Genome-Wide Characterization of Fructose 1,6-Bisphosphate Aldolase Genes and Expression Profile Reveals Their Regulatory Role in Abiotic Stress in Cucumber
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification of CsFBA Genes in Cucumber
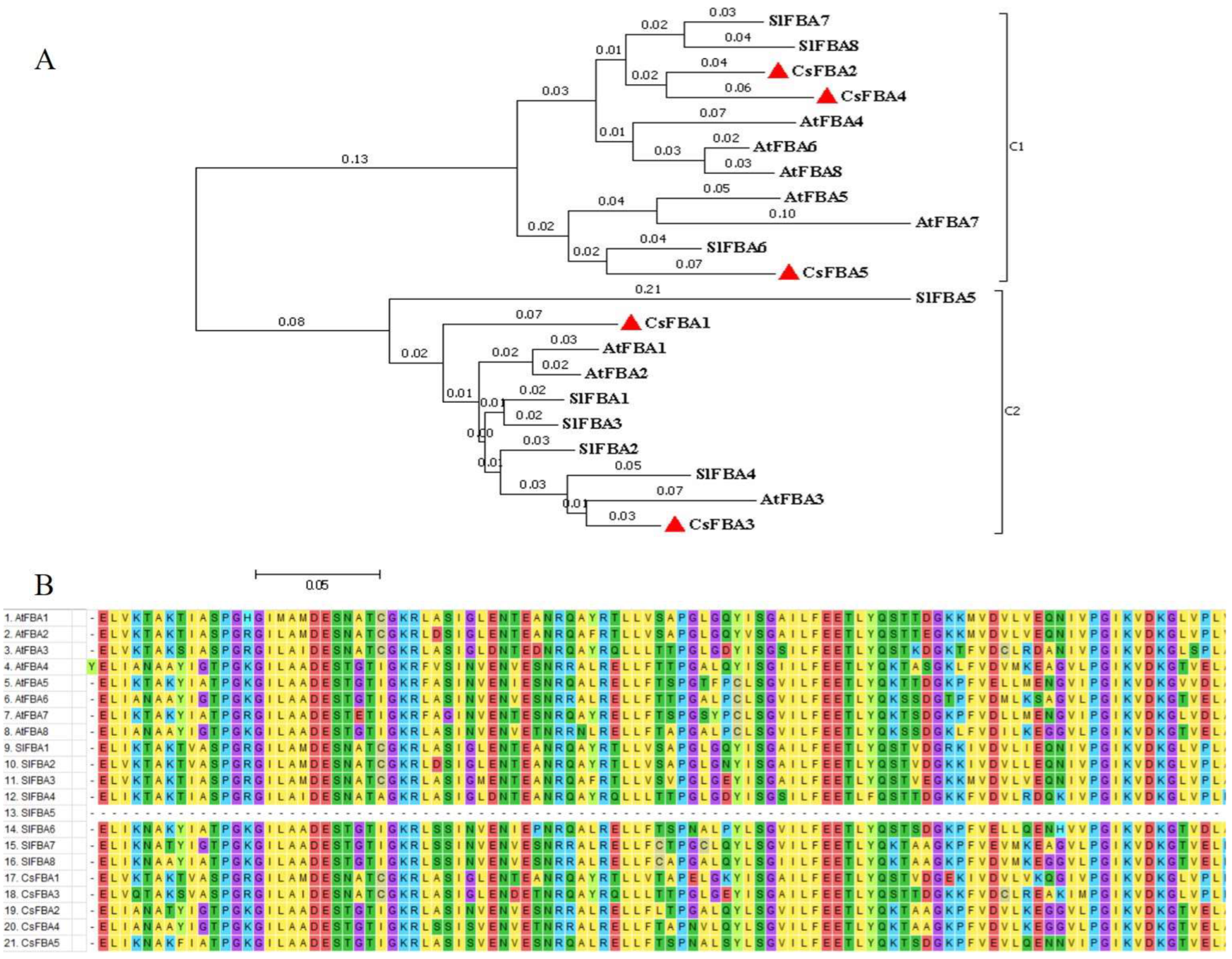
2.2. Phylogenetic Analysis of the FBA Family from Arabidopsis, Tomato, and Cucumber Genomes
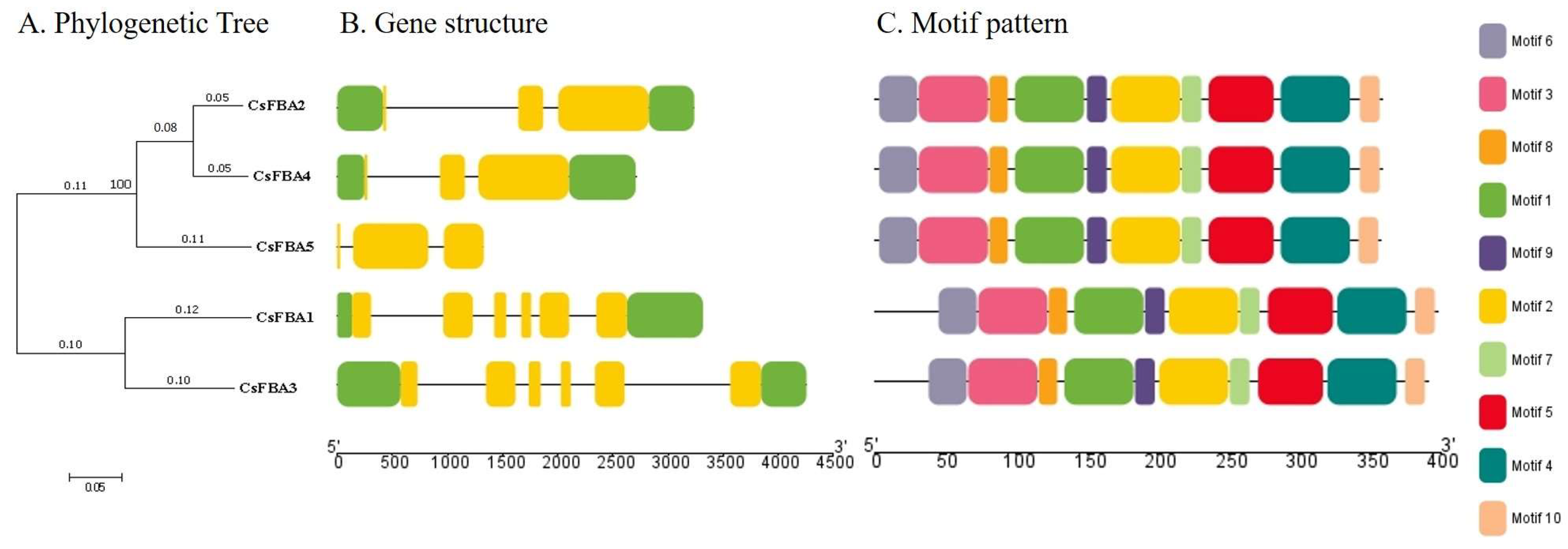
2.3. Gene Structure and Conserved Motif Analysis
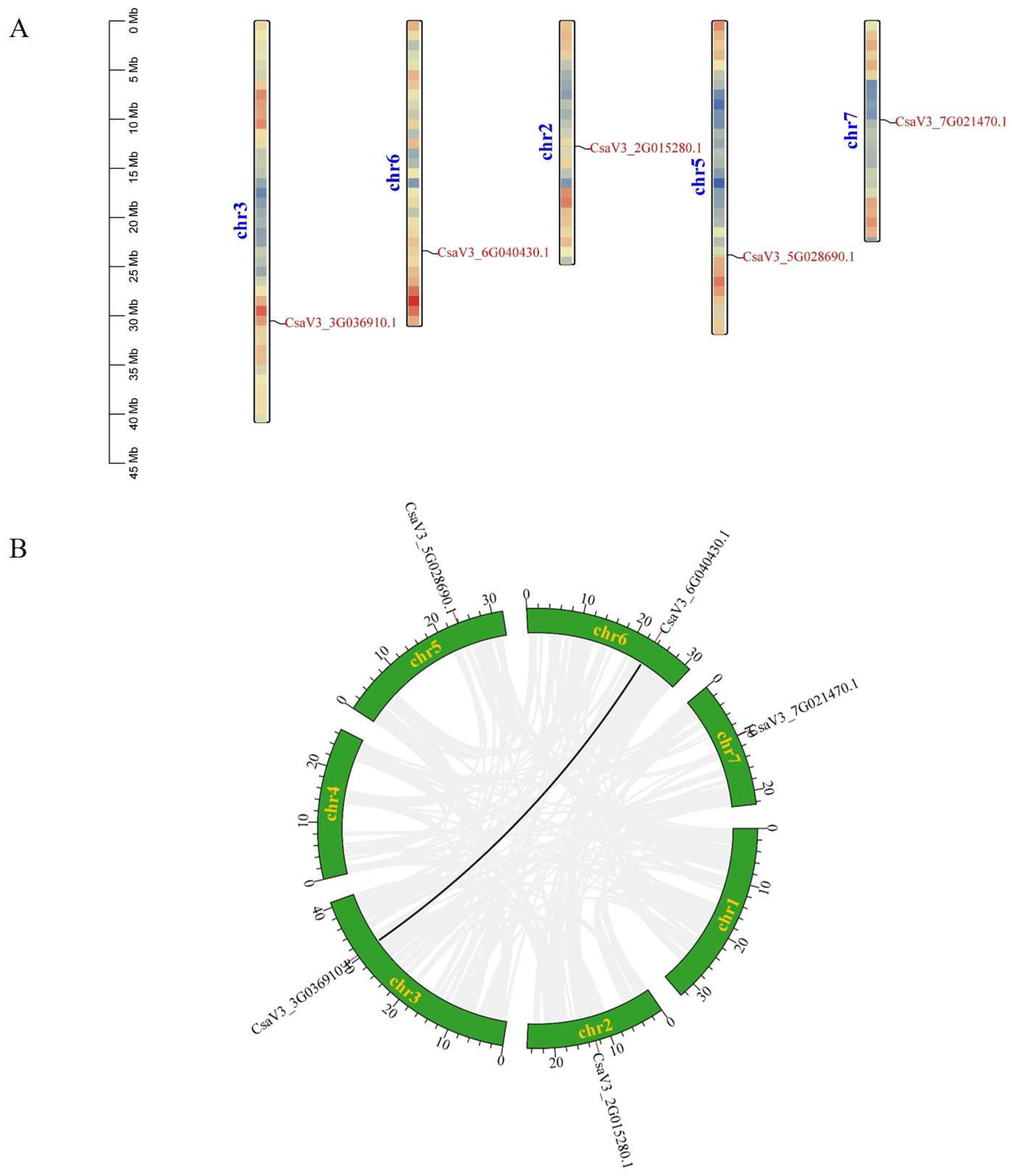
2.4. Chromosomal Distribution and Gene Duplication Analysis of CsFBA Genes
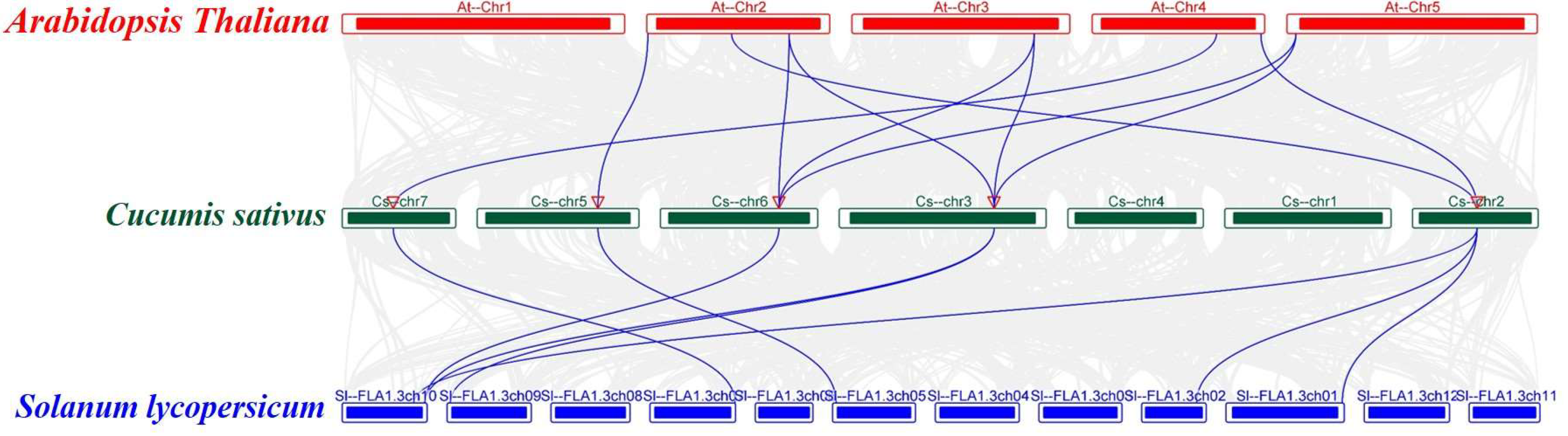
2.5. Analysis of Collinearity and Evolutionary Relationships between CsFBA and FBA Proteins in Arabidopsis and Tomato Genomes
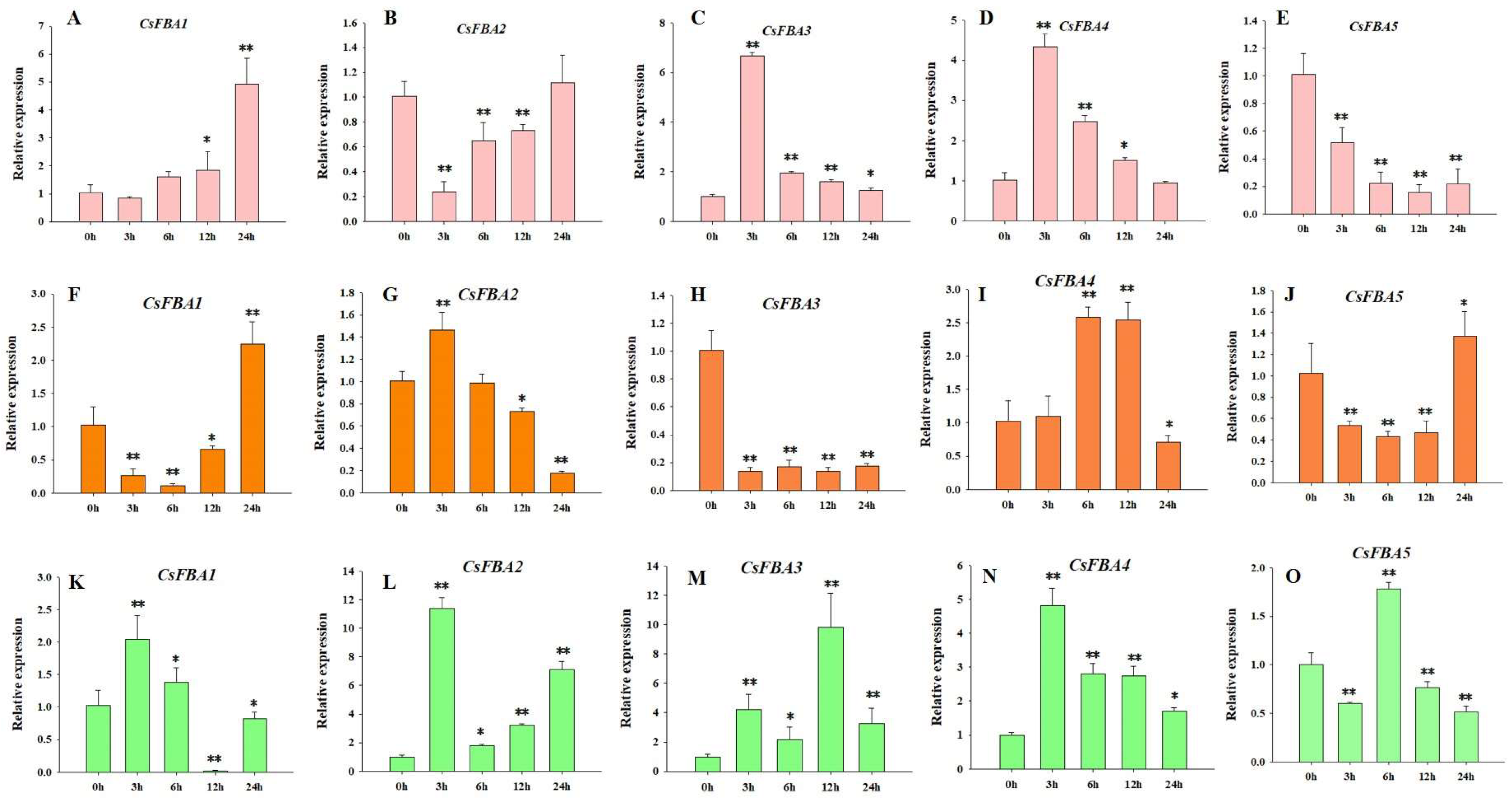
2.6. Expression Pattern of CsFBAs in Different Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Identification of CsFBA Genes in the Cucumber Genome
4.3. Phylogenetic Analysis
4.4. Gene Structure and Conserved Motif Analysis
4.5. Chromosomal Distribution and Synteny Analysis of CsFBA Genes
4.6. Total RNA Extraction, Reverse Transcription, and qRT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FBA | Fructose-1, 6-bisphosphate aldolase |
| ROS | Reactive oxygen species |
| FBPase | Fructose-1,6-diphosphatase |
| SBPase | Isoheptanone-1,7-diphosphatase |
| IAA | Indoleacetic acid |
| ABA | Abscisic acid |
| SA | Salicylic acid |
| JA | Jasmonic acid |
| ET | Ethylene |
| DREB1 | Dehydration-responsive element binding factor 1 |
| CBF | C-repeat binding factor |
| COR | Cold response |
| qRT-PCR | Quantitative real-time PCR |
| MW | Molecular weight |
| pI | Isoelectric point |
