The Role of Collagens in Atopic Dermatitis
Abstract
1. Introduction
1.1. Skin Barrier Defect
1.2. Immunologic Disorders
1.3. Microbiota in AD
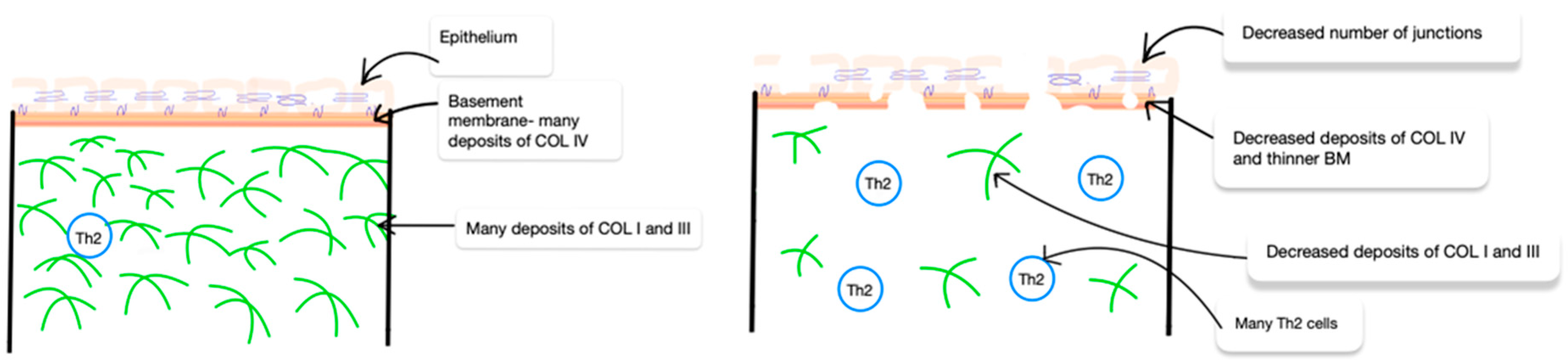
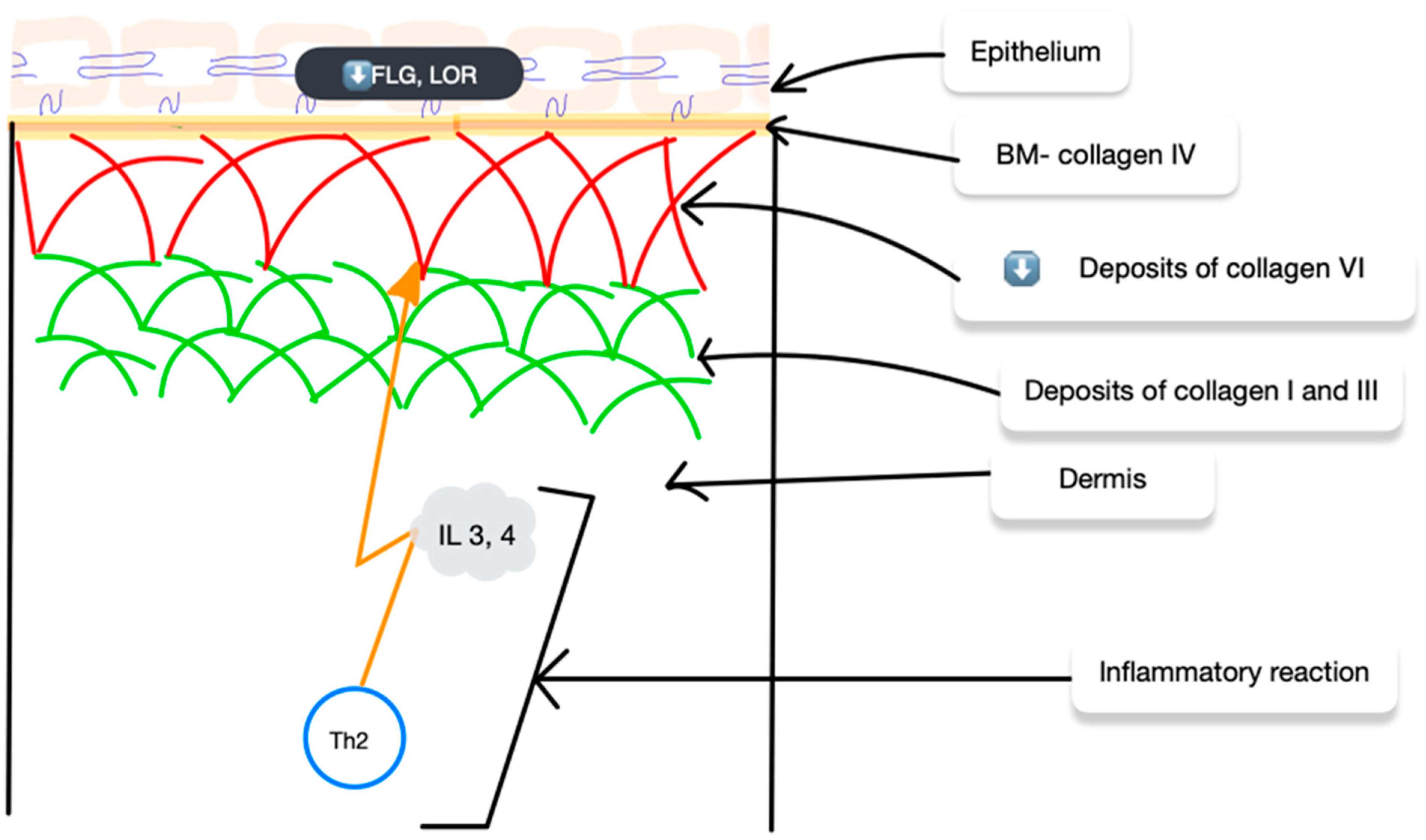
1.4. The Role of Collagens
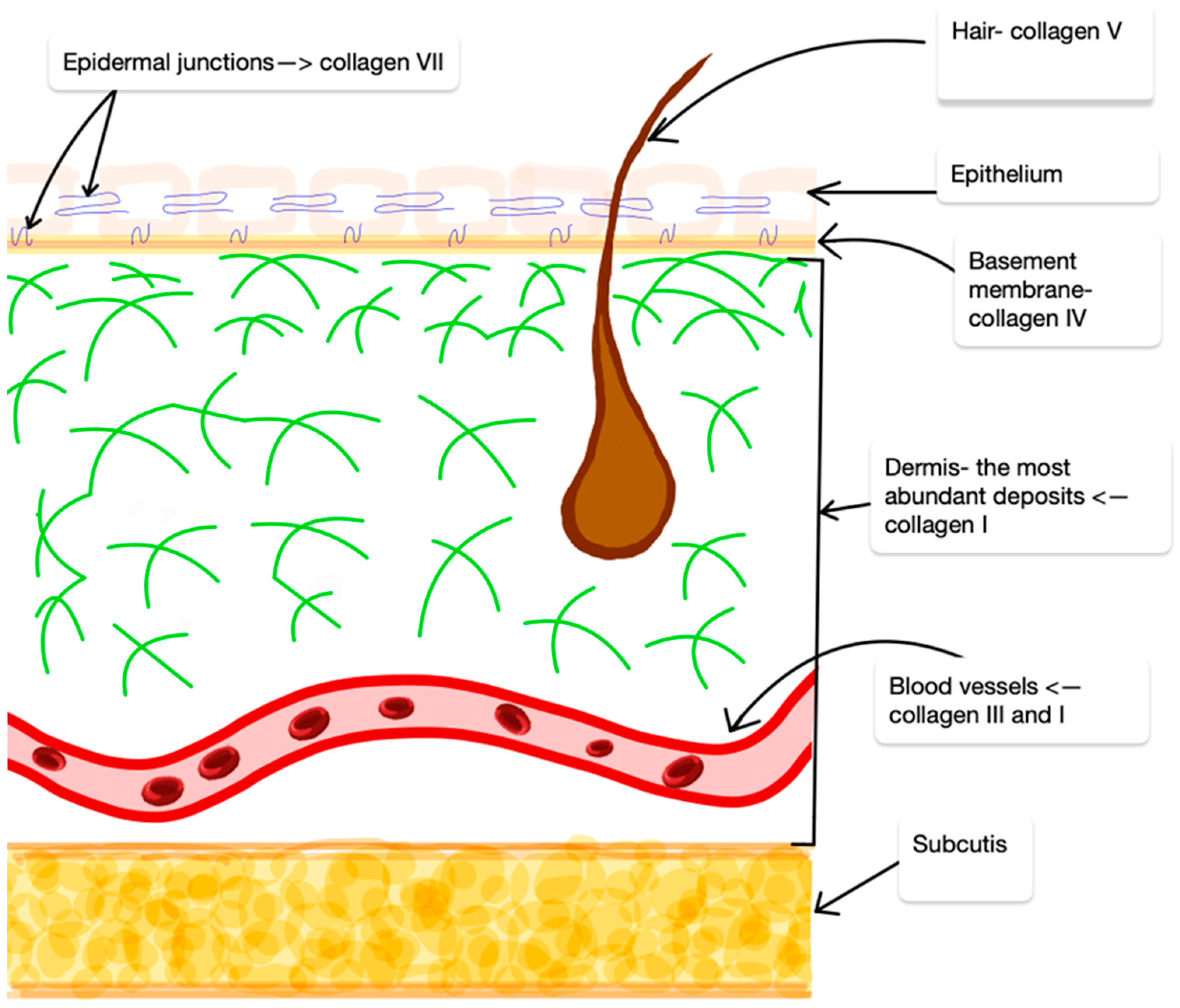
2. Collagens
3. Collagen in AD
4. Collagens and Possible AD Treatment
5. Conclusions
Funding
Conflicts of Interest
References
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.D.T.; Sodré, C.S.; Ferreira, D.C.; Abad, E.D.; Saintive, S.; Ribeiro, M.; Cavalcante, F.S.; Piciani, B.; Gonçalves, L.S. Oral Aspects Identified in Atopic Dermatitis Patients: A Literature Review. Open Dent. J. 2018, 12, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Leung, D.Y.M. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2018, 282, 256–267. [Google Scholar] [CrossRef]
- Hulshof, L.; Overbeek, S.A.; Wyllie, A.L.; Chu, M.L.J.N.; Bogaert, D.; de Jager, W.; Knippels, L.M.J.; Sanders, E.A.M.; van Aalderen, W.M.C.; Garssen, J.; et al. Exploring Immune Development in Infants with Moderate to Severe Atopic Dermatitis. Front. Immunol. 2018, 9, 630. [Google Scholar] [CrossRef]
- Murota, H.; Yamaga, K.; Ono, E.; Katayama, I. Sweat in the pathogenesis of atopic dermatitis. Allergol. Int. 2018, 67, 455–459. [Google Scholar] [CrossRef]
- Weidinger, S.; Illig, T.; Baurecht, H.; Irvine, A.D.; Rodriguez, E.; Diaz-Lacava, A.; Klopp, N.; Wagenpfeil, S.; Zhao, Y.; Liao, H.; et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J. Allergy Clin. Immunol. 2006, 118, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, O.; Sandhoff, K.; Kaiser, H.W. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J. Ivestig. Dermatol. 2002, 119, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, M.; Sakowicz-Burkiewicz, M.; Wasserling, M.; Dobaczewska, D.; Gleń, J.; Nowicki, R.; Pawelczyk, T. Expression of Cornified Envelope Proteins in Skin and Its Relationship with Atopic Dermatitis Phenotype. Acta Derm Venereol. 2017, 97, 36–41. [Google Scholar] [CrossRef]
- Wollenberg, A.; Bieber, T. Proactive therapy of atopic dermatitis—An emerging concept. Allergy 2009, 64, 276–278. [Google Scholar] [CrossRef]
- Simpson, E.L.; Hanifin, J.M. Atopic dermatitis. Med. Clin. N. Am. 2006, 90, 149–167. [Google Scholar] [CrossRef]
