Testosterone Nanoemulsion Prevents Prostate Cancer: PC-3 and LNCaP Cell Viability In Vitro
Abstract
1. Introduction
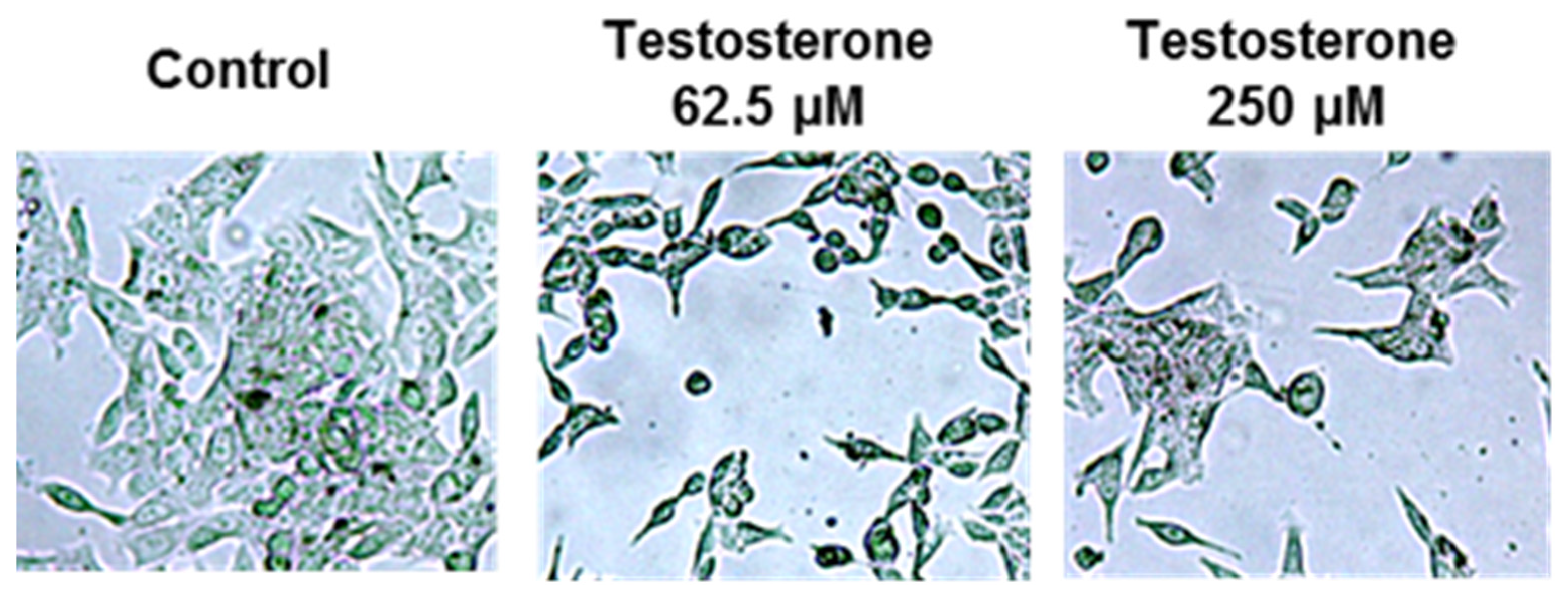
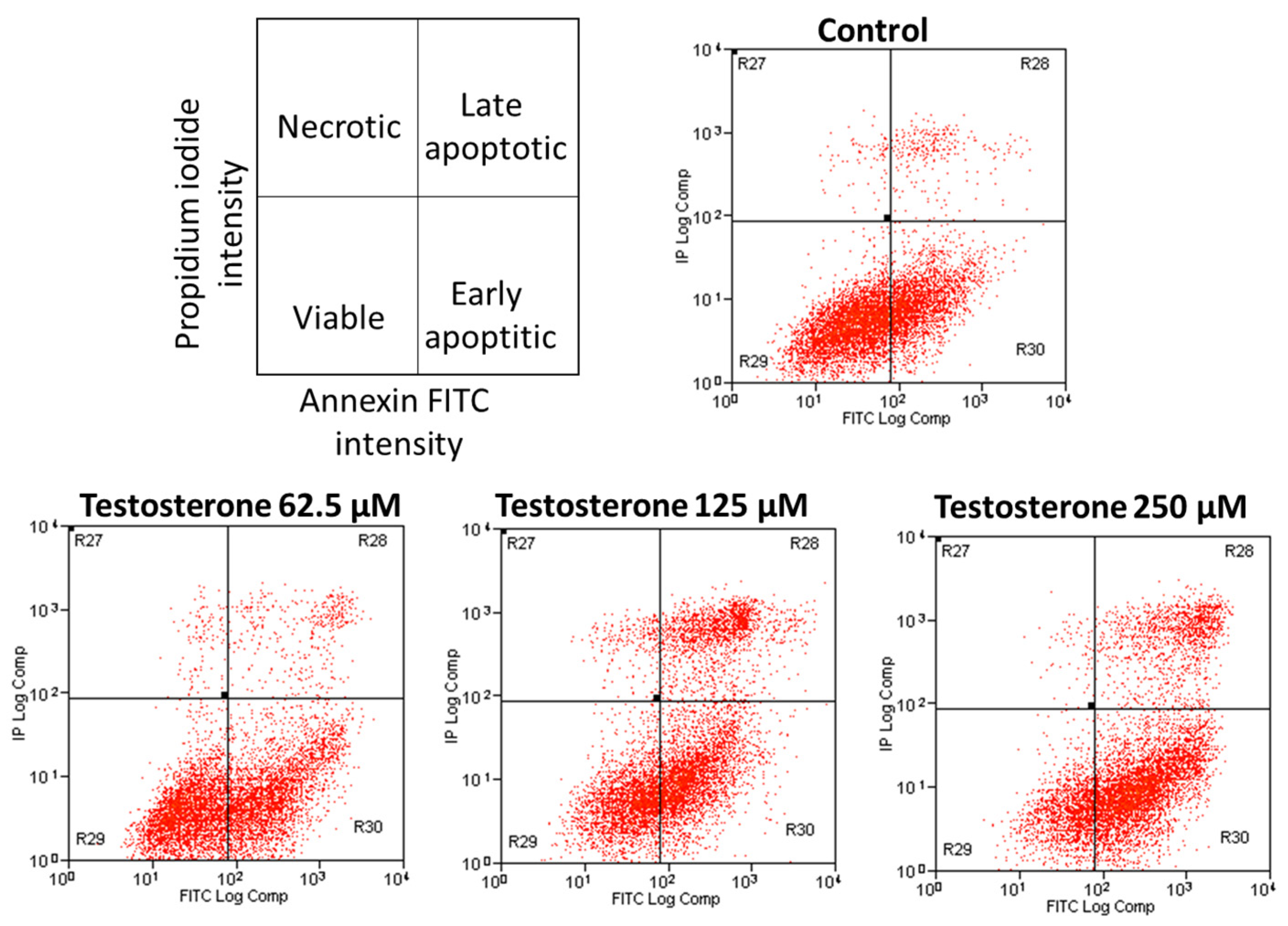
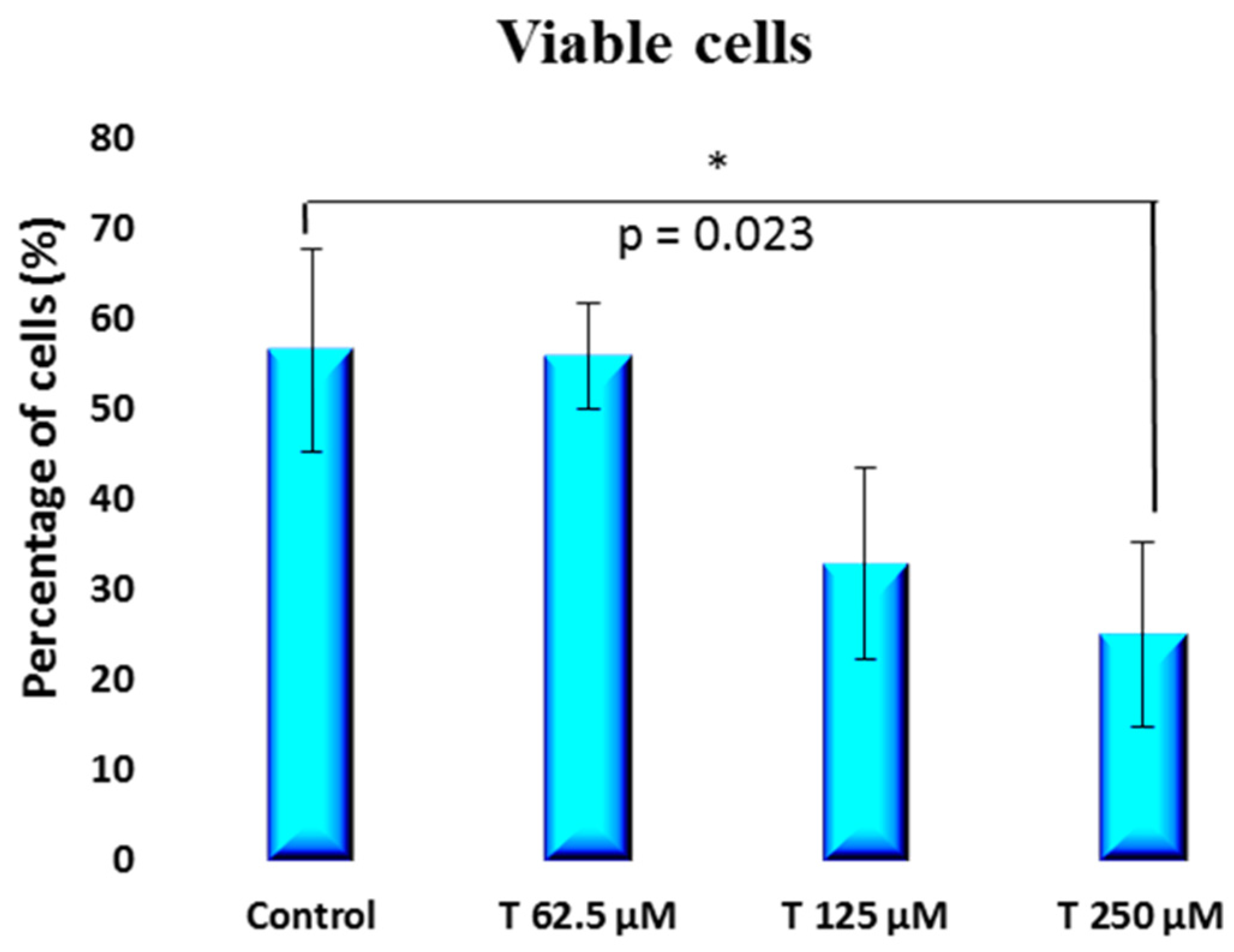
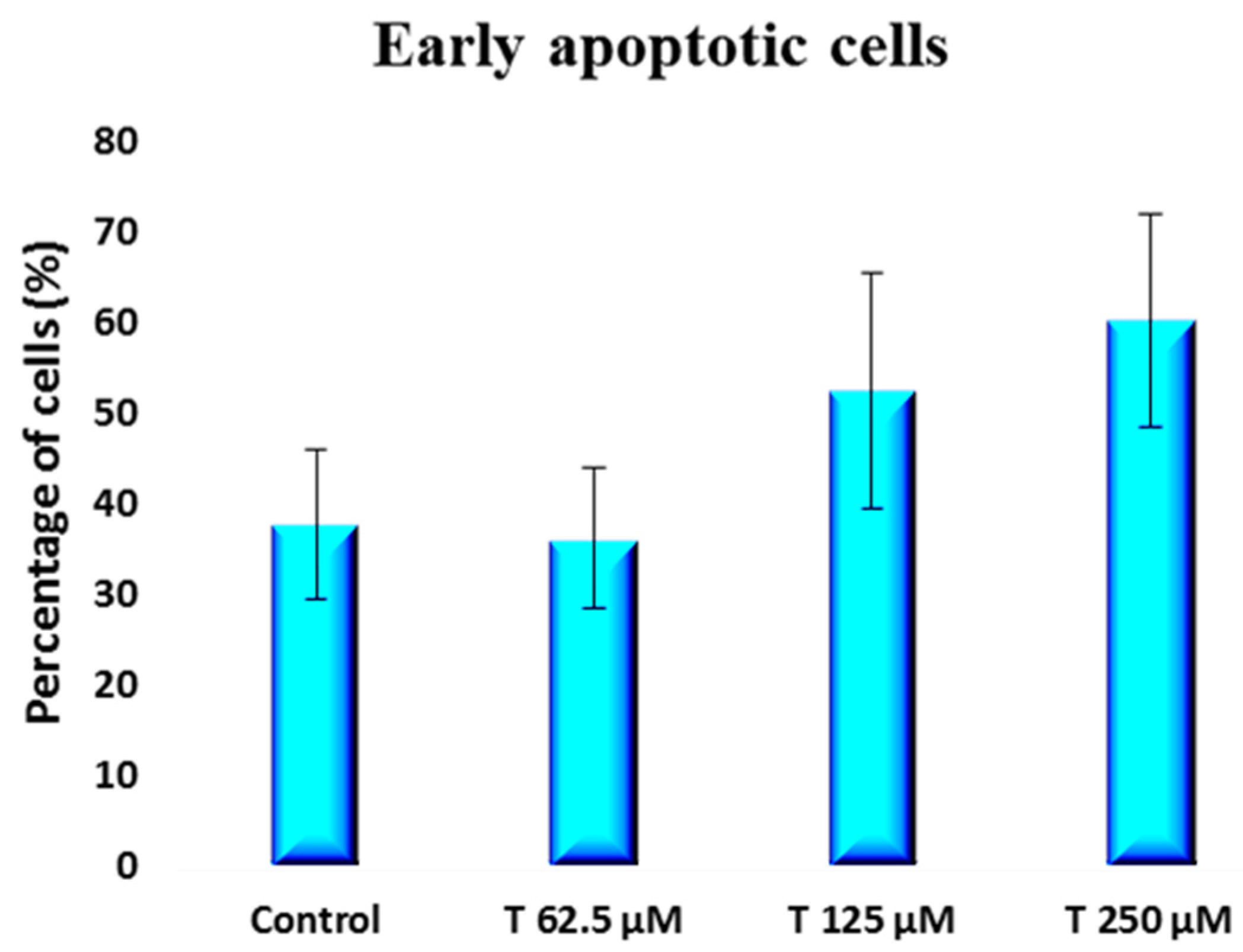
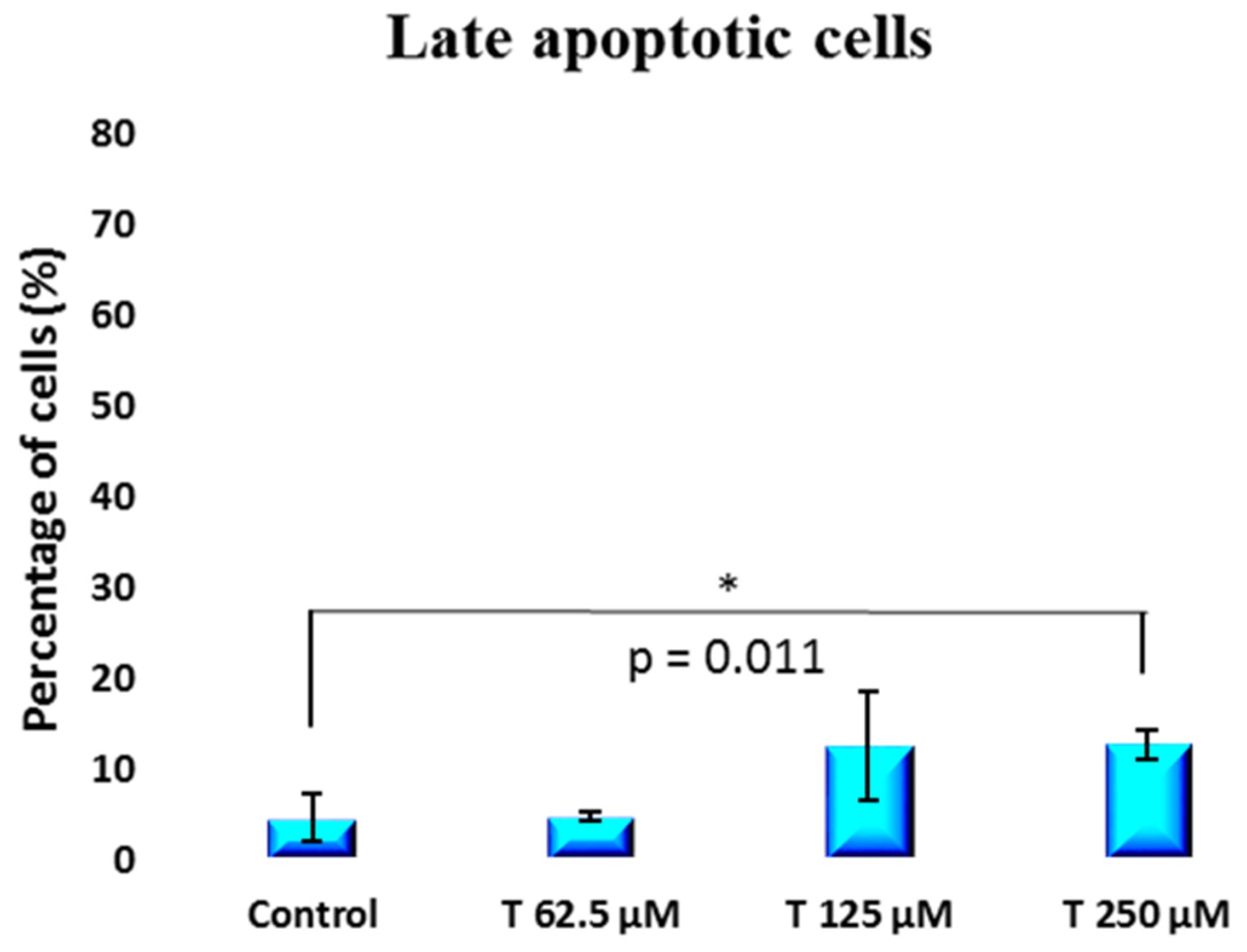
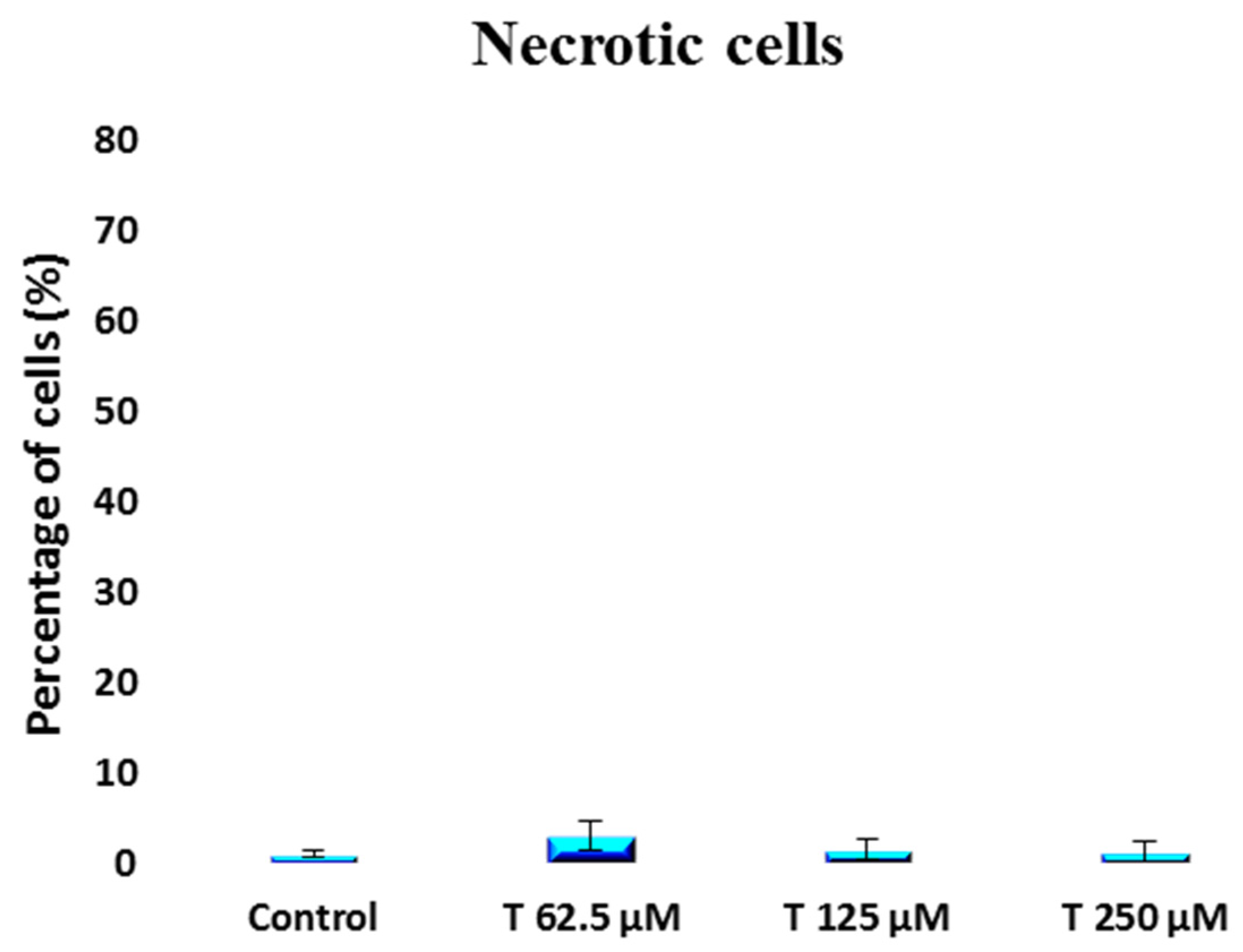
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Metabolic Activity Evaluation—Alamar Blue Test
4.3. Cell Viability Evaluation—Flow Cytometry
4.4. Particle Size Z-Average and Physical Stability
4.5. Nanoemulsion Preparation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Statistics Center. Available online: https://cancerstatisticscenter.cancer.org/#/cancer-site/Prostate (accessed on 25 August 2017).