References
- Wang, X.; Mi, S.; Miao, H. Transcriptomic responses to chilling reveal potential chilling tolerance mechanisms in cucumber. Int. J. Mol. Sci. 2022, 23, 12834. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Guo, L.; Li, D.; Liu, A.; Bilal, M.; Xie, C.; Yang, R.; Gu, Z.; Jiang, D.; et al. Antifreeze polysaccharides from wheat bran: The structural characterization and antifreeze mechanism. Biomacromolecules 2024, 10, 3c00958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, A.H.; Li, X.M.; Lu, C.M. The role of chloroplast gene expression in plant responses to environmental stress. Int. J. Mol. Sci. 2020, 21, 6082. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast immunity illuminated. New Phytol. 2021, 229, 3088–3107. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.; Lehotai, N.; Strand, Å. The role of retrograde signals during plant stress responses. J. Exp. Bot. 2018, 69, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Z.; Meyer, E.H.; Wu, S.; Bock, R. Extensive posttranscriptional regulation of nuclear gene expression by plastid retrograde signals. Plant Physiol. 2019, 180, 2034–2048. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.C.; Burch-Smith, T.M. Chloroplasts as mediators of plant biotic interactions over short and long distances. Curr. Opin. Plant Biol. 2019, 50, 148–155. [Google Scholar] [CrossRef]
- Gan, P.; Liu, F.; Li, R.B.; Wang, S.K.; Luo, J.J. Chloroplasts-beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef]
- KingstonSmith, A.H.; Harbinson, J.; Williams, J.; Foyer, C.H. Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol. 1997, 114, 1039–1046. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; Li, F.D.; Bi, H.G.; Ai, X.Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef]
