Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons
Abstract
:1. Introduction
2. Results
2.1. Blast of Chlorophyll-Related and Cellulose-Related Genes
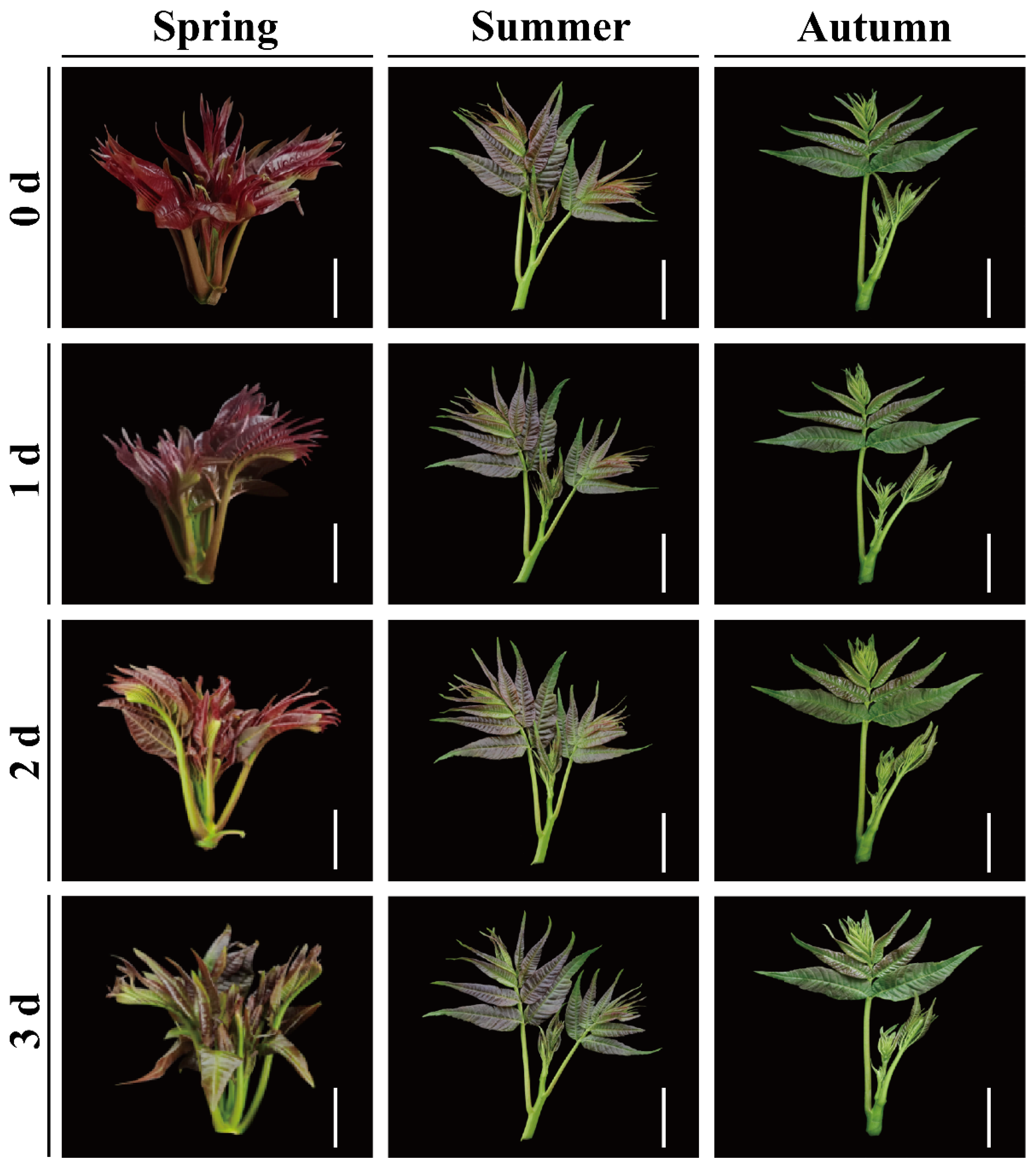
2.2. Color Changes of Red TSB during Postharvest Storage
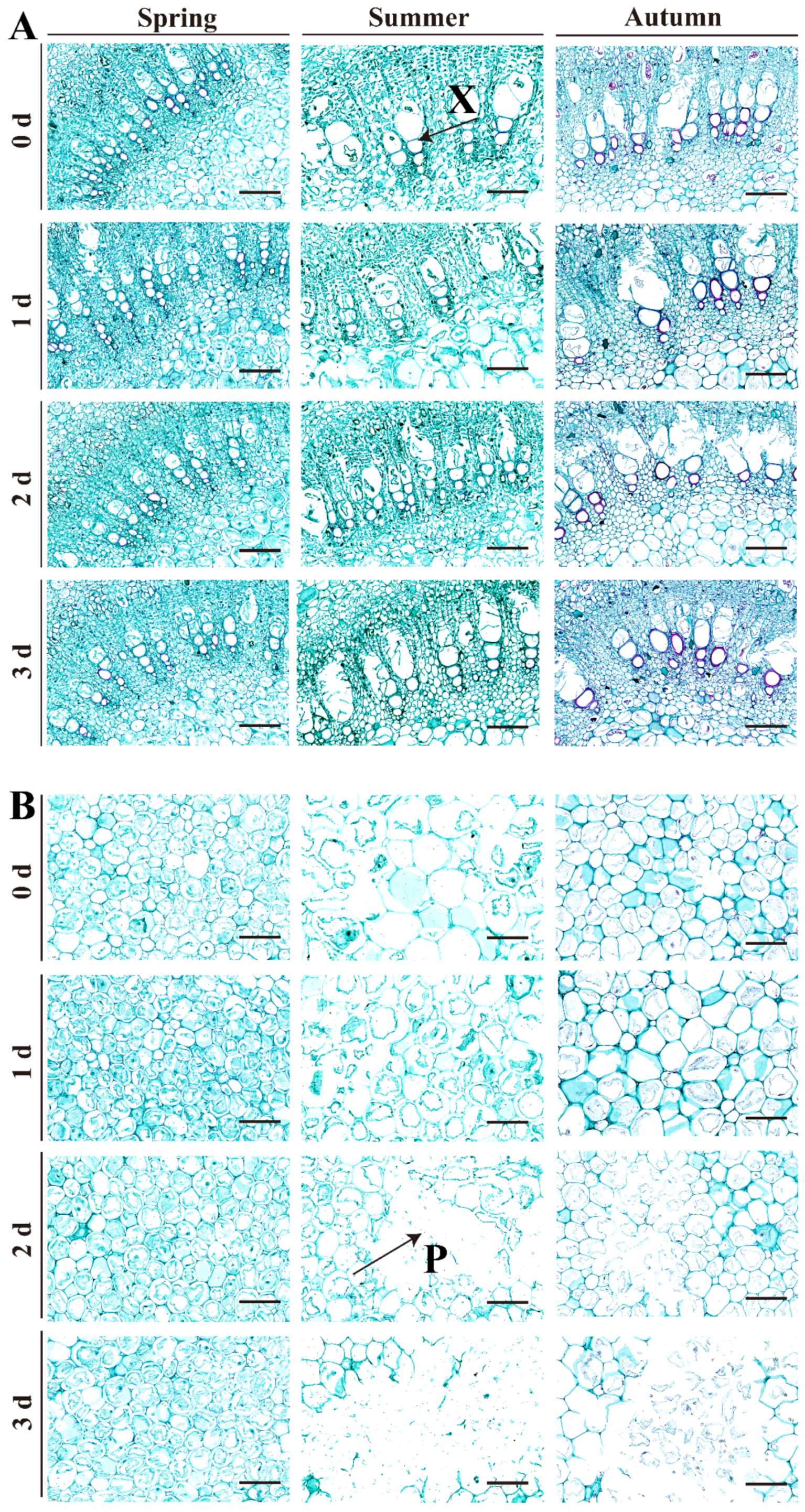
2.3. Structural Characteristics of Red TSB during Postharvest Storage
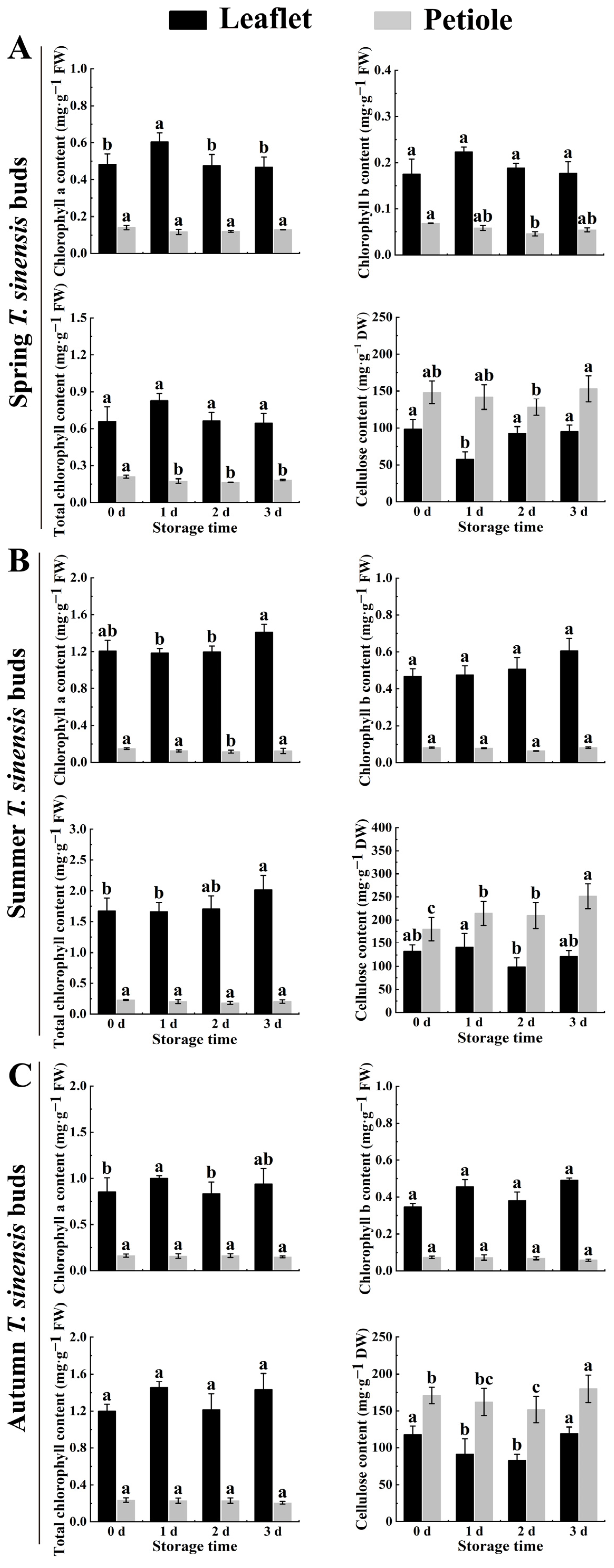
2.4. Chlorophyll and Cellulose Content of Red TSB during Postharvest Storage
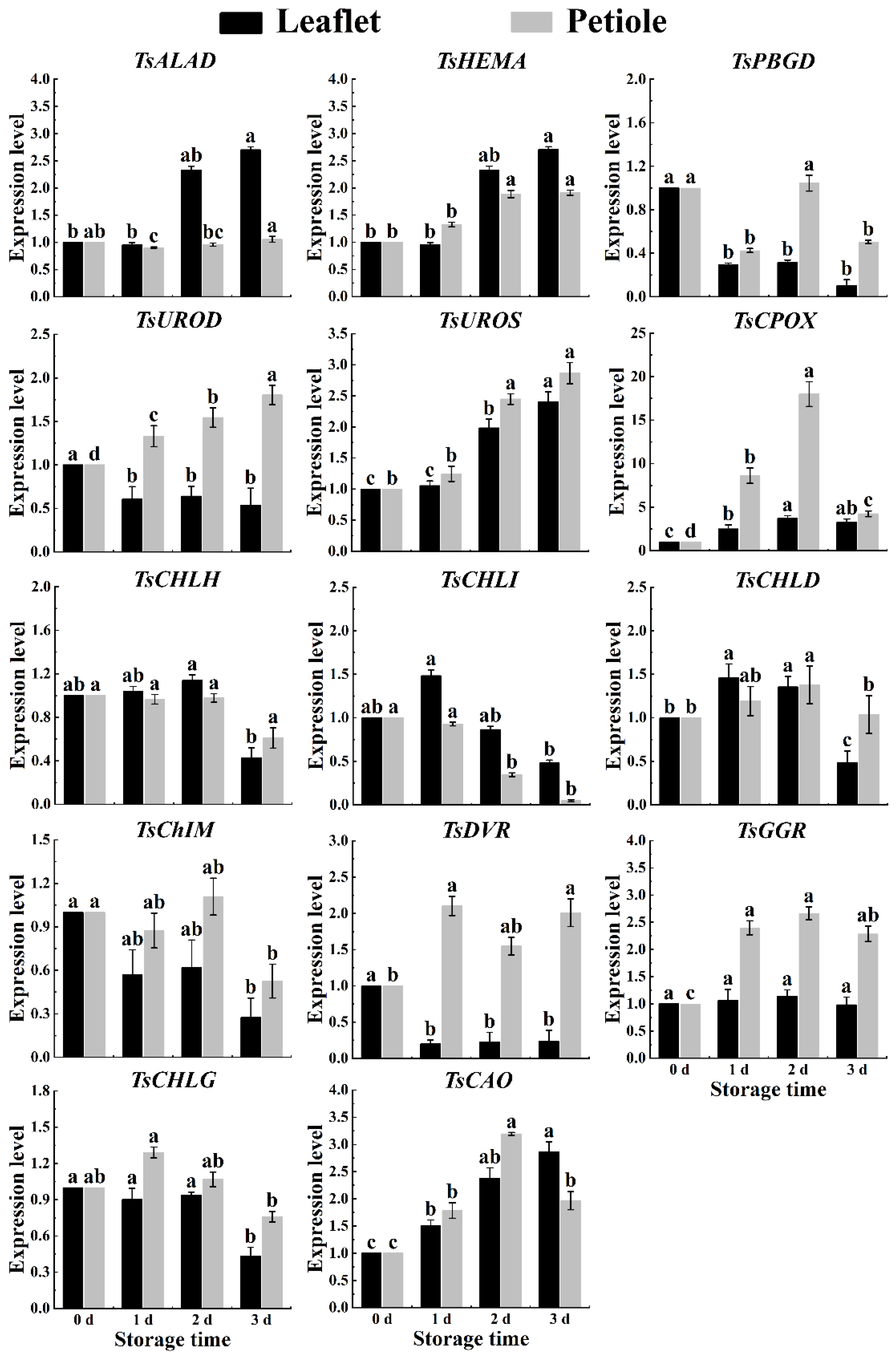
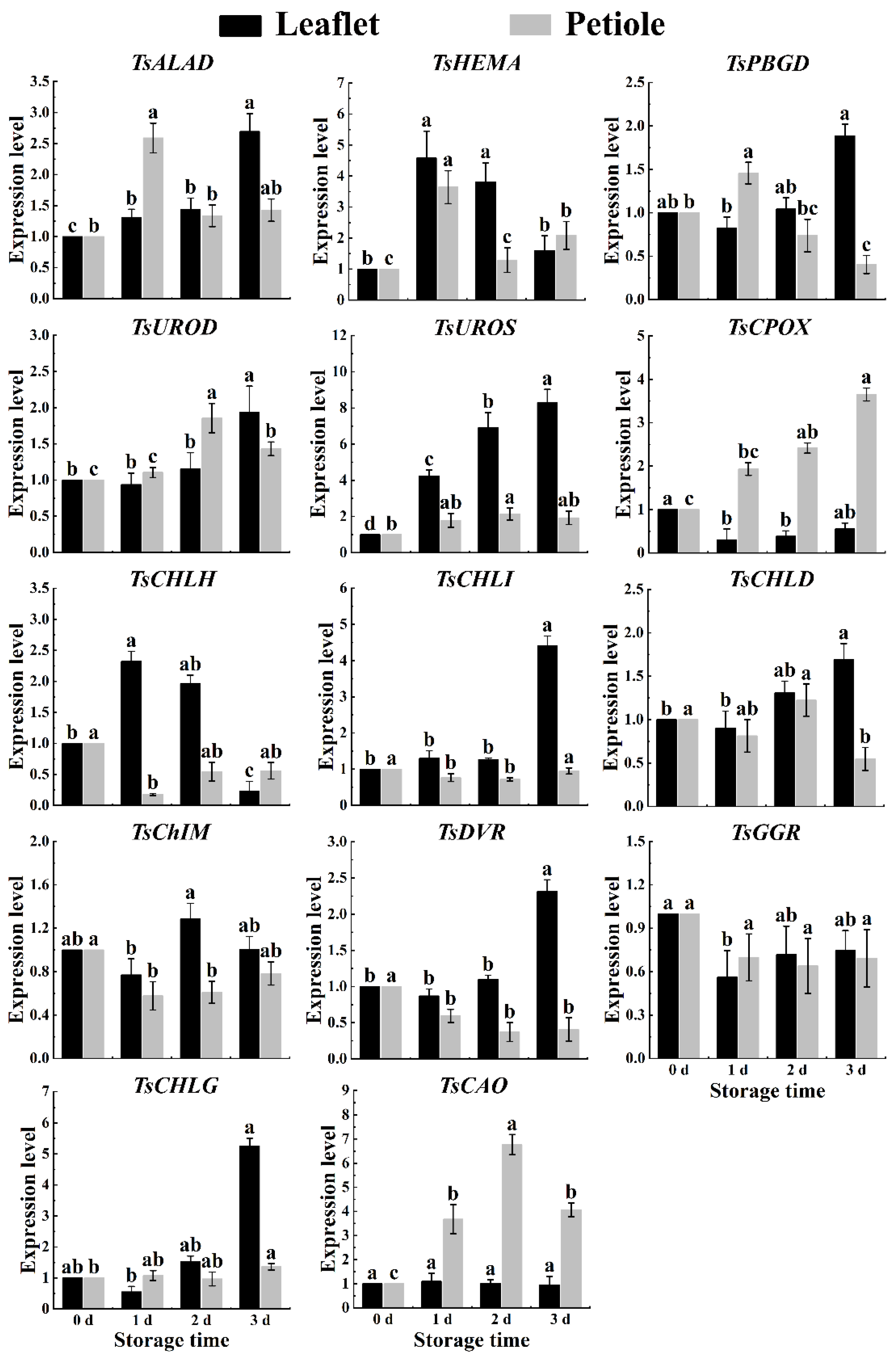
2.5. Chlorophyll-Related Genes Expression of Red TSB during Postharvest Storage
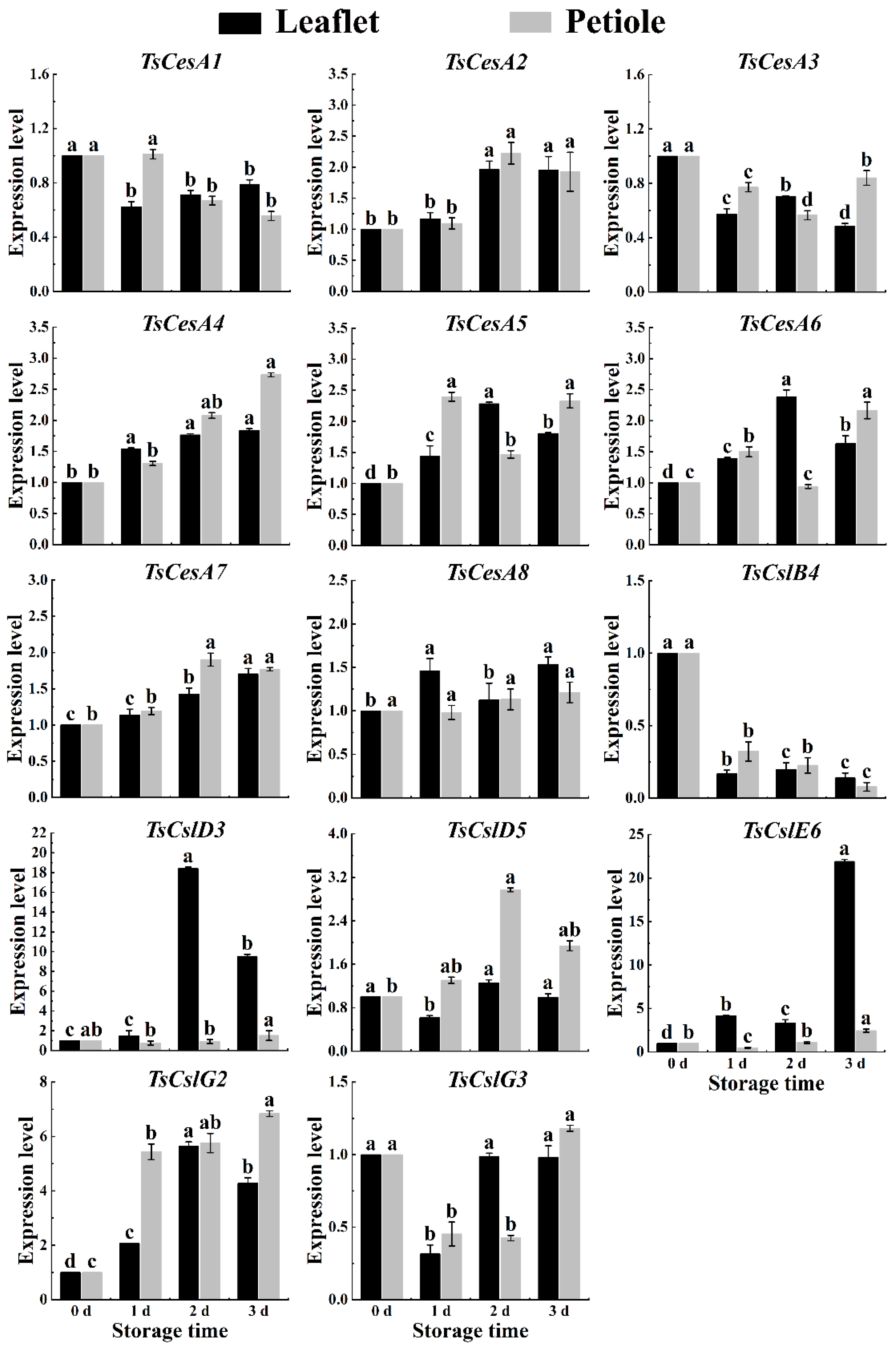
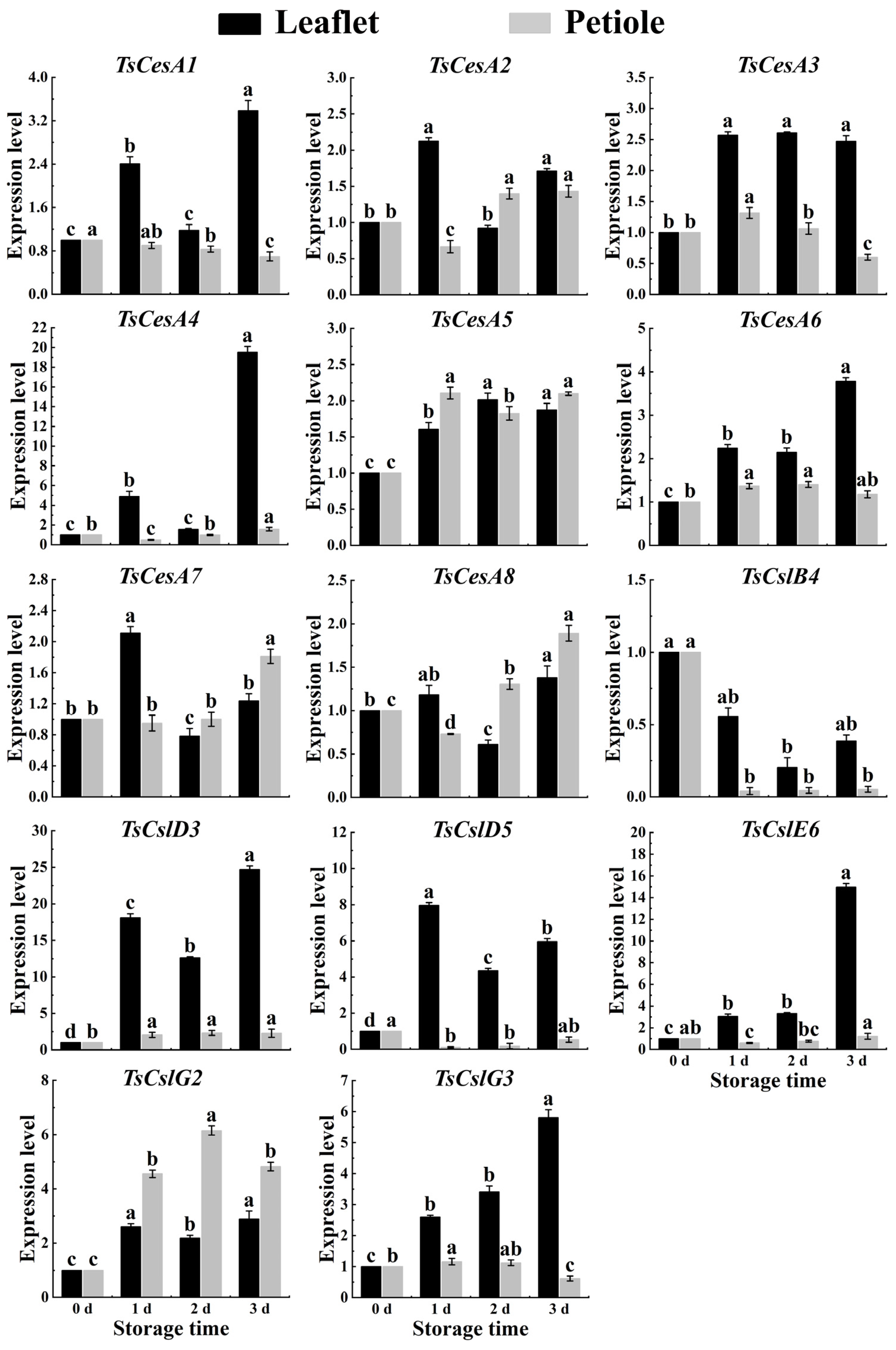
2.6. Cellulose-Related Genes Expression in Red TSB
3. Discussion
4. Materials and Methods
4.1. Material Collection and Pretreatment
4.2. Color and Structure Observation of Red TSB
4.3. Determination of Chlorophyll and Cellulose Content
4.4. Total RNA Extraction and cDNA Synthesis of Red TSB
4.5. Gene Identification and Primers Design
4.6. Expression Analysis of Chlorophyll-Related and Cellulose-Related Genes
4.7. Data Analysis
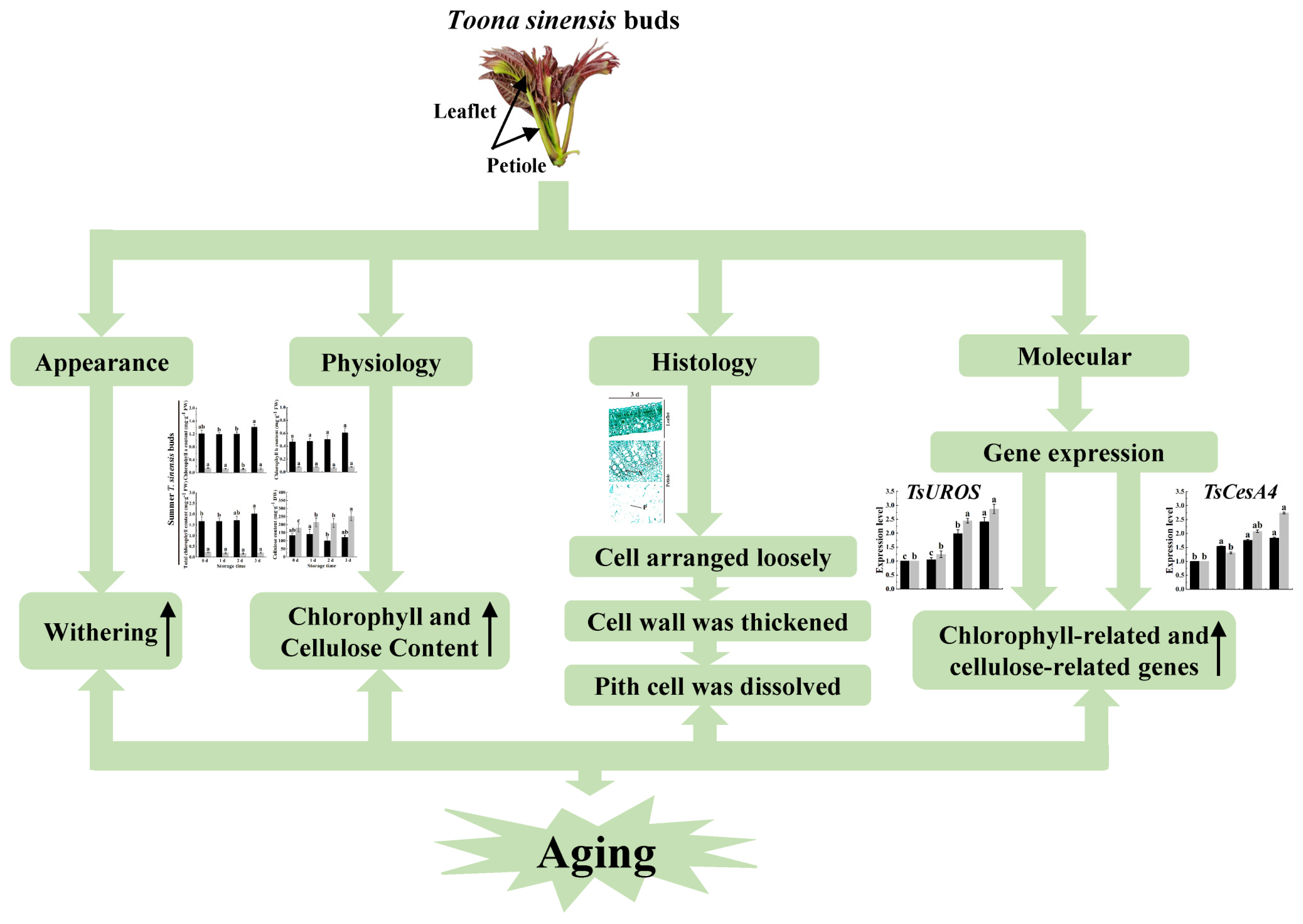
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, J.J.; Lv, Q.Q.; Zhang, B.; Chen, H.Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohydr. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, Y.J.; Hu, M.B.; Zhang, M.M.; Yang, J.; Liang, F.; Huang, Q.W.; Wu, C.J. Toona sinensis: A comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhong, X.L.; Cai, X.; Zhu, S.H.; Meng, P.H.; Zhang, J.; Tan, G.F. Comparative physiological analysis of lignification, anthocyanin metabolism and correlated gene expression in red Toona sinensis buds during cold storage. Agronomy 2023, 13, 119. [Google Scholar] [CrossRef]
