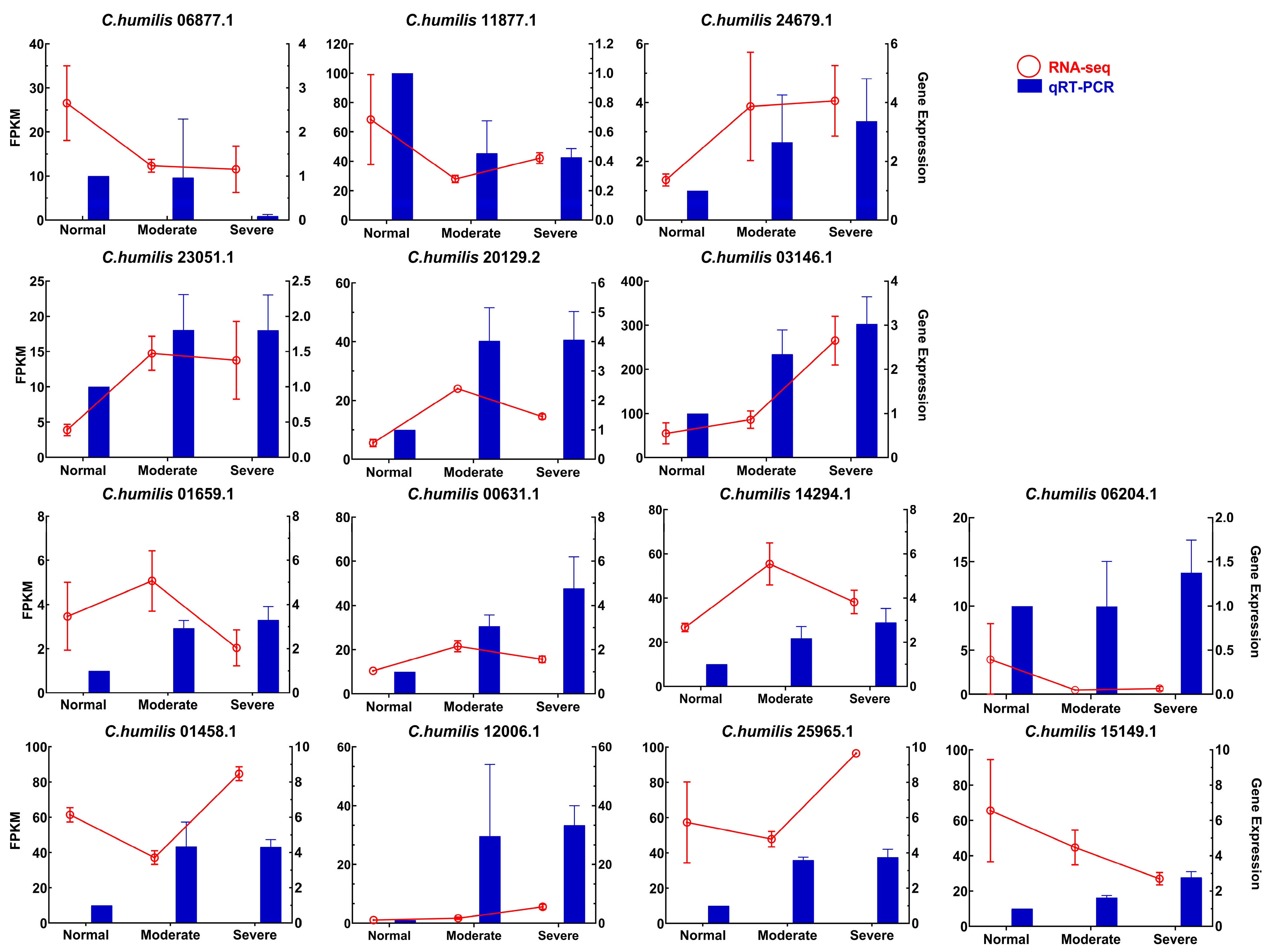
| Transcript_id | Description | Acronyms |
|---|---|---|
| C. humilis06877.1 | Secoisolariciresinol dehydrogenase-like | SIRD-like |
| C. humilis11877.1 | Beta-carotene hydroxylase 2, chloroplastic | CrtR-β2 |
| C. humilis24679.1 | (-)-Isopiperitenol/(-)-Carveol dehydrogenase mitochondrial | -i/-c DHM |
| C. humilis23051.1 | FAD-dependent urate hydroxylase-like | FAD-DUH-like |
| C. humilis20129.2 | Cytochrome P450 97B2, chloroplastic | Cyt P450 97B2 |
| C. humilis03146.1 | Probable carotenoid cleavage dioxygenase 4, chloroplastic | NCED 4 |
| C. humilis06204.1 | Probable protein phosphatase 2C 24 | PP2C 24 |
| C. humilis15149.1 | Protein phosphatase 2C 56 | PP2C 56 |
| C. humilis01659.1 | Abscisic acid receptor PYL2 | PYL2 |
| C. humilis00631.1 | Serine/threonine-protein kinase SAPK2 | SAPK2 |
| C. humilis14294.1 | SnRK2.2 | SnRK2.2 |
| C. humilis01458.1 | ABSCISIC ACID-INSENSITIVE 5-like protein 5 | ABI5-like 5 |
| C. humilis12006.1 | Probable protein phosphatase 2C 51 | PP2C 51 |
| C. humilis25965.1 | SnRK2.3 | SnRK2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, C.; Tang, X.; Wang, L.; Guo, R. Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress. Int. J. Mol. Sci. 2024, 25, 7635. https://doi.org/10.3390/ijms25147635
Liu Y, Zhao C, Tang X, Wang L, Guo R. Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress. International Journal of Molecular Sciences. 2024; 25(14):7635. https://doi.org/10.3390/ijms25147635
Chicago/Turabian StyleLiu, Yu, Chenxue Zhao, Xuedong Tang, Lianjun Wang, and Ruixue Guo. 2024. "Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress" International Journal of Molecular Sciences 25, no. 14: 7635. https://doi.org/10.3390/ijms25147635
APA StyleLiu, Y., Zhao, C., Tang, X., Wang, L., & Guo, R. (2024). Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress. International Journal of Molecular Sciences, 25(14), 7635. https://doi.org/10.3390/ijms25147635