- Chen, J.X.; Mao, L.C.; Mi, H.B.; Lu, W.J.; Ying, T.J.; Luo, Z.S. Involvement of three annexin genes in the ripening of strawberry fruit regulated by phytohormone and calcium signal transduction. Plant Cell Rep. 2016, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, S.; Wang, Y.; Lu, L.; Sun, M.; He, C.; Wang, J.; Li, Y.; Yu, X.; Li, Q.; et al. Physiological and molecular mechanisms of ABA and CaCl(2) regulating chilling tolerance of cucumber seedlings. Plants 2021, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, F.; Ai, X.; Bi, H. H2O2 participates in ABA regulation of grafting-induced chilling tolerance in cucumber. Plant Cell Rep. 2022, 41, 1115–1130. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, L.; Shang, Q.M.; Liu, X.Y.; Zhang, Z.G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, Y.Q.; Zhang, X.W.; Zhang, Y.Y.; Bi, H.G.; Ai, X.Z. Salicylic acid is involved in rootstock-scion communication in improving the chilling tolerance of grafted cucumber. Front. Plant Sci. 2021, 12, 693344. [Google Scholar] [CrossRef]
- Qi, C.D.; Dong, D.H.; Li, Y.F.; Wang, X.W.; Guo, L.Q.; Liu, L.; Dong, X.N.; Li, X.S.; Yuan, X.W.; Ren, S.X.; et al. Heat shock-induced cold acclimation in cucumber through CsHSFA1d-activated JA biosynthesis and signaling. Plant J. 2022, 111, 85–102. [Google Scholar] [CrossRef]
- Catalá, R.; López-Cobollo, R.; Castellano, M.M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 protein RARE COLD NDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef]