- Yang, Y.F.; Ma, Y.T.; Yang, S.H.; Yue, X.C.; Peng, W.X. Chemical components analysis of Toona sinensis bark and wood by pyrolisis–gas chromatography–mass spectrometry. Asia-Pac. J. Chem. Eng. 2020, 15, e2487. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, X.L.; Zhu, S.H.; Wang, K.; Tan, G.F.; Meng, P.H.; Zhang, J. Research advances in Toona sinensis, a traditional Chinese medicinal plant and popular vegetable in China. Diversity 2022, 14, 572. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Liu, X.X.; Zhou, J.Y.; Jiang, Y.J.; Wang, F.; Wang, R.S.; Li, W.Z. Terpenoids from the seeds of Toona sinensis and their ability to attenuate high glucose-induced oxidative stress and inflammation in rat glomerular mesangial cells. Molecules 2022, 27, 5784. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Food 2013, 5, 206–266. [Google Scholar] [CrossRef]
- Shen, Y.P.; Xu, M.H.; Chen, Y.F.; Wang, H.Y.; Zhou, Y.Y.; Zhu, Y.T.; Yang, H.; Yu, J.N. Integrated extraction and purification of total bioactive flavonoids from Toona sinensis leaves. Nat. Prod. Res. 2019, 33, 3025–3028. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, C.D.; Li, Y.F.; Zhang, Y.L.; Zhang, B.B.; Zhang, J.F. Integrated volatilomic profiles and chemometrics provide new insights into the spatial distribution and aroma differences of volatile compounds in seven Toona sinensis cultivars. Food Chem. 2023, 407, e135116. [Google Scholar] [CrossRef]
- Shi, G.Y.; Zhao, L.L.; Wang, X.M.; Zhang, L.; Jiang, P.F.; Wang, X.Z.; Wang, Z.G. Dynamic changes in major active substances and volatile components in Toona sinensis leaves during growth. Food Sci. 2022, 43, 276–284, (In Chinese with Abstract). [Google Scholar]
- Wang, C.; Zhang, B.B.; Li, Y.F.; Hou, J.; Fu, C.D.; Wang, Z.H.; Zhang, J.F. Integrated transcriptomic and volatilomic profiles to explore the potential mechanism of aroma formation in Toona sinensis. Food Res. Int. 2023, 165, e112452. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Han, Y.; Zhu, X.; Zhao, X.; Ma, X.; Jiang, D.; Zhang, Q. An efficient method for decoloration of polysacchairdes from the sprouts of Toonas sinensis (A. Juss.) Roem by anion exchange microporous. Food Chem. 2017, 217, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Tsai, Y.J.; Hsieh, Y.C.; Lin, R.J.; Lin, C. The aqueous extract from Toona sinensis leaves inhibits microglia-mediated neuroinflammation. Kaohsiung J. Med. Sci. 2014, 302, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Tsai, M.J.; Hsu, C.Y.; Wang, C.Y.; Hsu, H.K.; Weng, C.F. Toona sinensis Roem (Meliaceae) leaf extract alleviates hyperglycemia via altering adipose glucose transporter 4. Food Chem. Toxicol. 2008, 46, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, C.; You, L.J.; Fu, X.; Chen, Y.S.; Luo, Y.Q. Structural identification of compounds from Toona sinensis leaves with antioxidant and anticancer activities. J. Funct. Foods 2014, 10, 427–435. [Google Scholar] [CrossRef]
- Aurea Kuo, C.E.; Wu, S.Y.; Lee, C.H.; Lai, Y.R.; Lu, C.H.; Chen, P.C.; Cheng, J.H.; Tsail, L.Y.; Yen, K.T.; Tsao, Y.; et al. Toona sinensis modulates autophagy and cytokines in lipopolysaccharide induced RAW 264.7 macrophages. Biomed. Pharmacother. 2022, 129, e110386. [Google Scholar]
- Zhao, H.; Shi, X.P.; Shen, C.; Chen, C.F.; Liu, J.Y.; Qu, C.Q. High-throughput sequencing analysis reveals effects of short-term low-temperature storage on miRNA-mediated flavonoid accumulation in postharvest toon buds. Plant Gene 2021, 26, e100291. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, C.K.; Zhang, H.J.; Luo, H.X.; Wang, L.Q.; Cui, M.J.; Xu, W.T.; Deng, M.C. Analysis of microbial diversities dynamics of Toona sinensis (A. Juss.) M. Roem treated by three different preservation methods during storage based on nextgeneration sequencing. Food Res. Dev. 2019, 40, 42–50, (In Chinese with Abstract). [Google Scholar]
- Zhang, B.B.; Hao, L.F.; Zhang, J.; Feng, J.Z.; Wang, C.; Zhang, J.F. Integration of transcriptome, volatile and non-volatile metabolite profile reveals characteristic aroma formation in Toona sinensis. Food Chem. 2024, 436, e137788. [Google Scholar] [CrossRef]