- Agrawal, R.; Woodfolk, J.A. Skin Barrier Defects in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2014, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Waldman, A. Atopic Dermatitis: Pathogenesis. Semin. Cutan. Med. Surg. 2017, 36, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Witte, E.; Kokolakis, G. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J. Investig. Dermatol. 2014, 134, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Seok, J.; Park, K.Y.; Yoon, Y.; Kim, K.H.; Seo, S.J. Copy-number variation of the filaggrin gene in Korean patients with atopic dermatitis: What really matters, ‘number’ or ‘variation’? Br. J. Dermatol. 2016, 174, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu PZhao, Y. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin. Med. J. 2016, 129, 757–762. [Google Scholar]
- Chan, T.C.; Sanyal, R.D. Variable T(H)2/T(H)17-skewing places Chinese atopic dermatitis and psoriasis on an inflammatory spectrum. J. Investig. Dermatol. 2018, 138, S10. [Google Scholar] [CrossRef]
- Kong, H.H.; Segre, J.A. Skin microbiome: Looking back to move forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef]
- Gilani, S.J.K.; Gonzalez, M.; Hussain, I.; Finlay, A.; Patel, G.K. Staphylococcus aureus re-colonization in atopic dermatitis: Beyond the skin. Clin. Exp. Dermatol. 2005, 30, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Paller, A.; Traidl-Hoffmann, C.; Beck, L.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.; Spuls, P.; Su, J.; et al. The role of bacterial skin infections in atopic dermatitis: Expert statement and review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2019, 182, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef] [PubMed]
- Gunzer, M.; Friedl, P.; Niggemann, B.; Bröcker, E.B.; Kämpgen, E.; Zänker, K.S. Migration of dendritic cells within 3-D collagen lattices is dependent on tissue origin, state of maturation, and matrix structure and is maintained by proinflammatory cytokines. J. Leukoc. Biol. 2000, 67, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kisling, A.; Lust, R.M. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Vorstandlechner, V.; Laggner, M.; Kalinina, P.; Haslik, W.; Radtke, C.; Shaw, L.; Lichtenberger, B.M.; Tschachler, E.; Ankersmit, H.J.; Mildner, M. