Impact of Respiratory Dust on Health: A Comparison Based on the Toxicity of PM2.5, Silica, and Nanosilica
Abstract
:1. Introduction
2. Sources and Characteristics of PM2.5, Silica, and Nanosilica
3. Toxic Effects of PM2.5, Silica, and Nanosilica In Vivo
3.1. Animal Models
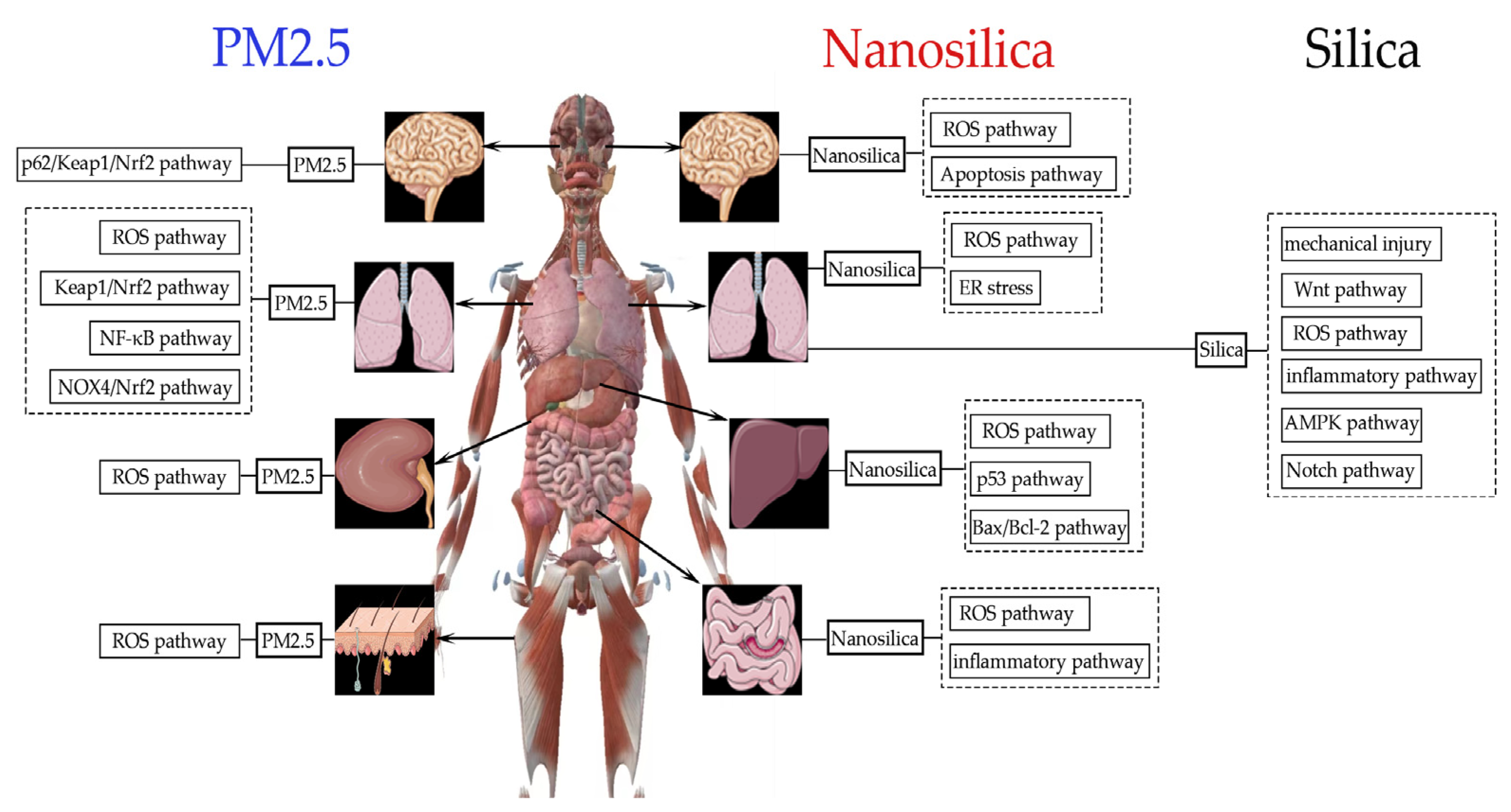
3.2. Organ Toxicity
4. The Cytotoxicity of PM2.5, Silica, and Nanosilica In Vitro
4.1. Cellular Models
4.2. Cytotoxicity
- Cause oxidative stress by increasing levels of lipid peroxidation through increased ROS, which can lead to intracellular oxidative damage and affect normal cellular function.
- Destroy mitochondria and disrupt their metabolism, causing mitochondrial autophagy and damage, which lead to an imbalance in cell energy and, ultimately, cell death.
- Disturb the formation of lysosomes, thereby impeding the autophagy and degradation of macromolecules, leading to the accumulation of intracellular waste and triggering apoptosis.
- Disrupt transcription and damage DNA, thereby accelerating mutagenesis, which further leads to abnormal cell proliferation and tumorigenesis.
- Disturb the normal mechanism of cellular metabolism, activate the synthesis of inflammatory mediators, and induce a series of inflammatory responses. These inflammatory responses may trigger tissue damage and the development of inflammatory diseases.
- Destroy the cell membrane via perforation, causing an imbalance between the internal and external environments of the cell, which in turn affects the normal function and survival of the cell.