- Huang, S.L.; Li, T.T.; Jiang, G.X.; Xie, W.P.; Chang, S.D.; Jiang, Y.M.; Duan, X.W. 1-Methylcyclopropene reduces chilling injury of harvested okra (Hibiscus esculentus L.) pods. Sci. Hortic. 2012, 141, 42–46. [Google Scholar] [CrossRef]
- Liu, J.X.; Liu, H.; Tao, J.P.; Tan, G.F.; Dai, Y.; Yang, L.L.; Feng, K.; Wang, H.; Li, T.; Liu, Y.H.; et al. High-quality genome sequence reveals a young polyploidization and provides insights into cellulose and lignin biosynthesis in water dropwort (Oenanthe sinensis). Ind. Crop. Prod. 2023, 193, e116203. [Google Scholar] [CrossRef]
- Pintos, F.; Rodoni, L.; Patrignani, M.; Ixtaina, P.; Vicente, A.; Martínez, G.; Hasperué, J. Advances in the use of white light on broccoli and kale postharvest shelf life. Innov. Food Sci. Emerg. Technol. 2023, 86, e103373. [Google Scholar] [CrossRef]
- Ning, M.; Tang, F.X.; Chen, J.L.; Song, W.; Cai, W.C.; Zhang, Q.; Zhao, X.X.; Yang, X.Q.; Shan, C.H.; Hao, G.F. Low-temperature adaptation and preservation revealed by changes in physiological-biochemical characteristics and proteome expression patterns in post-harvest Hami melon during cold storage. Planta 2022, 255, e91. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.T.; Zhang, J.Y.; Shi, D.F.; Yang, C.; Zeng, M.; Li, X.; Zhou, K.; Xi, W.P. Postharvest application of methyl jasmonate alleviates lignin accumulation in stone cells of pear fruit during low-temperature storage. Postharvest Biol. Technol. 2024, 209, e112692. [Google Scholar] [CrossRef]
- Xiao, Y.N.; Xie, L.H.; Li, Y.L.; Li, C.Y.; Yu, Y.T.; Hu, J.G.; Li, G.K. Impact of low temperature on the chemical profile of sweet corn kernels during post-harvest storage. Food Chem. 2023, 431, e137079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Liu, Y.; Hu, W.J.; Sun, B.; Chen, Q.; Tang, H.R. Anthocyanin accumulation and related gene expression affected by low temperature during strawberry coloration. Acta Physiol. Plant. 2018, 40, e192. [Google Scholar] [CrossRef]