- Dong, Y.F.; Tang, M.J.; Huang, Z.L.; Song, J.N.; Xu, J.; Ahammed, G.J.; Yu, J.Q.; Zhou, Y.H. The miR164a-NAM3 module confers cold tolerance by inducing ethylene production in tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef]
- Shi, Y.T.; Tian, S.W.; Hou, L.Y.; Huang, X.Z.; Zhang, X.Y.; Guo, H.W.; Yang, S.H. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Wang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. Embo J. 2021, 40, e104559. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, H.; Wu, S.; Fu, D.; Li, M.; Gong, Z.; Yang, S. CPK28-NLP7 module integrates cold-induced Ca(2+) signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. 2022, 8, eabn7901. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Q.; Liu, Y.M.; Mu, Y.; Anwar, A.; He, C.X.; Yan, Y.; Li, Y.S.; Yu, X.C. Heterotrimeric G-protein γ subunit CsGG3.2 positively regulates the expression of genes and chilling tolerance in Cucumber. Front. Plant Sci. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Li, H.M.; Quan, X.Y.; Shan, Q.L.; Wang, W.B.; Yin, N.; Wang, S.Q.; Wang, Z.H.; He, W.X. Comprehensive analysis of cucumber C-repeat/dehydration-responsive element binding factor family genes and their potential roles in cold tolerance of cucumber. BMC Plant Biol. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tang, X.L.; Huo, Y.Q.; Xu, R.; Qi, S.D.; Huang, J.G.; Zheng, C.C.; Wu, C.A. Identification and characterization of fructose 1,6-bisphosphate aldolase genes in Arabidopsis reveal a gene family with diverse responses to abiotic stresses. Gene 2012, 503, 65–74. [Google Scholar] [CrossRef]
- Cai, B.B.; Li, Q.; Xu, Y.C.; Yang, L.; Bi, H.G.; Ai, X.Z. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol. Biochem. 2016, 108, 251–265. [Google Scholar] [CrossRef]