- Stepan, J.J.; Lachman, M.; Zverina, J.; Pacovsky, V.; Baylink, D.J. Castrated men exhibit bone loss: Effect of calcitonin treatment on biochemical indices of bone remodeling. J. Clin. Endocrinol. Metab. 1989, 69, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bonfiglio, R.; Varone, F.; Ciuffa, S.; Mauriello, A.; Bonanno, E. Calcifications in prostate cancer: An active phenomenon mediated by epithelial cells with osteoblast-phenotype. Microsc. Res. Tech. 2018, 81, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Hofbauer, L.C.; Heufelder, A.E.; Roth, S.; Delmas, P.D. Osteoprotegerin serum levels in men: Correlation with age, estrogen, and testosterone status. J. Clin. Endocrinol. Metab. 2001, 86, 3162–3165. [Google Scholar] [CrossRef] [PubMed]
- Mearini, L.; Zucchi, A.; Nunzi, E.; Villirillo, T.; Bini, V.; Porena, M. Low serum testosterone levels are predictive of prostate cancer. World J. Urol. 2013, 31, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A. Testosterone replacement therapy and prostate cancer. Urol. Clin. N. Am. 2007, 34, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.D.; Chen, Y.H.; Xu, S.; Zhang, C.; Wang, D.M.; Wang, H.; Chen, L.; Zhang, Z.H.; Xia, M.Z.; Xu, D.X.; et al. Low vitamin D status is associated with inflammation in patients with prostate cancer. Oncotarget 2017, 8, 22076–22085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Chen, H.; Zhang, S.; Chen, X.; Sheng, Y.; Pang, J. Association of cigarette smoking habits with the risk of prostate cancer: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bandini, M.; Gandaglia, G.; Briganti, A. Obesity and prostate cancer. Curr. Opin. Urol. 2017, 27, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.E.; Sierra-Fonseca, J.A.; Davalos, O.; Saenz, L.A.; Castellanos, M.M.; Zavala, J.K.; Gosselink, K.L. Stress