- Gao, M.F.; He, R.; Shi, R.; Zhang, Y.T.; Song, S.W.; Su, W.; Liu, H.C. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, S.E.; Park, S.U.; Lim, Y.H.; Kwak, S.S. Tuberous roots of transgenic sweetpotato overexpressing IbCAD1 have enhanced low-temperature storage phenotypes. Plant Physiol. Biochem. 2021, 166, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.R.; Sun, L.; Wang, X.M.; Liu, C.F. Leaf responses to iron nutrition and low cadmium in peanut: Anatomical properties in relation to gas exchange. Plant Soil 2014, 375, 99–111. [Google Scholar] [CrossRef]
- Li, Z.X.; Shen, L.; Xu, L.F.; Wan, L.; Zhao, D.Y.; Sheng, J.P. Effects of 6-benzyladenine treatment on postharvest respiration and quality of Toona sinensis Roem sprout. Food Sci. 2009, 30, 282–285, (In Chinese with Abstract). [Google Scholar]
- Li, X.R.; Wang, J.X.; Qu, Y.H.; Li, Y.P.; Humaira, Y.; Muhammad, S.; Pu, H.M.; Yu, L.J.; Li, H. Comparison of storage and lignin accumulation characteristics between two types of snow pea. PLoS ONE 2022, 17, e0268776. [Google Scholar] [CrossRef] [PubMed]
- Garrido, Y.; Tudela, J.A.; Marín, A.; Mestre, T.; Martínez, V.; Gil, M.I. Physiological, phytochemical and structural changes of multi-leaf lettuce caused by salt stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Liu, S.W.; Yu, H.T.; Yu, Z.F. Exogenous melatonin ameliorates yellowing of postharvest pak choi (Brassica rapa subsp. chinensis) by modulating chlorophyll catabolism and antioxidant system during storage at 20 °C. Sci. Hortic. 2023, 311, e111808. [Google Scholar] [CrossRef]