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J. 2020, 34, 3677–3692. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Ashok, D.; Raghavan, S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front. Cell Devel. Biol. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Müller, A.K.; Yang, J.; Ŝulcová, J.; Werner, S. The role of chronic inflammation in cutaneous fibrosis: Fibroblast growth factor receptor deficiency in keratinocytes as an example. J. Investig. Dermatol. Symp. Proc. 2011, 15, 48–52. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Fidler, A.L. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Mouw, J.K. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Nielsen, S.H. A fragment of type VI collagen alpha-6 chain is elevated in serum from patients with atopic dermatitis, psoriasis, hidradenitis suppurativa, systemic lupus erythematosus and melanoma. Sci. Rep. 2023, 13, 3056. [Google Scholar] [CrossRef]
- Uitto, J. Connective Tissue Biochemistry of the Aging Dermis: Age-Associated Alterations in Collagen and Elastin. Clin. Geriatr. Med. 1989, 5, 127–148. [Google Scholar] [CrossRef]
- Lee, J.; Jang, A.; Seo, S.J.; Myung, S.C. Epigenetic regulation of filaggrin gene expression in human epidermal keratinocytes. Ann. Dermatol. 2020, 32, 122–129. [Google Scholar] [CrossRef]
- Nyström, A. Transmembrane collagens-Unexplored mediators of epidermal-dermal communication and tissue homeostasis. Exp. Dermatol. 2021, 30, 10–16. [Google Scholar] [CrossRef]
- Harrison, I.P. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Suarez-Farinas, M.; Dhingra, N. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Gonzalez, J. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 2015, 136, 104–115. [Google Scholar] [CrossRef]
- Pfisterer, K. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Szalus, K. The Associations of Single Nucleotide Polymorphisms of the COL3A1, COL6A5, and COL8A1 Genes with Atopic Dermatitis. J. Pers. Med. 2023, 13, 661. [Google Scholar] [CrossRef]
- Huang, F.; Wachi, S. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: Important implications in asthma. J. Allergy Clin. Immunol. 2008, 121, 1415–1421. [Google Scholar] [CrossRef]