5. The Population Health Effects of PM2.5, Silica, and Nanosilica
6. Combined Toxicity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 16HBE cells | Normal human bronchial epithelial cells |
| A549 cells | Human lung adenocarcinoma epithelial cells |
| AHR | Aryl hydrocarbon receptor |
| AM | Alveolar macrophage |
| AMP | Adenosine 5′-monophosphate |
| AMPK | AMP-activated protein kinase |
| ANS | Autonomic nervous system |
| Apaf1 | Apoptotic protease-activating factor-1 |
| ARE | Antioxidant response element |
| BALF | Bronchoalveolar lavage fluid |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| Beas-2b cells | Human normal lung epithelial cells |
| BRL-3A cells | Rat normal liver cells |
| Caco-2 cells | Human intestinal epithelial cells |
| CD36 | Platelet glycoprotein 4 |
| CNS | Central nervous system |
| COL I | Collagen I |
| CVD | Cardiovascular disease |
| Cyt C | Cytochrome C |
| EBf-H9 cells | Human embryonic stem cells |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERK1 | Extracellular signal-regulated kinase 1 |
| FDA | Food and Drug Administration |
| GSDMD | Gasdermin D |
| GSH | Glutathione |
| HaCaT cells | Human keratinocyte-forming cells |
| HEK-293 cells | Human embryonic renal cells |
| HepG2 cells | Human liver cancer cells |
| HK-2 cells | Human renal cortical proximal tubule epithelial cells |
| HL-7702 cells | Human normal liver cells |
| HRV | Heart rate variability |
| Hs-CRP | High-sensitivity C-reactive protein |
| HT-29 cells | Human intestinal epithelial cells |
| HTR-8/SVneo cells | Human chorionic trophoblast cells |
| HUVECs | Human umbilical vein endothelial cells |
| IARC | International Agency for Research on Cancer |
| IER | integrated exposure–response |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde. |
| MMP | Mitochondrial membrane potential |
| MRC-5 cells | Human lung fibroblasts |
| M-SNPs | Mesoporous silica nanoparticles |
| mtROS | Mitochondrial reactive oxygen species |
| MTX | Methothexate |
| N2a cells | Mouse neuroblastoma cells |
| N9 cells | Microglia cells |
| NF-κB | Nuclear factor kappa-B |
| NO | Nitric oxide |
| NOX4 | NADPH oxidase |
| Nrf2 | NF-E2 p45-related factor 2 |
| OELs | Occupational exposure limits |
| OSHA | Occupational Safety and Health Administration |
| p62 | Sequestosome 1 |
| PAHs | Polycyclic aromatic hydrocarbons |
| PBEC | Primary human bronchial epithelial cells |
| PC-TWA | Permissible concentration time-weighted average |
| P-SNPs | PEGylated silica nanoparticles |
| RAW264.7 | Mouse monocyte macrophages |
| ROS | Reactive oxygen species |
| SH-SY5Y | Human neuroblastoma cells |
| SPM | Suspended particulate matter |
| S-SNPs | Spherical silica nanoparticles |
| TGF-β1 | Transforming growth factor 1 |
| THP-1 cells | Human leukemia monocytic cells |
| TNF-α | Tumor necrosis factor α |
| VCAM-1 | Vascular cell adhesion molecule-l |
| WHO | World Health Organization |
| Wnt | Wingless-type MMTV-integration site |
| α-SMA | α-smooth muscle actin |
References
- Pouri, N.; Karimi, B.; Kolivand, A.; Mirhoseini, S.H. Ambient dust pollution with all-cause, cardiovascular and respiratory mortality: A systematic review and meta-analysis. Sci. Total Environ. 2024, 912, 168945. [Google Scholar] [CrossRef] [PubMed]
- Poinen-Rughooputh, S.; Rughooputh, M.S.; Guo, Y.; Rong, Y.; Chen, W. Occupational exposure to silica dust and risk of lung cancer: An updated meta-analysis of epidemiological studies. BMC Public Health 2016, 16, 1137. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Zeger, S.L.; White, R.H.; Samet, J.M. Particulate air pollution and mortality in the United States: Did the risks change from 1987 to 2000? Am. J. Epidemiol. 2007, 166, 880–888. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Ryou, H.G.; Heo, J.; Kim, S.Y. Source apportionment of PM10 and PM2.5 air pollution, and possible impacts of study characteristics in South Korea. Environ. Pollut. 2018, 240, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, J.; Kim, J.Y.; Lee, J.Y.; Kim, H.; Kim, B.M. Characterization of PM2.5 and identification of transported secondary and biomass burning contribution in Seoul, Korea. Environ. Sci. Pollut. Res. Int. 2018, 25, 4330–4343. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Almeida, S.M.; Manousakas, M.; Diapouli, E.; Kertesz, Z.; Samek, L.; Hristova, E.; Šega, K.; Alvarez, R.P.; Belis, C.A.; Eleftheriadis, K. Ambient particulate matter source apportionment using receptor modelling in European and Central Asia urban areas. Environ. Pollut. 2020, 266, 115199. [Google Scholar] [CrossRef]
- Coleman, N.C.; Burnett, R.T.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Pope, C.A., 3rd. Fine Particulate Matter Exposure and Cancer Incidence: Analysis of SEER Cancer Registry Data from 1992–2016. Environ. Health Perspect. 2020, 128, 107004. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, J.; Tao, Y.; Dai, L.; Lu, S.E.; Hou, B.; Wang, Z.; Zhu, T. Seasonal variation of chemical species associated with short-term mortality effects of PM2.5 in Xi’an, a Central City in China. Am. J. Epidemiol. 2012, 175, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova, J.; Mokra, D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021, 22, 4162. [Google Scholar] [CrossRef] [PubMed]
- Bredeck, G.; Busch, M.; Rossi, A.; Stahlmecke, B.; Fomba, K.W.; Herrmann, H.; Schins, R.P.F. Inhalable Saharan dust induces oxidative stress, NLRP3 inflammasome activation, and inflammatory cytokine release. Environ. Int. 2023, 172, 107732. [Google Scholar] [CrossRef] [PubMed]
- Mołocznik, A. Qualitative and quantitative analysis of agricultural dust in working environment. Ann. Agric. Environ. Med. 2002, 9, 71–78. [Google Scholar] [PubMed]
- Li, R.; Kang, H.; Chen, S. From Basic Research to Clinical Practice: Considerations for Treatment Drugs for Silicosis. Int. J. Mol. Sci. 2023, 24, 8333. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Zhou, T.; Cheng, W.; Guo, J.; Cui, X.; Liu, Y.; Chen, W. Particle-size-dependent cytokine responses and cell damage induced by silica particles and macrophages-derived mediators in endothelial cell. Environ. Toxicol. Pharmacol. 2013, 36, 921–928. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert. Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Morris, R.; Cheng, F. Signaling Pathways Regulated by Silica Nanoparticles. Molecules 2021, 26, 1398. [Google Scholar] [CrossRef] [PubMed]
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.; Yu, S.; Hong, S. pH- and temperature-responsive radially porous silica nanoparticles with high-capacity drug loading for controlled drug delivery. Nanotechnology 2020, 31, 335103. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Shi, F.; Yang, P.; Sun, K.; Zhang, J.; Yang, X.; Li, X.; Shen, F.; Liu, H.; et al. Silica nanoparticle induces pulmonary fibroblast transdifferentiation via macrophage route: Potential mechanism revealed by proteomic analysis. Toxicol. In Vitro 2021, 76, 105220. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.S.; Ignacio, R.M.; Kim, D.H.; Sajo, M.E.; Maeng, E.H.; Qi, X.F.; Park, S.E.; Kim, Y.R.; Kim, M.K.; et al. Immunotoxicity of silicon dioxide nanoparticles with different sizes and electrostatic charge. Int. J. Nanomed. 2014, 9 (Suppl. S2), 183–193. [Google Scholar] [CrossRef]