- Fang, H.X.; Zhou, Q.; Cheng, S.C.; Zhou, X.; Wei, B.D.; Zhao, Y.B.; Ji, S.J. 24-epibrassinolide alleviates postharvest yellowing of broccoli via improving its antioxidant capacity. Food Chem. 2021, 365, e130529. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.L.; Zhao, H.H.; Zhang, L.G.; Zhou, H.S.; Li, P.X. The application of 1-methylcyclopropene preserves the postharvest quality of cabbage by inhibiting ethylene production, delaying chlorophyll breakdown and increasing antioxidant capacity. Sci. Hortic. 2021, 281, e109986. [Google Scholar] [CrossRef]
- Song, L.L.; Yi, R.X.; Luo, H.B.; Jiang, L.; Gu, S.M.; Yu, Z.F. Postharvest 1-methylcyclopropene application delays leaf yellowing of pakchoi (Brassica rapa subsp. chinensis) by improving chloroplast antioxidant capacity and maintaining chloroplast structural integrity during storage at 20 °C. Sci. Hortic. 2020, 270, e109466. [Google Scholar] [CrossRef]
- Tian, Y.; Sheng, Y.A.; Wu, T.; Wang, C.Y. Effect of modified okara insoluble dietary fibre on the quality of yoghurt. Food Chem. X 2024, 21, e101064. [Google Scholar] [CrossRef] [PubMed]
- Meents, M.J.; Watanabe, Y.; Samuels, A.L. The cell biology of secondary cell wall biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wu, C.; Ren, G.R.; Liang, X.L.; Wang, X.Y.; Huang, J.Y. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 2014, 155, 105–111. [Google Scholar] [CrossRef]
- Yu, Z.M.; Yang, Z.Y.; Teixeira da Silva, J.A.; Luo, J.P.; Duan, J. Influence of low temperature on physiology and bioactivity of postharvest Dendrobium officinale stems. Postharvest Biol. Technol. 2019, 148, 97–106. [Google Scholar] [CrossRef]
- Zhou, F.H.; Zuo, J.H.; Xu, D.Y.; Gao, L.P.; Wang, Q.; Jiang, A.L. Low intensity white light-emitting diodes (LED) application to delay senescence and maintain quality of postharvest pakchoi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Sci. Hortic. 2020, 262, e109060. [Google Scholar]
