Nordalbergin Synergizes with Novel β-Lactam Antibiotics against MRSA Infection
Abstract
1. Introduction
2. Results
2.1. Nordalbergin Inhibited the Activity of β-Lactamase
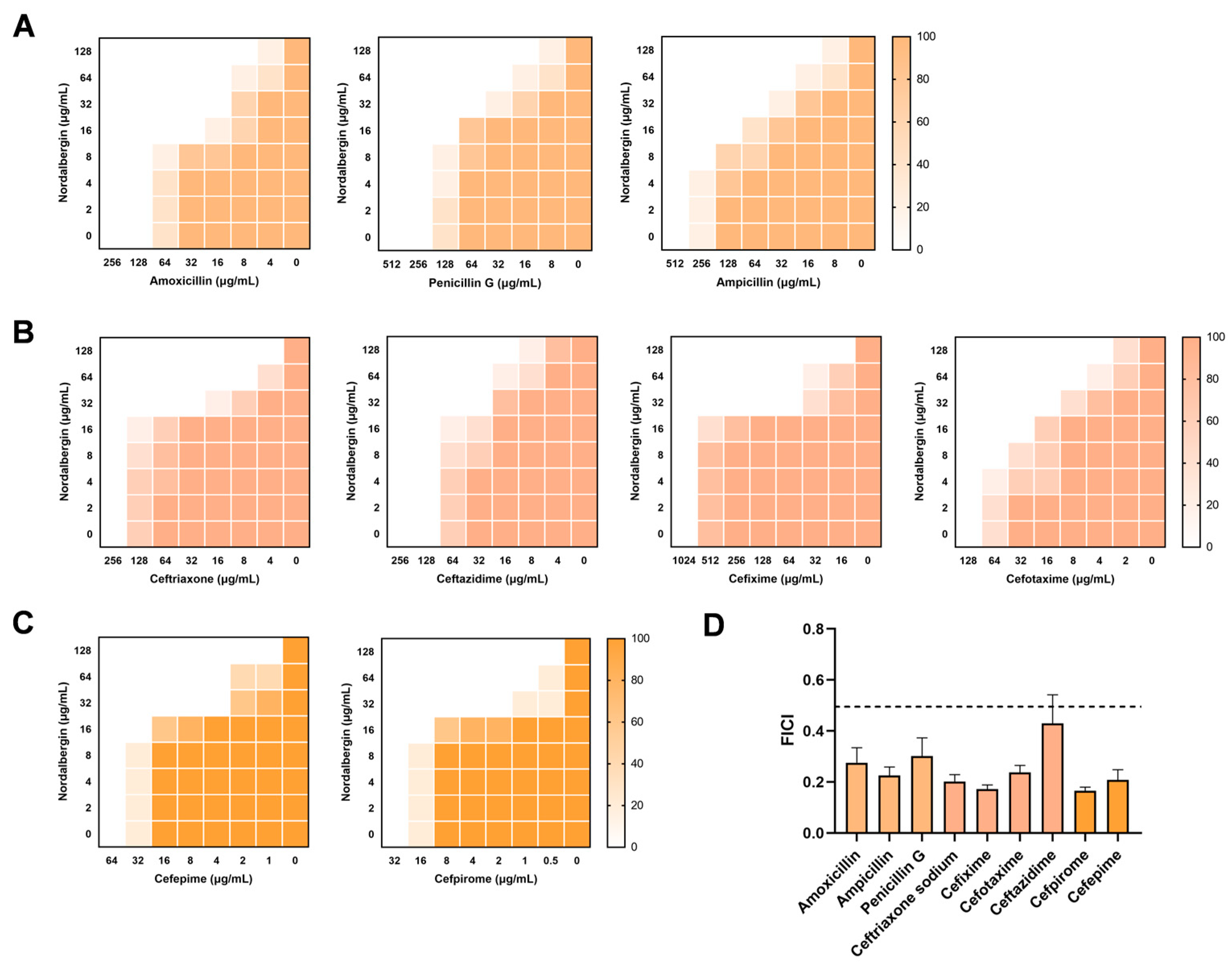
2.2. Synergistic Efficacy of Nordalbergin and β-Lactam Antibiotics against MRSA
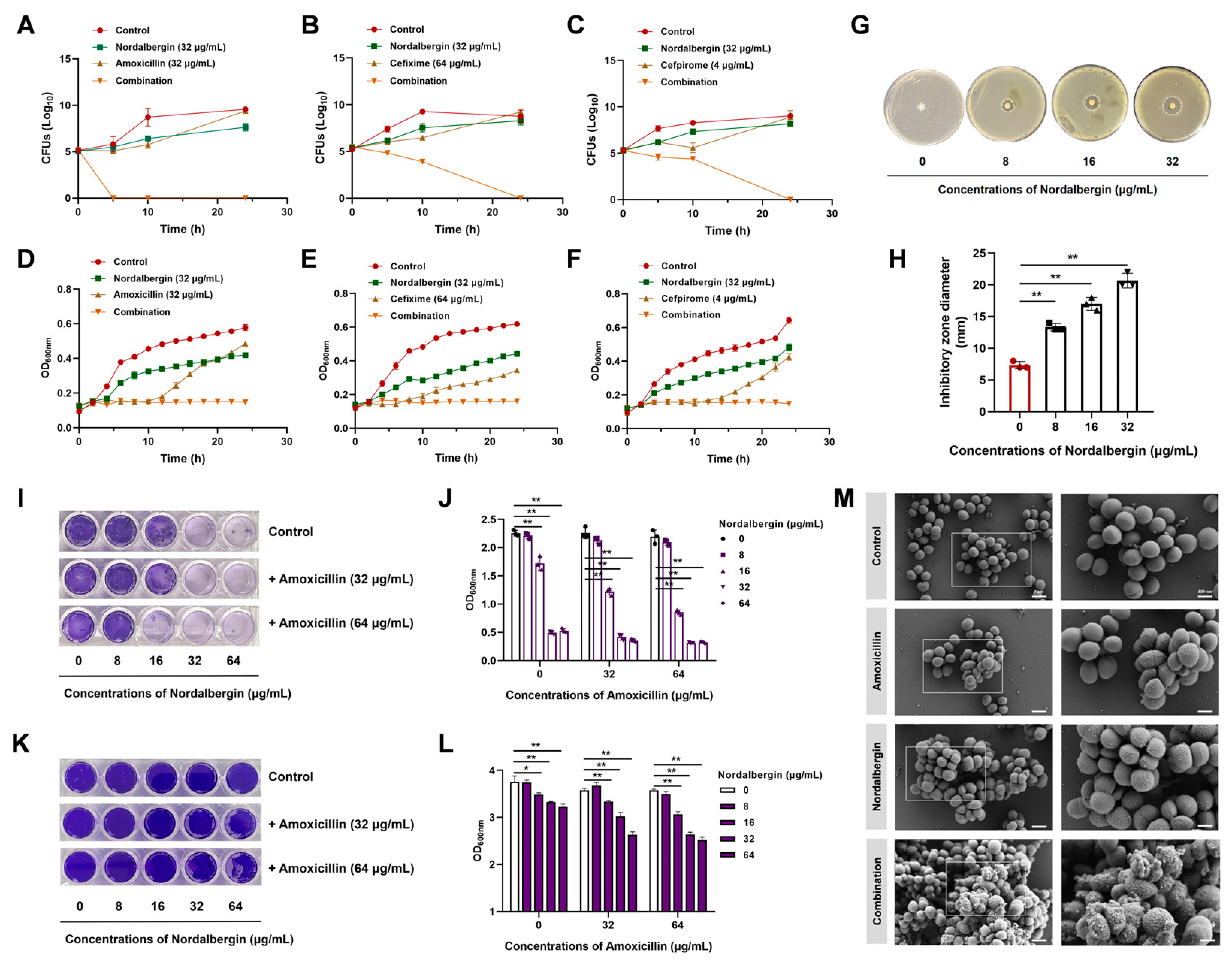
2.3. Nordalbergin Synergized with β-Lactam Antibiotics against MRSA by Enhancing Cell Membrane Permeability
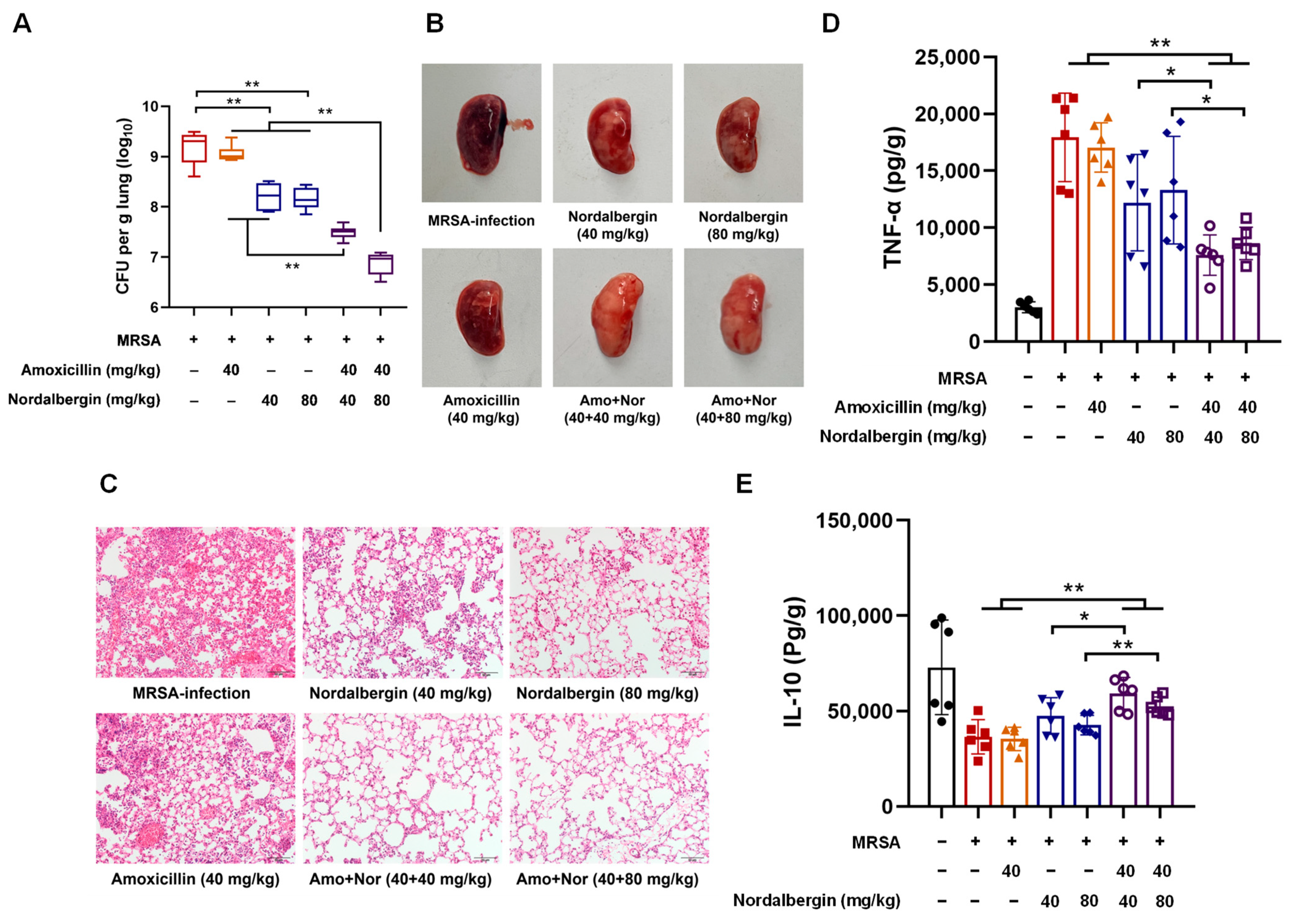
2.4. Nordalbergin Improved Therapeutic Efficacy of Amoxicillin against MRSA Pneumonia in Mice
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Reagents
4.2. Enzyme Inhibition Assays
4.3. Molecular Docking and Molecular Dynamics (MD) Simulations
4.4. Checkerboard Assays
4.5. Time-Dependent Killing Curves
4.6. Growth Curves
4.7. Combined Disk Tests
4.8. Biofilm Inhibition Assays
4.9. Scanning Electron Microscopy (SEM) Observation
4.10. Live/Dead Bacteria Staining
4.11. Membrane Permeability Assay
4.12. Membrane Depolarization Assay
4.13. NAD+/NADH Level Detection
4.14. ATP Determination
4.15. Animal Experiments
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; De Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T.G. Methicillin-resistant emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K.Y. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Franco, D.S.P.; Meili, L.; Dehmani, Y.; Reis, G.S.D.; Lima, E.C. Main advances and future prospects in the remediation of the antibiotic amoxicillin with a focus on adsorption technology: A critical review. J. Water Process Eng. 2023, 56, 104417. [Google Scholar] [CrossRef]