- Sabatelli, P.; Gara, S.K.; Grumati, P.; Urciuolo, A.; Gualandi, F.; Curci, R.; Squarzoni, S.; Zamparelli, A.; Martoni, E.; Merlini, L.; et al. Expression of the collagen VI α5 and α6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J. Investig. Dermatol. 2011, 131, 99–107. [Google Scholar] [CrossRef]
- Jung, H.J. The Role of Collagen VI α6 Chain Gene in Atopic Dermatitis. Ann. Dermatol. 2022, 34, 46–54. [Google Scholar] [CrossRef]
- Lee, Y.I. Proteoglycan Combined with Hyaluronic Acid and Hydrolyzed Collagen Restores the Skin Barrier in Mild Atopic Dermatitis and Dry, Eczema-Prone Skin: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 10189. [Google Scholar] [CrossRef]
- Makmatov-Rys, M. The Effect of single nucleotide polymorphism in COL23A1 gene on increased HSV-1 susceptibility of human macrophages. Acta Derm. Venereol. 2023, 103 (Suppl. S226). Available online: https://document.isad.org/meeting/2023/Gda%C5%84sk/ActaDV-103-Suppl226-ISAD-2023-Gdansk_abstract-book.pdf (accessed on 7 July 2024).
- Hakuta, A. Anti-inflammatory effect of collagen tripeptide in atopic dermatitis. J. Dermatol. Sci. 2017, 88, 357–364. [Google Scholar] [CrossRef]
- Chopra, S. Identification and characterization of a novel possible genetic risk factor for Eczema Herpeticum. Acta Derm. Venereol. 2023, 103 (Suppl. S226). Available online: https://document.isad.org/meeting/2023/Gda%C5%84sk/ActaDV-103-Suppl226-ISAD-2023-Gdansk_abstract-book.pdf (accessed on 7 July 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalus, K.; Trzeciak, M. The Role of Collagens in Atopic Dermatitis. Int. J. Mol. Sci. 2024, 25, 7647. https://doi.org/10.3390/ijms25147647
Szalus K, Trzeciak M. The Role of Collagens in Atopic Dermatitis. International Journal of Molecular Sciences. 2024; 25(14):7647. https://doi.org/10.3390/ijms25147647
Chicago/Turabian StyleSzalus, Krzysztof, and Magdalena Trzeciak. 2024. "The Role of Collagens in Atopic Dermatitis" International Journal of Molecular Sciences 25, no. 14: 7647. https://doi.org/10.3390/ijms25147647
APA StyleSzalus, K., & Trzeciak, M. (2024). The Role of Collagens in Atopic Dermatitis. International Journal of Molecular Sciences, 25(14), 7647. https://doi.org/10.3390/ijms25147647