- Zhang, Y. Functional Analysis of a Fructose-1, 6-Diphosphatase Aldolase Gene ALDY in Rice. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2014; pp. 34–37. [Google Scholar]
- Kelley, P.M.; Freeling, M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J. Biol. Chem. 1984, 259, 14180–14183. [Google Scholar] [CrossRef]
- Lebherz, H.G.; Leadbetter, M.M.; Bradshaw, R.A. Isolation and characterization of the cytosolic and chloroplast forms of spinach leaf fructose diphosphate aldolase. J. Biol. Chem. 1984, 259, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A.; Wong, D.M.; Sachs, M.M. The anaerobic response of soybean. Plant Physiol. 1990, 92, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Haake, V.; Zrenner, R.; Sonnewald, U.; Stitt, M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998, 14, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Michelis, R.; Gepstein, S. Identification and characterization of a heat-induced isoform of aldolase in oat chloroplast. Plant Mol. Biol. 2000, 44, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Komori, T.; Hashimoto, A.; Kuwata, S.; Imaseki, H.; Kubo, T. Differential expression of plastidic aldolase genes in plants under salt stress. Plant Sci. 2000, 154, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, Z.; Zhang, Y. Cloning and molecular characterization of fructose-1,6-bisphosphate aldolase gene regulated by high-salinity and drought in Sesuvium portulacastrum. Plant Cell Rep. 2009, 28, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Lv, K. The Primary Study of the Wheat Fructose-1,6-Bisphoshate Aldolase from Albinism Line. Master’s Dissertation, Northwest Agriculture & Forestry University, Yangling, China, 2011; pp. 35–37. [Google Scholar]