- Honnons, S.; Porcher, J.M. In vivo experimental model for silicosis. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 391–400. [Google Scholar]
- Tyrkalska, S.D.; Pedoto, A.; Martínez-López, A.; Ros-Lucas, J.A.; Mesa-Del-Castillo, P.; Candel, S.; Mulero, V. Silica crystals activate toll-like receptors and inflammasomes to promote local and systemic immune responses in zebrafish. Dev. Comp. Immunol. 2023, 138, 104523. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, D.; Tyrkalska, S.D.; Valera-Pérez, A.; Gómez-Abenza, E.; Pérez-Oliva, A.B.; Mulero, V. The zebrafish: A research model to understand the evolution of vertebrate immunity. Fish. Shellfish. Immunol. 2019, 90, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Al Mahrooqi, J.H.; Khutoryanskiy, V.V.; Williams, A.C. Thiolated and PEGylated silica nanoparticle delivery to hair follicles. Int. J. Pharm. 2021, 593, 120130. [Google Scholar] [CrossRef] [PubMed]
- Koskimäki, J.; Tarkia, M.; Ahtola-Sätilä, T.; Saloranta, L.; Simola, O.; Forsback, A.P.; Laakso, A.; Frantzén, J. Intracranial biodegradable silica-based nimodipine drug release implant for treating vasospasm in subarachnoid hemorrhage in an experimental healthy pig and dog model. Biomed. Res. Int. 2015, 2015, 715752. [Google Scholar] [CrossRef] [PubMed]
- Suedmeyer, W.K.; Johnson, G.; Lovell, M.A. Pulmonary silicosis in three North American river otters (Lutra canadensis). J. Zoo. Wildl. Med. 1999, 30, 564–572. [Google Scholar] [PubMed]
- Gutierrez, C.; Corbera, J.A.; Doreste, F.; Padrón, T.R.; Morales, M. Silica urolithiasis in the dromedary camel in a subtropical climate. Vet. Res. Commun. 2002, 26, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.W.; Knight, H.D.; Whittig, L.D.; Malloy, R.L.; Abraham, J.L.; Tyler, N.K. Silicate pneumoconiosis and pulmonary fibrosis in horses from the Monterey-Carmel peninsula. Chest 1981, 80, 82–85. [Google Scholar] [CrossRef]
- Hannothiaux, M.H.; Scharfman, A.; Wastiaux, A.; Cornu, L.; van Brussel, E.; Lafitte, J.J.; Sebastien, P.; Roussel, P. An attempt to evaluate lung aggression in monkey silicosis: Hydrolases, peroxidase and antiproteases activities in serial bronchoalveolar lavages. Eur. Respir. J. 1991, 4, 191–204. [Google Scholar] [CrossRef]
- Pinkerton, K.E.; Harbaugh, M.; Han, M.K.; Jourdan Le Saux, C.; Van Winkle, L.S.; Martin, W.J., 2nd; Kosgei, R.J.; Carter, E.J.; Sitkin, N.; Smiley-Jewell, S.M.; et al. Women and Lung Disease. Sex Differences and Global Health Disparities. Am. J. Respir. Crit. Care Med. 2015, 192, 11–16. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Jia, H.; Liu, Y.; Guo, D.; He, W.; Zhao, L.; Xia, S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ. Toxicol. 2021, 36, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, J.; Wang, B.; Liu, Y.; Xiao, L.; Ye, Z.; Fan, L.; Wang, D.; Mu, G.; Chen, W. Long-term effect of personal PM2.5 exposure on lung function: A panel study in China. J. Hazard. Mater. 2020, 393, 122457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Shaffer, M.L.; Li, X.; Rodriguez-Colon, S.; Wolbrette, D.L.; Williams, R.; Cascio, W.E.; Liao, D. Individual-level PM₂.₅ exposure and the time course of impaired heart rate variability: The APACR Study. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Xu, X.; Bai, Y.; Zhong, J.; Chen, M.; Liang, Y.; Zhao, J.; Liu, D.; Morishita, M.; Sun, Q.; et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ. Health Perspect. 2014, 122, 79–86. [Google Scholar] [CrossRef]
- Long, M.H.; Zhu, X.M.; Wang, Q.; Chen, Y.; Gan, X.D.; Li, F.; Fu, W.L.; Xing, W.W.; Xu, D.Q.; Xu, D.G. PM2.5 exposure induces vascular dysfunction via NO generated by iNOS in lung of ApoE-/- mouse. Int. J. Biol. Sci. 2020, 16, 49–60. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Z.M.; Chen, L.; Zhao, X.; Cai, M.; Wang, C.; Zou, H.; Wu, Y.; Zhang, Z.; Li, H.; et al. Exposure to Air Pollution during Pre-Hypertension and Subsequent Hypertension, Cardiovascular Disease, and Death: A Trajectory Analysis of the UK Biobank Cohort. Environ. Health Perspect. 2023, 131, 17008. [Google Scholar] [CrossRef]
- Lee, H.; Myung, W.; Kim, D.K.; Kim, S.E.; Kim, C.T.; Kim, H. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci. Rep. 2017, 7, 44741. [Google Scholar] [CrossRef]
- Kasdagli, M.I.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E. Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 2019, 222, 402–409. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292, 118320. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, G.; Zhang, L.; Chen, K. Particulate matter pollution and hospital outpatient visits for endocrine, digestive, urological, and dermatological diseases in Nanjing, China. Environ. Pollut. 2020, 261, 114205. [Google Scholar] [CrossRef]
- Pritchett, N.; Spangler, E.C.; Gray, G.M.; Livinski, A.A.; Sampson, J.N.; Dawsey, S.M.; Jones, R.R. Exposure to Outdoor Particulate Matter Air Pollution and Risk of Gastrointestinal Cancers in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Evidence. Environ. Health Perspect. 2022, 130, 36001. [Google Scholar] [CrossRef] [PubMed]
- Van Pee, T.; Nawrot, T.S.; van Leeuwen, R.; Hogervorst, J. Ambient particulate air pollution and the intestinal microbiome; a systematic review of epidemiological, in vivo and, in vitro studies. Sci. Total Environ. 2023, 878, 162769. [Google Scholar] [CrossRef] [PubMed]
- Rasking, L.; Vanbrabant, K.; Bové, H.; Plusquin, M.; De Vusser, K.; Roels, H.A.; Nawrot, T.S. Adverse Effects of fine particulate matter on human kidney functioning: A systematic review. Environ. Health 2022, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Ustáriz, J.; Marín, R.; Carrasco-Wong, I.; Farías, M.; Giordano, A.; Gallardo, F.S.; Illanes, S.E.; Gutiérrez, J. Cellular mechanisms linking to outdoor and indoor air pollution damage during pregnancy. Front. Endocrinol. 2023, 14, 1084986. [Google Scholar] [CrossRef]
- GBD 2019 Diabetes and Air Pollution Collaborators. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM(2·5) air pollution, 1990-2019: An analysis of data from the Global Burden of Disease Study 2019. Lancet Planet Health 2022, 6, e586–e600. [Google Scholar] [CrossRef]
- Zare Sakhvidi, M.J.; Lequy, E.; Goldberg, M.; Jacquemin, B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: A systematic review. Environ. Pollut. 2020, 267, 115328. [Google Scholar] [CrossRef]