- Niu, N.Z.; Zhang, Y.; Li, S.J.; Meng, X.R.; Liu, M.J.; Wang, H.B.; Zhao, J. Genome-wide characterization of the cellulose synthase gene family in Ziziphus jujuba reveals its function in cellulose biosynthesis during fruit development. Int. J. Biol. Macromol. 2023, 239, e124360. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.T.; Zhang, N.; Ji, H.P.; Zhang, X.J.; Dong, C.H.; Yu, J.Z.; Yan, S.J.; Chen, C.K.; Liang, L.Y. Effects of atmospheric cold plasma treatment on the storage quality and chlorophyll metabolism of postharvest tomato. Foods 2022, 11, 4088. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.M.R.; Gómez-Lobato, M.E.; Civello, P.M.; Martínez, G.A. Expression of BoNOL and BoHCAR genes during postharvest senescence of broccoli heads. J. Sci. Food Agric. 2021, 101, 1629–1635. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, P.Y.; Gao, M.D.; Zhao, Y.; Zhang, C.J.; Zhu, H.L. Integrative metabolomics and transcriptomics analyses reveal pivotal regulatory mechanisms of 1-methylcyclopropene in maintaining postharvest storage quality of ‘Fuji’ apples. Food Qual. Saf. 2023, 7, fyac063. [Google Scholar] [CrossRef]
- Njie, A.; Dong, X.Q.; Liu, Q.G.; Lu, C.Y.; Pan, X.J.; Zhang, W.E. Melatonin treatment inhibits mango fruit (Cv. ‘Guiqi’) softening by maintaining cell wall and reactive oxygen metabolisms during cold storage. Postharvest Biol. Tech. 2023, 205, e112500. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhao, Y.T.; Zhang, Z.C.; He, H.; Shi, L.; Zhu, X.; Cui, K.B. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, e132921. [Google Scholar] [CrossRef]
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 2795. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Q.; Pu, Y.; Liu, S.H.; Wu, Q.S.; Yao, Y.L.; Yang, Y.M.; Zhang, X.M.; Sun, W.S. Genome-wide identification and expression patterns of AcSWEET family in pineapple and AcSWEET11 mediated sugar accumulation. Int. J. Mol. Sci. 2022, 23, 13875. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Cruz, R.M.S.; Abreu, M.; Brandao, T.R.S.; Silva, C.L.M. Biochemical and colour changes of watercress (Nasturtium officinale R. Br.) during freezing and frozen storage. J. Food Eng. 2009, 93, 32–39. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.; Song, X.J.; Duan, X.; Zhou, L.Q.; Sun, J.C. Effect of pretreatments and storage conditions on the quality of frozen kiwifruit slices. Int. J. Food Prop. 2008, 11, 68–78. [Google Scholar] [CrossRef]