- Abramovic, B.F.; Uzelac, M.M.; Armakovic, S.J.; Gasic, U.; Cetojevic-Simin, D.D.; Armakovic, S. Experimental and computational study of hydrolysis and photolysis of antibiotic ceftriaxone: Degradation kinetics, pathways, and toxicity. Sci. Total Environ. 2021, 768, 144991. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Belati, A.; Bussini, L.; Cento, V.; Diella, L.; Gatti, M.; Saracino, A.; Pea, F.; Viale, P.; Bartoletti, M. Safety and effectiveness of fifth generation cephalosporins for the treatment of methicillin-resistant Staphylococcus aureus bloodstream infections: A narrative review exploring past, present, and future. Expert Opin. Drug Saf. 2024, 23, 9–36. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Elings, W.; Chikunova, A.; van Zanten, D.B.; Drenth, R.; Ahmad, M.U.D.; Blok, A.J.; Timmer, M.; Perrakis, A.; Ubbink, M. Two β-lactamase variants with reduced clavulanic acid inhibition display different millisecond dynamics. Antimicrob. Agents Chemother. 2021, 65, e02628-20. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Wang, C.J.; Han, J.; Li, Y.Q.; Wang, G.C. Synergism of coumarins from the Chinese drug with antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2016, 23, 1814–1820. [Google Scholar] [CrossRef]
- Mun, S.H.; Kang, O.H.; Joung, D.K.; Kim, S.B.; Seo, Y.S.; Choi, J.G.; Lee, Y.S.; Cha, S.W.; Ahn, Y.S.; Han, S.H.; et al. Combination therapy of sophoraflavanone B against MRSA: In vitro synergy testing. Evid. Based Complement. Alternat. Med. 2013, 2013, 823794. [Google Scholar] [CrossRef]