- Bai, J.; Pugh, S.L.; Eldridge, R.; Yeager, K.A.; Zhang, Q.; Lee, W.R.; Shah, A.B.; Dayes, I.S.; D’Souza, D.P.; Michalski, J.M.; et al. Neighborhood Deprivation and Rurality Associated With Patient-Reported Outcomes and Survival in Men With Prostate Cancer in NRG Oncology RTOG 0415. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Omidianidost, A.; Gharavandi, S.; Azari, M.R.; Hashemian, A.H.; Ghasemkhani, M.; Rajati, F.; Jabari, M. Occupational Exposure to Respirable Dust, Crystalline Silica and Its Pulmonary Effects among Workers of a Cement Factory in Kermanshah, Iran. Tanaffos 2019, 18, 157–162. [Google Scholar] [PubMed]
- Tan, S.; Chen, S. Macrophage Autophagy and Silicosis: Current Perspective and Latest Insights. Int. J. Mol. Sci. 2021, 22, 453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; You, Y.; He, Y.; Wei, Y.; Zhang, Y.; Min, H.; Li, C.; Chen, J. Crystalline Silica-Induced Proinflammatory Interstitial Macrophage Recruitment through Notch3 Signaling Promotes the Pathogenesis of Silicosis. Environ. Sci. Technol. 2023, 57, 14502–14514. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Colby, T.V. Histiocytic lesions and proliferations in the lung. Semin. Diagn. Pathol. 2007, 24, 162–182. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Chen, S. The Mechanism and Effect of Autophagy, Apoptosis, and Pyroptosis on the Progression of Silicosis. Int. J. Mol. Sci. 2021, 22, 8110. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yuan, H.; Min, H.; Li, C.; Chen, J. Fibroblast-derived CXCL14 aggravates crystalline silica-induced pulmonary fibrosis by mediating polarization and recruitment of interstitial macrophages. J. Hazard. Mater. 2023, 460, 132489. [Google Scholar] [CrossRef] [PubMed]
- Pairon, J.C.; Brochard, P.; Jaurand, M.C.; Bignon, J. Silica and lung cancer: A controversial issue. Eur. Respir. J. 1991, 4, 730–744. [Google Scholar] [CrossRef]
- Gliga, A.R.; Grahn, K.; Gustavsson, P.; Ljungman, P.P.; Albin, M.; Selander, J.; Broberg, K. Short and long-term associations between serum proteins linked to cardiovascular disease and particle exposure among constructions workers. Scand. J. Work. Environ. Health 2023, 49, 145–154. [Google Scholar] [CrossRef]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef]
- Seaton, A.; MacNee, W.; Donaldson, K.; Godden, D. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Zhu, J.; Ritzenthaler, J.D.; Roman, J. Pulmonary hypertension and vascular remodeling in mice exposed to crystalline silica. Respir. Res. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.D.; Atwood, C.W., Jr.; Ross, S.B.; Eichhorn, K.A.; Olszewski, J.W.; Doyle, P.J. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia 2008, 23, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ahn, Y.S.; Lee, S.; Song, B.M.; Hong, S.; Yoon, J.H. Occupational exposure to crystalline silica and gastric cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2016, 73, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Möhner, M.; Pohrt, A.; Gellissen, J. Occupational exposure to respirable crystalline silica and chronic non-malignant renal disease: Systematic review and meta-analysis. Int. Arch. Occup. Environ. Health 2017, 90, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, N. Silica nephropathy. Int. J. Occup. Environ. Med. 2010, 1, 108–115. [Google Scholar] [PubMed]
- Mourad, B.H.; Ashour, Y.A. Demonstration of Subclinical Early Nephrotoxicity Induced by Occupational Exposure to Silica among Workers in Pottery Industry. Int. J. Occup. Environ. Med. 2020, 11, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, Q.Y.; Li, M.Y.; Lao, C.S.; Zhang, Y.J. Pulmonary Toxicity in Rats Caused by Exposure to Intratracheal Instillation of SiO2 Nanoparticles. Biomed. Environ. Sci. 2017, 30, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Lee, H.J.; Shin, N.R.; Seo, Y.S.; Kim, S.H.; Shin, I.S.; Kim, J.S. Silicon Dioxide Nanoparticles Enhance Endotoxin-Induced Lung Injury in Mice. Molecules 2018, 23, 2247. [Google Scholar] [CrossRef]
- Ko, J.W.; Shin, N.R.; Je-Oh, L.; Jung, T.Y.; Moon, C.; Kim, T.W.; Choi, J.; Shin, I.S.; Heo, J.D.; Kim, J.C. Silica dioxide nanoparticles aggravate airway inflammation in an asthmatic mouse model via NLRP3 inflammasome activation. Regul. Toxicol. Pharmacol. 2020, 112, 104618. [Google Scholar] [CrossRef]
- Großgarten, M.; Holzlechner, M.; Vennemann, A.; Balbekova, A.; Wieland, K.; Sperling, M.; Lendl, B.; Marchetti-Deschmann, M.; Karst, U.; Wiemann, M. Phosphonate coating of SiO2 nanoparticles abrogates inflammatory effects and local changes of the lipid composition in the rat lung: A complementary bioimaging study. Part. Fibre Toxicol. 2018, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Sohn, J.H.; Kim, Y.J.; Park, Y.H.; Han, H.; Park, K.H.; Lee, K.; Choi, H.; Um, K.; Choi, I.H.; et al. Acute exposure to silica nanoparticles aggravate airway inflammation: Different effects according to surface characteristics. Exp. Mol. Med. 2015, 47, e173. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Liu, S.; He, W.; Wang, L.; Gan, J.; Huang, Z.; Wang, Z.; Wei, H.; Zhang, J.; et al. Specifically Formed Corona on Silica Nanoparticles Enhances Transforming Growth Factor β1 Activity in Triggering Lung Fibrosis. ACS Nano 2017, 11, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Yukina, G.Y.; Polovnikov, I.V.; Sukhorukova, E.G.; Zhuravskii, S.G.; Galagudza, M.M. Morphological Analysis of the Respiratory Tract of Rats after Parenteral Administration of Silicon Dioxide Nanoparticles. Bull. Exp. Biol. Med. 2020, 170, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhao, D.; Jing, L.; Cui, G.; Jin, M.; Li, Y.; Liu, X.; Liu, Y.; Du, H.; Guo, C.; et al. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc. Toxicol. 2013, 13, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, X.; Liang, S.; Xu, Q.; Miller, M.R.; Duan, J.; Sun, Z. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part. Fibre Toxicol. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Xue, S.M.; Zhang, P.; Xu, L.N.; Wang, D.P.; Li, G.; Cao, J.M. Silica Nanoparticles Disturb Ion Channels and Transmembrane Potentials of Cardiomyocytes and Induce Lethal Arrhythmias in Mice. Int. J. Nanomed. 2020, 15, 7397–7413. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qi, Y.; Zhao, X.; Li, X.; Sun, X.; Niu, P.; Li, Y.; Guo, C.; Chen, R.; Sun, Z. Amorphous silica nanoparticles accelerated atherosclerotic lesion progression in ApoE-/- mice through endoplasmic reticulum stress-mediated CD36 up-regulation in macrophage. Part. Fibre Toxicol. 2020, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xia, Y.; Niu, P.; Jiang, L.; Duan, J.; Yu, Y.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling. Int. J. Nanomed. 2015, 10, 1463–1477. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein-nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Kenesei, K.; Murali, K.; Voetz, M.; Eiden, S.; Puntes, V.F.; Duschl, A.; Madarász, E. Interaction of differently functionalized fluorescent silica nanoparticles with neural stem- and tissue-type cells. Nanotoxicology 2014, 8 (Suppl. S1), 138–148. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, J.; Sun, J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol. Lett. 2012, 214, 91–98. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Gaiser, B.K.; Johnston, H.; Brown, D.M.; Stone, V. Toxicological effect of engineered nanomaterials on the liver. Br. J. Pharmacol. 2014, 171, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Sun, M.; Ma, Y.; Wang, F.; Sun, Z.; Duan, J. Adverse effects and underlying mechanism of amorphous silica nanoparticles in liver. Chemosphere 2023, 311, 136955. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Fu, C.; Li, L.; Chen, D.; Zhang, Y.; Tang, F. Smaller silica nanorattles reabsorbed by intestinal aggravate multiple organs damage. J. Nanosci. Nanotechnol. 2013, 13, 6506–6516. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.D.; Zhang, X.D.; Yang, X.S.; Huang, Z.L.; Wei, X.; Yang, X.F.; Liao, W.Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Sasai, F.; Rogers, K.L.; Orlicky, D.J.; Stem, A.; Schaeffer, J.; Garcia, G.; Fox, J.; Ray, M.S.; Butler-Dawson, J.; Gonzalez-Quiroz, M.; et al. Inhaled silica nanoparticles cause chronic kidney disease in rats. Am. J. Physiol. Renal Physiol. 2022, 323, F48–F58. [Google Scholar] [CrossRef] [PubMed]
- Passagne, I.; Morille, M.; Rousset, M.; Pujalté, I.; L’Azou, B. Implication of oxidative stress in size-dependent toxicity of silica nanoparticles in kidney cells. Toxicology 2012, 299, 112–124. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Sarkar, A. Overview on biological implications of metal oxide nanoparticle exposure to human alveolar A549 cell line. Nanotoxicology 2017, 11, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Yang, S.; Li, W.; Ming, Z.; Zhang, Y.; Hou, Y.; Niu, Z.; Rong, B.; Zhang, X.; et al. Differential mitochondrial proteome analysis of human lung adenocarcinoma and normal bronchial epithelium cell lines using quantitative mass spectrometry. Thorac. Cancer 2013, 4, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Sha, S.; Li, J.; Zhou, Z.; Cao, Y. Evaluation of in vitro toxicity of silica nanoparticles (NPs) to lung cells: Influence of cell types and pulmonary surfactant component DPPC. Ecotoxicol. Environ. Saf. 2019, 186, 109770. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J.; Blanchard, R.K.; Popp, M.P.; Liu, L.; Cao, J.; Moore, J.B.; Green, C.L. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6952–6957. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.D.; Thornton, J.; Barker, K.S.; McDaniel, D.O.; Sacks, G.S.; Swiatlo, E.; McDaniel, L.S. Pneumolysin-dependent and -independent gene expression identified by cDNA microarray analysis of THP-1 human mononuclear cells stimulated by Streptococcus pneumoniae. Infect. Immun. 2003, 71, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, R.; Yan, X. Airway hyperresponsiveness development and the toxicity of PM2.5. Environ. Sci. Pollut. Res. Int. 2021, 28, 6374–6391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Zhu, J.; Li, C.; Zhang, T.; Liu, H.; Xu, Q.; Ye, X.; Zhou, L.; Ye, L. Effect of Atmospheric PM2.5 on Expression Levels of NF-κB Genes and Inflammatory Cytokines Regulated by NF-κB in Human Macrophage. Inflammation 2018, 41, 784–794. [Google Scholar] [CrossRef]
- Fan, X.; Dong, T.; Yan, K.; Ci, X.; Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 2023, 59, 102587. [Google Scholar] [CrossRef]
- Shim, I.; Kim, W.; Kim, H.; Lim, Y.M.; Shin, H.; Park, K.S.; Yu, S.M.; Kim, Y.H.; Sung, H.K.; Eom, I.C.; et al. Comparative Cytotoxicity Study of PM2.5 and TSP Collected from Urban Areas. Toxics 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, Y.; Sun, J.; Feng, J.; Chen, L.; Chen, H.; Zeng, S.; Chen, C.; Li, X.; Zhu, H.; et al. Flavone protects HBE cells from DNA double-strand breaks caused by PM2.5. Human Cell 2018, 31, 116–126. [Google Scholar] [CrossRef]
- Wei, M.; Bao, G.; Li, S.; Yang, Z.; Cheng, C.; Le, W. PM2.5 exposure triggers cell death through lysosomal membrane permeabilization and leads to ferroptosis insensitivity via the autophagy dysfunction/p62-KEAP1-NRF2 activation in neuronal cells. Ecotoxicol. Environ. Saf. 2022, 248, 114333. [Google Scholar] [CrossRef]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of Ambient Fine Particles PM2.5 on Human HaCaT Cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Liu, W.S.; Wan, C.; Wang, H.H. Pentraxin 3 mediates early inflammatory response and EMT process in human tubule epithelial cells induced by PM2.5. Int. Immunopharmacol. 2022, 112, 109258. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Wang, S.; Jiang, L.; Sun, X.; Liu, X.; Yao, X.; Zhang, C.; Wang, N.; Yang, G. 2-Undecanone Protects against Fine Particle-Induced Kidney Inflammation via Inducing Mitophagy. J. Agric. Food Chem. 2021, 69, 5206–5215. [Google Scholar] [CrossRef] [PubMed]
- Nääv, Å.; Erlandsson, L.; Isaxon, C.; Åsander Frostner, E.; Ehinger, J.; Sporre, M.K.; Krais, A.M.; Strandberg, B.; Lundh, T.; Elmér, E.; et al. Urban PM2.5 Induces Cellular Toxicity, Hormone Dysregulation, Oxidative Damage, Inflammation, and Mitochondrial Interference in the HRT8 Trophoblast Cell Line. Front. Endocrinol. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.M.; Zhao, Z.M.; Zhou, W.; Yuan, X.Y.; Jia, L.; Zhao, J.; Peng, S.Q. Oxidative damage related to PM2.5 exposure in human embryonic stem cell-derived fibroblasts. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.I.; Waksman, J.; Curtis, J. Silicosis: A review. Dis. Mon. 2007, 53, 394–416. [Google Scholar] [CrossRef]
- Castranova, V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: Role of reactive oxygen/nitrogen species. Free Radic. Biol. Med. 2004, 37, 916–925. [Google Scholar] [CrossRef]
- Zhao, J.H.; Li, S.; Du, S.L.; Zhang, Z.Q. The role of mitochondrial dysfunction in macrophages on SiO2-induced pulmonary fibrosis: A review. J. Appl. Toxicol. 2024, 44, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sun, Y.; Zhou, N.; Wu, W.; Zheng, W.; Wang, Y. Dihydroquercetin Attenuates Silica-Induced Pulmonary Fibrosis by Inhibiting Ferroptosis Signaling Pathway. Front. Pharmacol. 2022, 13, 845600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xu, Q.; Wang, Y.; Li, G.; Sun, W.; Ma, D.; Zhou, S.; Liu, Y.; Han, L.; Ni, C. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J. Transl. Med. 2021, 19, 349. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chakraborty, P.; Jolly, M.K.; Levine, H. A Theoretical Approach to Coupling the Epithelial-Mesenchymal Transition (EMT) to Extracellular Matrix (ECM) Stiffness via LOXL2. Cancers 2021, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.N.; Dentener, M.A.; Stassen, F.R.; Rohde, G.G.; Mossman, B.T.; Wouters, E.F.; Reynaert, N.L. Alteration of canonical and non-canonical WNT-signaling by crystalline silica in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2016, 301, 61–70. [Google Scholar] [CrossRef]