- Lao, X.T.; Azuma, J.; Sakamoto, M. Two cytosolic aldolases show different expression patterns during shoot elongation in Moso bamboo, Phyllostachys pubescens Mazel. Physiol. Plant. 2013, 149, 422–431. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Tan, X.F.; Zhang, L.; Jiang, N.; Cao, H.P. Identification and expression of fructose-1,6-bisphosphate aldolase genes and their relations to oil content in developing seeds of tea oil tree (Camellia oleifera). PLoS ONE 2014, 9, e107422. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.M.; Taxak, P.C.; Jain, P.K.; Saini, R.; Srinivasan, R. Glycolytic enzyme activities and gene expression in cicer arietinum exposed to water-deficit stress. Appl. Biochem. Biotechnol. 2014, 173, 2241–2253. [Google Scholar] [CrossRef]
- Cai, B.B.; Li, Q.; Liu, F.J.; Bi, H.A.; Ai, X.Z. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018, 163, 247–258. [Google Scholar] [CrossRef]
- Cai, B.B.; Ning, Y.; Li, Q.; Li, Q.Y.; Ai, X.Z. Effects of the Chloroplast Fructose-1,6-Bisphosphate Aldolase Gene on Growth and Low-Temperature Tolerance of Tomato. Int. J. Mol. Sci. 2022, 23, 728. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, M.T.; Liu, Q.Y.; Niu, B. Hydrological cycling optimization-based multiobjective feature-selection method for customer segmentation. Int. J. Intell. Syst. 2021, 36, 2347–2366. [Google Scholar] [CrossRef]