- Sharaf, M.H.; El-Sherbiny, G.M.; Moghannem, S.A.; Abdelmonem, M.; Elsehemy, I.A.; Metwaly, A.M.; Kalaba, M.H. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2021, 11, 4240. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Effect of coumarins on HL-60 cell differentiation. Anticancer Res. 2000, 20, 2505–2512. [Google Scholar]
- Lo, J.; Wu, H.E.; Liu, C.C.; Chang, K.C.; Lee, P.Y.; Liu, P.L.; Huang, S.P.; Wu, P.C.; Lin, T.C.; Lai, Y.H.; et al. Nordalbergin exerts anti-neuroinflammatory effects by attenuating MAPK signaling pathway, NLRP3 inflammasome activation and ROS production in LPS-stimulated BV2 microglia. Int. J. Mol. Sci. 2023, 24, 7300. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, H.T.; Yang, M.G.; Ding, R.; Gao, Y.W.; Niu, X.D.; Deng, X.M.; Wang, J.F.; Feng, H.H.; Qiu, J.Z. Novel metallo-ß-lactamases inhibitors restore the susceptibility of carbapenems to New Delhi metallo-lactamase-1 (NDM-1)-harbouring bacteria. Br. J. Pharmacol. 2024, 181, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef]
- Grande, R.; Puca, V.; Muraro, R. Antibiotic resistance and bacterial biofilm. Expert Opin. Ther. Pat. 2020, 30, 897–900. [Google Scholar] [CrossRef]
- Bush, K.; Macielag, M.J. New β-lactam antibiotics and β-lactamase inhibitors. Expert Opin. Ther. Pat. 2010, 20, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Zhang, A.H.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, e1900380. [Google Scholar] [CrossRef]