- Greish, K.; Thiagarajan, G.; Herd, H.; Price, R.; Bauer, H.; Hubbard, D.; Burckle, A.; Sadekar, S.; Yu, T.; Anwar, A.; et al. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology 2012, 6, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, J.; Chai, X.; Yang, M.; Wang, J.; Chen, R.; Sun, Z. Microarray-assisted size-effect study of amorphous silica nanoparticles on human bronchial epithelial cells. Nanoscale 2019, 11, 22907–22923. [Google Scholar] [CrossRef]
- Wottrich, R.; Diabaté, S.; Krug, H.F. Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono- and co-culture. Int. J. Hyg. Environ. Health 2004, 207, 353–361. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef]
- Blechinger, J.; Bauer, A.T.; Torrano, A.A.; Gorzelanny, C.; Bräuchle, C.; Schneider, S.W. Uptake kinetics and nanotoxicity of silica nanoparticles are cell type dependent. Small 2013, 9, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257–5276. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Wang, Z.J.; Zhao, R.; Lin, C.X.; Sun, Q.Y.; Yan, C.P.; Zhou, X.; Cao, J.M. Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part. Fibre Toxicol. 2020, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ge, D.; Mirshafiee, V.; Chen, C.; Li, M.; Xue, C.; Ma, X.; Sun, B. Assessment of neurotoxicity induced by different-sized Stöber silica nanoparticles: Induction of pyroptosis in microglia. Nanoscale 2019, 11, 12965–12972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Wang, J.; Li, Y.; Li, Y.; Wei, J.; Zheng, T.; Jin, M.; Sun, Z. Silica nanoparticles induced intrinsic apoptosis in neuroblastoma SH-SY5Y cells via CytC/Apaf-1 pathway. Environ. Toxicol. Pharmacol. 2017, 52, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Jeong, J.; Yoon, D.; Kim, S.; Choi, J. Global metabolomics approach in in vitro and in vivo models reveals hepatic glutathione depletion induced by amorphous silica nanoparticles. Chem. Biol. Interact. 2018, 293, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar] [CrossRef]
- Zuo, D.; Duan, Z.; Jia, Y.; Chu, T.; He, Q.; Yuan, J.; Dai, W.; Li, Z.; Xing, L.; Wu, Y. Amphipathic silica nanoparticles induce cytotoxicity through oxidative stress mediated and p53 dependent apoptosis pathway in human liver cell line HL-7702 and rat liver cell line BRL-3A. Colloids Surf. B Biointerfaces 2016, 145, 232–240. [Google Scholar] [CrossRef]
- Guo, Z.; Martucci, N.J.; Liu, Y.; Yoo, E.; Tako, E.; Mahler, G.J. Silicon dioxide nanoparticle exposure affects small intestine function in an in vitro model. Nanotoxicology 2018, 12, 485–508. [Google Scholar] [CrossRef]
- Abounit, K.; Scarabelli, T.M.; McCauley, R.B. Autophagy in mammalian cells. World J. Biol. Chem. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Rafieepour, A.; Azari, M.R.; Khodagholi, F. Cytotoxic effects of crystalline silica in form of micro and nanoparticles on the human lung cell line A549. Toxicol. Ind. Health 2023, 39, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tao, S.; Huang, L.; Du, J.; Liu, C.; Jiang, Y.; Jiang, T.; Lv, H.; Lu, Q.; Meng, Q.; et al. Maternal PM2.5 exposure during gestation and offspring neurodevelopment: Findings from a prospective birth cohort study. Sci. Total Environ. 2022, 842, 156778. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, R.; Yin, P.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Cohen, A.J.; Brauer, M.; Liu, C.; Wang, W.; et al. Associations of long-term exposure to fine particulate matter and its constituents with cardiovascular mortality: A prospective cohort study in China. Environ. Int. 2022, 162, 107156. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- West, J.J.; Cohen, A.; Dentener, F.; Brunekreef, B.; Zhu, T.; Armstrong, B.; Bell, M.L.; Brauer, M.; Carmichael, G.; Costa, D.L.; et al. What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health. Environ. Sci. Technol. 2016, 50, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Oftedal, B.; Chen, J.; Rodopoulou, S.; Renzi, M.; Atkinson, R.W.; Bauwelinck, M.; Klompmaker, J.O.; Mehta, A.; Vienneau, D.; et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: Results from seven large European cohorts within the ELAPSE project. Lancet Planet. Health 2022, 6, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, J.; Huang, J.; Kelly, F.J.; Li, G. Long-term Exposure to Multiple Ambient Air Pollutants and Association With Incident Depression and Anxiety. JAMA Psychiatry 2023, 80, 305–313. [Google Scholar] [CrossRef]
- Misra, S.; Sussell, A.L.; Wilson, S.E.; Poplin, G.S. Occupational exposure to respirable crystalline silica among US metal and nonmetal miners, 2000-2019. Am. J. Ind. Med. 2023, 66, 199–212. [Google Scholar] [CrossRef]
- De Matteis, S.; Heederik, D.; Burdorf, A.; Colosio, C.; Cullinan, P.; Henneberger, P.K.; Olsson, A.; Raynal, A.; Rooijackers, J.; Santonen, T.; et al. Current and new challenges in occupational lung diseases. Eur. Respir. Rev. 2017, 26, 170080. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Carey, R.N.; Reid, A.; Driscoll, T.; Glass, D.C.; Peters, S.; Benke, G.; Darcey, E.; Fritschi, L. The Australian Work Exposures Study: Prevalence of Occupational Exposure to Respirable Crystalline Silica. Ann. Occup. Hyg. 2016, 60, 631–637. [Google Scholar] [CrossRef]
- Sharma, N.; Kundu, D.; Dhaked, S.; Das, A. Silicosis and silicotuberculosis in India. Bull. World Health Organ. 2016, 94, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Fowler, P.; Kirkland, D. An updated review of the genotoxicity of respirable crystalline silica. Part. Fibre Toxicol. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Saers, J.; Andersson, L.; Janson, C.; Sundh, J. Respiratory symptoms, lung function, and fraction of exhaled nitric oxide before and after assignment in a desert environment-a cohort study. Respir. Med. 2021, 189, 106643. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes. Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Mannetje, A.; Boffetta, P.; Stayner, L.; Attfield, M.; Chen, J.; Dosemeci, M.; DeKlerk, N.; Hnizdo, E.; Koskela, R.; et al. Pooled exposure-response analyses and risk assessment for lung cancer in 10 cohorts of silica-exposed workers: An IARC multicentre study. Cancer Causes Control 2001, 12, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.F.; Jeebhay, M.F.; Cavalin, C.; Chen, W.; Cohen, R.A.; Fireman, E.; Go, L.H.T.; León-Jiménez, A.; Menéndez-Navarro, A.; Ribeiro, M.; et al. Current global perspectives on silicosis-Convergence of old and newly emergent hazards. Respirology 2022, 27, 387–398. [Google Scholar] [CrossRef]
- Boudigaard, S.H.; Schlünssen, V.; Vestergaard, J.M.; Søndergaard, K.; Torén, K.; Peters, S.; Kromhout, H.; Kolstad, H.A. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: A nationwide cohort study. Int. J. Epidemiol. 2021, 50, 1213–1226. [Google Scholar] [CrossRef]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef]