alters the expression of cancer-related genes in the prostate. BMC Cancer 2017, 17, 621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morales, A.; Siemens, D.R. Testosterone Therapy and Prostate Cancer: Incorporating Low-Level Evidence into Practical Recommendations. Urol. Clin. N. Am. 2022, 49, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Caliber, M. Safety of testosterone therapy in men with prostate cancer. Expert Opin. Drug Saf. 2019, 18, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Bosland, M.C.; Schlicht, M.J.; Horton, L.; McCormick, D.L. The MNU Plus Testosterone Rat Model of Prostate Carcinogenesis. Toxicol. Pathol. 2022, 50, 478–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botelho, M.A.; Martins, J.G.; Ruela, R.S. Nanotechnology in ligature-induced periodontitis: Protective effect of a doxycycline gel with nanoparticules. J. Appl. Oral Sci. 2010, 18, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, L.W.; Botelho, M.A.; Queiroz, D.B. Nanotechnology in Hormone Replacement Therapy: Safe and Efficacy of Transdermal Estriol and Estradiol Nanoparticles after 5 Years Follow-Up Study. Lat. Am. J. Pharm. 2012, 31, 442–450. [Google Scholar]
- Botelho, M.A.; Barros, G.; Queiroz, D.B. Nanotechnology in Phytotherapy: Antiinflammatory Effect of a Nanostructured Thymol Gel from Lippia sidoides in Acute Periodontitis in Rats. Phytother. Res. 2016, 30, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.A.; Queiroz, D.B.; Barros, G. Nanostructured transdermal hormone replacement therapy for relieving menopausal symptoms: A confocal Raman spectroscopy study. Clinics 2014, 69, 