- Sandhu, S.; Bansal, Y.; Bansal, G.; Kaur, M.; Kohli, S. Coumarin: A promising scaffold for anticancer agents. Anticancer Agents Med. Chem. 2015, 15, 1032–1048. [Google Scholar]
- Xia, D.G.; Liu, H.; Cheng, X.; Maraswami, M.; Chen, Y.T.; Lv, X.H. Recent developments of coumarin-based hybrids in drug discovery. Curr. Top. Med. Chem. 2022, 22, 269–283. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Torres, V.J. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol. Spectr. 2019, 7, GPP3-0039-2018. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hu, S.; Pei, Y.; Sun, H. Nordalbergin Synergizes with Novel β-Lactam Antibiotics against MRSA Infection. Int. J. Mol. Sci. 2024, 25, 7704. https://doi.org/10.3390/ijms25147704
Wang H, Hu S, Pei Y, Sun H. Nordalbergin Synergizes with Novel β-Lactam Antibiotics against MRSA Infection. International Journal of Molecular Sciences. 2024; 25(14):7704. https://doi.org/10.3390/ijms25147704
Chicago/Turabian StyleWang, Haiting, Sangyu Hu, Yuzhu Pei, and Hongxiang Sun. 2024. "Nordalbergin Synergizes with Novel β-Lactam Antibiotics against MRSA Infection" International Journal of Molecular Sciences 25, no. 14: 7704. https://doi.org/10.3390/ijms25147704
APA StyleWang, H., Hu, S., Pei, Y., & Sun, H. (2024). Nordalbergin Synergizes with Novel β-Lactam Antibiotics against MRSA Infection. International Journal of Molecular Sciences, 25(14), 7704. https://doi.org/10.3390/ijms25147704