- Huang, H.H.; Wu, N.Q.; Liang, Y.; Peng, X.D.; Jun, S. SLNL: A novel method for gene selection and phenotype classification. Int. J. Intell. Syst. 2022, 37, 6283–6304. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford; UK; New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; Liu, Y.K.; Xue, Z.J.; Li, Q.Y.; Cai, B.B. Structures, characteristics and functions of fructose-1,6-bisphosphate aldolase in various tissues. Acta Soc. Bot. Pol. 2023, 92, 174253. [Google Scholar] [CrossRef]
- Xing, H.Y.; Pudake, R.N.; Guo, G.G.; Xing, G.F.; Hu, Z.R.; Zhang, Y.R.; Sun, Q.X.; Ni, Z.F. Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genom. 2011, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Carrera, D.A.; George, G.M.; Fischer-Stettler, M.; Galbier, F.; Eicke, S.; Truernit, E.; Streb, S.; Zeeman, S.C. Distinct plastid fructose bisphosphate aldolases function in photosynthetic and non-photosynthetic metabolism in Arabidopsis. J. Exp. Bot. 2021, 72, 3739–3755. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.M. Fructose Biphosphate Aldolase Regulates the Stem Cell Function in Arabidopsis Thaliana. Master’s Dissertation, Xiamen University, Xiamen, China, 2020. [Google Scholar]
- Liu, W.; Deng, Y.; Li, Y.; Yang, L.; Zhu, L.; Jiang, L. Coupling protein scaffold and biosilicification: A sustainable and recyclable approach for d-mannitol production via one-step purification and immobilization of multienzymes. Int. J. Biol. Macromol. 2024, 269, 132196. [Google Scholar] [CrossRef]
- Liu, F.J.; Zhang, X.W.; Cai, B.B.; Pan, D.Y.; Fu, X.; Bi, H.G.; Ai, X.Z. Physiological response and transcription profiling analysis reveal the role of glutathione in HS-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Sequence ID | Number of Amino Acids | Molecular Weight (KD) | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|---|
| CsFBA1 | CsaV3_2G015280 | 397 | 42.89 | 6.39 | 33.92 | 92.42 | −0.126 |