- Johnson, R.L., Jr. Relative effects of air pollution on lungs and heart. Circulation 2004, 109, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.; Jacobson, J.; Kroening, Z.; Pierce, C. PM 2.5 Airborne Particulates Near Frac Sand Operations. J. Environ. Health 2015, 78, 8–12. [Google Scholar] [PubMed]
- Westberg, H.; Hedbrant, A.; Persson, A.; Bryngelsson, I.L.; Johansson, A.; Ericsson, A.; Sjögren, B.; Stockfelt, L.; Särndahl, E.; Andersson, L. Inflammatory and coagulatory markers and exposure to different size fractions of particle mass, number and surface area air concentrations in Swedish iron foundries, in particular respirable quartz. Int. Arch. Occup. Environ. Health 2019, 92, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Nie, J.; Duan, J.; Shi, Y.; Feng, L.; Yang, X.; An, Y.; Sun, Z. The chronic effect of amorphous silica nanoparticles and benzo[a]pyrene co-exposure at low dose in human bronchial epithelial BEAS-2B cells. Toxicol. Res. 2019, 8, 731–740. [Google Scholar] [CrossRef]
| Political Bodies | United States | European Union | Japan | Korea | China | ||
|---|---|---|---|---|---|---|---|
| Primary * | Secondary ** | Primary *** | Secondary **** | ||||
| Daily (µg/m3) | 35 | 35 | - | 35 | 35 | 35 | 75 |
| Annual (µg/m3) | 9 | 15 | 25 | 15 | 15 | 15 | 35 |
| PC TWA (mg/m³) | United States | European Union | China | ||
|---|---|---|---|---|---|
| 10% ≤ Free SiO2 * ≤ 50% | 50% < Free SiO2 ≤ 80% | Free SiO2 > 80% | |||
| Total dust | - | - | 1 | 0.7 | 0.5 |
| Respirable dust | 0.05 | 0.1 | 0.7 | 0.3 | 0.2 |
| Animal Models | Advantages | Disadvantages |
|---|---|---|
| Mice | Diminutive size, ease of breeding and maintenance in large numbers, rapid life cycle, and convenient sample collection | Physical characteristics are not exactly the same as humans; the reproducibility of experimental results is poor; limited expression of human disease genes |
| Rats | Medium size, docile, high blood volume, and fertility | |
| Zebrafish | Ease of rearing, low maintenance costs, external fertilization and development, transparency of early life, and adaptability to chemical and genetic screening | |
| Pigs | Similar to humans in anatomical size and structure, physiology, immunity, and genome | |
| Dogs | Ease of compliance and high test fit |
| Animal Models | Types of Dust | Treatment | Main Results | Reference |
|---|---|---|---|---|
| C57BL/6N mice | PM2.5 | 1.8, 5.4, and 16.2 mg/kg PM2.5 through intratracheal instillation on day 1, day 4, and day 7 | Exposure to PM2.5 significantly altered the abundance, homogeneity, and composition of the lung microbiota | [43] |
| C57BL/6J mice | PM2.5 | Exposed to concentrated ambient PM2.5 for 6 months | Long-term PM2.5 exposure increased blood pressure through sympathetic nervous system activation | [45] |
| ApoE−/− mice | PM2.5 | Instilled with PM2.5 at a dose of 4 mg/kg | PM2.5 exposure directly activated inducible nitric oxide synthase (iNOS) in the lungs to produce excess nitric oxide (NO) as the initiating factor of vascular dysfunction | [46] |
| C57BL/6JGpt mice | Crystalline silica | 30 mg/kg silica through intratracheal instillation | Crystalline silica induced pulmonary inflammation and fibrosis in mice | [64] |
| C57BL6 mice | Crystalline silica | Intratracheally injected with crystalline silica at doses of 0.2, 0.3, and 0.4 g/kg | Silica promoted the damage of the pulmonary vasculature through mechanisms that might involve endothelial dysfunction, inflammation, and vascular remodeling | [72] |
| Male Wistar rats | Synthetic silica nanoparticles | Instilled intratracheally with 1 mL of saline containing 6.25, 12.5, and 25.0 mg of nanosilica for 30 d | The nanosilica resulted in pulmonary fibrosis by means of increased lipid peroxidation and the high expression of cytokines | [78] |
| Female C57BL/6 mice | Nanosilica | Instillation at doses of 0.1 mg/kg and 0.05 mg/kg on days 2–4 | The respiratory tract exposure to nanosilica led to inflammatory responses, including an increase in the number of inflammatory cells and the production of proinflammatory cytokines, which is related to the elevation of MAPK phosphorylation | [79] |
| Female BALB/c mice | Nanosilica | 5, 10, and 20 mg/kg nanosilica through intranasal instillation | Exposure to nanosilica significantly elevated the characteristic markers of asthma including aryl hydrocarbon receptor (AHR), levels of inflammatory mediators and IgE, inflammatory cell infiltration, and mucus production | [80] |
| Female BALB/c mice | S-SNPs *; M-SNPs **; P-SNPs *** | Intranasally inoculated with nanosilica | Acute nanosilica exposure induced significant airway inflammation and further aggravated airway inflammation | [82] |
| Wistar rats | Amorphous silica nanoparticles | 2, 5, and 10 mg/kg nanosilica through intratracheal instillation | Nanosilica exposure significantly decreased the levels of superoxide dismutase, glutathione peroxidase, and NO production, while increasing the production of malondialdehyde (MDA) | [85] |
| ApoE−/− mice | Amorphous silica nanoparticles | 1.5, 3.0, and 6.0 mg/kg nanosilica through intratracheal instillation once every 7 days and 12 times in total | The serum levels of total triglycerides and low-density lipoprotein cholesterol were elevated after nanosilica exposure | [88] |
| Male Tuck-Ordinary mice | Amorphous silica nanoparticles | Intraperitoneally administered with nanosilica at a dose of 0.25 mg/kg | Acute systemic exposure to nanosilica caused oxidative stress, inflammation, and DNA damage in multiple major organs, including the lungs, heart, liver, and brain | [93] |
| Male C57BI/6 mice | M-SNPs | 50, 100, and 200 mg/kg of nanosilica through intragastric administration | Nanosilica affected the expression of metabolites involved in a series of metabolic pathways, including pyrimidine metabolism, purine metabolism, central carbon metabolism in cancer, protein digestion and absorption, and mineral absorption | [97] |
| Wistar rats | Amorphous silica nanoparticles | 4 mg/dose nanosilica oropharyngeal aspiration twice a week | Nanosilica exposure caused kidney damage, with early tubular injury and inflammation | [98] |
| Cell Models | Types of Dust and Treatment | Main Results | Reference |
|---|---|---|---|
| Macrophages | Treated with 100, 200, and 400 μg/mL PM2.5 for 12, 24, and 48 h | The survival rate of macrophages decreased with increasing PM2.5 exposure time and dose; the levels of TNF-α and CRP in macrophages elevated | [109] |
| Beas-2b cells | Treated with 50, 100, 200, and 400 μg/mL PM2.5 for 24 h | PM2.5 significantly increased the levels of ROS in a dose-dependent manner, leading to NOX4/Nrf2 redox imbalance | [110] |
| A549 cells | Treated with 25, 50, 100, 200, and 400 μg/mL for PM2.5 24 h | PM2.5 activated the Keap1/Nrf2 pathway, leading to excessive intracellular ROS production and significant cytotoxicity in a dose-dependent manner | [111] |
| HBE cells | Treated with 16, 32, 64, and 128 μg/mL PM2.5 for 24 h | PM2.5 evidently induced viability inhibition, DNA damage, and part of apoptosis compared with the normal control in a forward concentration-dependent manner | [112] |