- Xie, J.; Wang, G.Z.; Ren, T.Y.; Li, J.L.; Chen, S.; Shi, L.L.; Liu, H.J. The gradient distribution of pigment metabolites provided insights into the uneven colouration of pulp in cold-stored blood orange. Postharvest Biol. Tec. 2023, 198, e112234. [Google Scholar] [CrossRef]
- Lv, J.Y.; Han, X.Z.; Bai, L.; Xu, D.L.; Ding, S.Y.; Ge, Y.H.; Li, C.Y.; Li, J.R. Effects of calcium chloride treatment on softening in red raspberry fruit during low-temperature storage. J. Food Biochem. 2020, 44, e13419. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real- time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wang, F.; Wang, Y.; Zhong, X.; Zhu, S.; Zhang, X.; Li, S.; Lei, X.; Zang, Z.; Tan, G.; et al. Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons. Int. J. Mol. Sci. 2024, 25, 7719. https://doi.org/10.3390/ijms25147719
Zhao Q, Wang F, Wang Y, Zhong X, Zhu S, Zhang X, Li S, Lei X, Zang Z, Tan G, et al. Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons. International Journal of Molecular Sciences. 2024; 25(14):7719. https://doi.org/10.3390/ijms25147719
Chicago/Turabian StyleZhao, Qian, Fu Wang, Yifei Wang, Xiulai Zhong, Shunhua Zhu, Xinqi Zhang, Shuyao Li, Xiujuan Lei, Zhenyuan Zang, Guofei Tan, and et al. 2024. "Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons" International Journal of Molecular Sciences 25, no. 14: 7719. https://doi.org/10.3390/ijms25147719
APA StyleZhao, Q., Wang, F., Wang, Y., Zhong, X., Zhu, S., Zhang, X., Li, S., Lei, X., Zang, Z., Tan, G., & Zhang, J. (2024). Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons. International Journal of Molecular Sciences, 25(14), 7719. https://doi.org/10.3390/ijms25147719