| CsFBA3 | CsaV3_5G028690 | 390 | 42.38 | 8.69 | 41.65 | 84.87 | −0.247 |
| CsFBA2 | CsaV3_3G036910 | 358 | 38.60 | 6.49 | 29.59 | 90.84 | −0.199 |
| CsFBA4 | CsaV3_6G040430 | 358 | 38.41 | 7.57 | 28.19 | 94.08 | −0.164 |
| CsFBA5 | CsaV3_7G021470 | 357 | 37.97 | 6.07 | 34.28 | 91.6 | −0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Zhou, Z.; Yang, L.; Xue, Z.; Li, Q.; Cai, B. Genome-Wide Characterization of Fructose 1,6-Bisphosphate Aldolase Genes and Expression Profile Reveals Their Regulatory Role in Abiotic Stress in Cucumber. Int. J. Mol. Sci. 2024, 25, 7687. https://doi.org/10.3390/ijms25147687
Zhang J, Liu Y, Zhou Z, Yang L, Xue Z, Li Q, Cai B. Genome-Wide Characterization of Fructose 1,6-Bisphosphate Aldolase Genes and Expression Profile Reveals Their Regulatory Role in Abiotic Stress in Cucumber. International Journal of Molecular Sciences. 2024; 25(14):7687. https://doi.org/10.3390/ijms25147687
Chicago/Turabian StyleZhang, Jinlong, Yike Liu, Zhenpeng Zhou, Lina Yang, Zhanjun Xue, Qingyun Li, and Bingbing Cai. 2024. "Genome-Wide Characterization of Fructose 1,6-Bisphosphate Aldolase Genes and Expression Profile Reveals Their Regulatory Role in Abiotic Stress in Cucumber" International Journal of Molecular Sciences 25, no. 14: 7687. https://doi.org/10.3390/ijms25147687
APA StyleZhang, J., Liu, Y., Zhou, Z., Yang, L., Xue, Z., Li, Q., & Cai, B. (2024). Genome-Wide Characterization of Fructose 1,6-Bisphosphate Aldolase Genes and Expression Profile Reveals Their Regulatory Role in Abiotic Stress in Cucumber. International Journal of Molecular Sciences, 25(14), 7687. https://doi.org/10.3390/ijms25147687