| N2a cells | Treated with 25, 50, 100, 200, and 400 μg/mL PM2.5 for 24 h | PM2.5 exposure induced a significant increase in intracellular ROS and disruption of physiological functions of macromolecules in a concentration-dependent manner | [113] |
| HaCaT cells | Treated with 5, 10, 25, 50, 100, 200, 300, 400, 500, and 800 μg/mL for 24 h | PM2.5 stimulated HaCaT cells to release a series of cytokines and proinflammatory cytokines, further stimulating epithelial cells, fibroblasts, and endothelial cells to secrete cytokines and adhesion molecules | [114] |
| HK-2 cells | Treated with 50 μg/mL PM2.5 for 0, 6, 12, and 24 h | The concentrations of IL-1β, TNF-α, and IL-6 in culture supernatants were significantly increased in HK-2 cells following PM2.5 exposure time-dependently | [115] |
| HEK-293 cells | Treated with 50, 100, and 200 μg/mL PM2.5 for 24 h. | The levels of IL-6, IL-18, TNF-α, and IL-1β were increased significantly relative to controls | [116] |
| HTR-8 cells | Treated with 1000, 5000, 10,000 ng/mL PM2.5 for 48 h. | The levels of IL-6 increased and the levels of hCG in cells decreased dose-dependently, resulting in a decrease in cellular viability and ER stress | [117] |
| EBf-H9 cells | Treated with 3.91, 7.81, 15.63, 31.25, 62.50, and 125.00 μg/cm2 of PM2.5 for 6 h | PM2.5 exposure reduced cell viability, increased lipid peroxidation levels, and induced ROS production, causing oxidative stress and cellular oxidative damage | [118] |
| HBE cells and MRC-5 cells | HBE cells were treated with 50 μg/mL silica for 24 h; the obtained supernatant was used to treat MRC-5 cells for 48 h | Silica increased the levels of collagen I (COL I) and α-smooth muscle actin (α-SMA) in MRC-5 cells and activated ferroptosis in HBE cells | [122] |
| A549, HBE and THP-1 cells | HBE and A549 cells were treated with 30, 50, 10, 150, and 200 μg/mL silica for 24 h or treated with 200 μg/mL silica for 12, 24, and 48 h separately; THP-1 cells were treated with 200 μg/mL silica for 12 h | Silica increased the expression of α-SMA and waveform protein and decreased the expression of E-calmodulin dose-dependently | [123] |
| Beas-2b cells and PBEC | BEAS-2B were exposed to 150 × 106 μm2/cm2 and PBECs were exposed to 100 × 106 μm2/cm2 crystalline silica | Crystalline silica induced canonical Wnt signaling (β-catenin) and decreased non-canonical (Wnt5A) signaling, contributing to proliferative, fibrogenic, and inflammatory responses in lung epithelial cells | [125] |
| BEAS-2B cells | Exposed to 25 μg/mL 41, 61, and 200 nm amorphous silica nanoparticles for 24 h | The genes involved in the immune and inflammatory response, gene expression, signal transduction, ER stress, oxidative stress, cell metabolism, and cell proliferation were gradually upregulated with the particle size decreasing | [128] |
| A549 cells | Exposed to 10, 50, and 100 μg/mL 15 nm and 46 nm amorphous silica nanoparticles for 12, 24, and 48h | Exposure to 15 nm nanosilica generated oxidative stress in A549 cells as reflected by reduced GSH levels, elevated production of MDA, and lactate dehydrogenase (LDH) leakage | [130] |
| HUVECs | Exposed to 50 μg/mL 60 nm nanosilica for 24 h | Nanosilica induced LDH release and oxidative injury, promoted the activation of Nrf2 signaling, promoted endothelial cell apoptosis and autophagy, and induced MMP collapse | [131] |
| HUVECs | Exposed to 12.5, 25, 50, and 100 μg/mL 58 nm Stöber silica nanoparticles for 24 h | Nanosilica induced ROS generation and caused redox imbalance, oxidative stress, and NO/NOS system disorder, leading to oxidative damage and inflammation response, further resulting in endothelial cytotoxicity and endothelial dysfunction via the MAPK-Nrf2 and NF-κB signaling pathways | [89] |
| HUVECs | Exposed to 25, 50, 100, and 200 μg/mL 20 and 100 nm S-SNPs * for 24 h | Nanosilica-100 at 100 μg/mL and 200 μg/mL was more toxic in inducing LDH release, decreasing the viability of HUVECs, and damaging the membrane integrity than nanosilica-20 at the same concentrations | [133] |
| N9 cells | Exposed to 50, 100, 150, and 200 μg/mL 50, 100, and 300 nm Stöber silica nanoparticles for 24 h | Nanosilica exhibited particle-size-dependent toxicity, leading to mitochondrial ROS production, expression of proinflammatory cytokines, and gaseous protein-d cleavage and pyroptosis | [134] |
| SH-SY5Y cells | Exposed to 3.125, 6.25, 12.5, 25, and 50 μg/mL 70 nm Stöber silica nanoparticles for 24 h | Nanosilica led to MMP loss, mitochondria damage, decreased cell viability, caused neural cell membrane damage, increased intracellular Ca2+ level, and elevated the expression of Apaf1 and Cyt C, which triggered caspase cascades of caspase-9 and caspase-3, ultimately resulting in neural apoptosis | [135] |
| HepG2 | Exposed to 10, 25, 50, 100, and 200 μg/mL 15 nm amorphous silica nanoparticles for 24 h | Nanosilica exposure caused alterations in global metabolomics, specifically, the depletion of glutathione, NADPH oxidase-mediated ROS formation, and alteration in the antioxidant enzyme system | [136] |
| HepG2 | Exposed to 1, 5, 10, 25, 50, 100, and 200 μg/mL 14nm amorphous silica nanoparticles for 72 h | Nanosilica-induced apoptosis in HepG2 may be mediated and regulated through the p53, Bax/Bcl-2, and cysteine asparaginase pathways | [137] |
| HL-7702 and BRL-3A cells | Exposed to 31.25, 62.5, 125, 250, and 500 μg/mL 20 nm amphipathic silica nanoparticles for 72 h | Nanosilica induced cytotoxicity and oxidative stress in HL-7702 and BRL-3A cells in dose-dependent manners through oxidative stress-mediated and p53, Bax/Bcl-2, and caspase-dependent pathways | [138] |
| Caco-2 and HT29-MTX co-cultures | Exposed to 2.65 g/cm3 30 nm amorphous silica nanoparticles for 4 h or 5 d | Nanosilica significantly affected nutrient absorption and barrier function, decreased the number of intestinal microvilli, and significantly increased the brush border membrane enzyme intestinal alkaline phosphatase, further changed the expression levels of nutrient transport proteins, and induced ROS and proinflammatory signaling | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, A.; Li, R.; Chen, G.; Chen, S. Impact of Respiratory Dust on Health: A Comparison Based on the Toxicity of PM2.5, Silica, and Nanosilica. Int. J. Mol. Sci. 2024, 25, 7654. https://doi.org/10.3390/ijms25147654
Hu A, Li R, Chen G, Chen S. Impact of Respiratory Dust on Health: A Comparison Based on the Toxicity of PM2.5, Silica, and Nanosilica. International Journal of Molecular Sciences. 2024; 25(14):7654. https://doi.org/10.3390/ijms25147654
Chicago/Turabian StyleHu, Aoxiang, Rou Li, Guo Chen, and Shi Chen. 2024. "Impact of Respiratory Dust on Health: A Comparison Based on the Toxicity of PM2.5, Silica, and Nanosilica" International Journal of Molecular Sciences 25, no. 14: 7654. https://doi.org/10.3390/ijms25147654