75–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botelho, M.A.; Queiroz, D.B.; Freitas, A.; Guerreiro, S.; Umbelino, S.; Barros, G. Effects of a new testosterone transdermal delivery system, Biolipid B2-testosterone in healthy middle aged men: A Confocal Raman Spectroscopy Study. J. Pharm. Sci. Innov. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Gleave, M.E.; Hsieh, J.-T.; Wu, H.-C.; von Eschenbach, A.C.; Chung, L.W. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 1992, 52, 1598–1605. [Google Scholar]
- Kaighn, M.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.; Jones, L. Establishment and characterization of a human prostatic carcinoma cell line [PC-3]. Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Nunez, R. Flow cytometry: Principles and instrumentation. Curr. Issues Mol. Biol. 2001, 3, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.; Kutzner, B.; Truong, M.; Sanchez-Dardon, J.; McLean, J. Analysis of radiation-induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytom. Part A 2002, 48, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Del Buono, C.; Baldracchini, F.; Talesa, V.; Cottini, E.; Brancadoro, C.; Zucchi, A. Alteration of glyoxalases gene expression in response to testosterone in LNCaP and PC3 human prostate cancer cells. Cancer Biol. Ther. 2007, 6, 1880–1888. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho, M.A.; Queiroz, D.B. Testosterone Nanoemulsion Prevents Prostate Cancer: PC-3 and LNCaP Cell Viability In Vitro. Int. J. Mol. Sci. 2024, 25, 7729. https://doi.org/10.3390/ijms25147729
Botelho MA, Queiroz DB. Testosterone Nanoemulsion Prevents Prostate Cancer: PC-3 and LNCaP Cell Viability In Vitro. International Journal of Molecular Sciences. 2024; 25(14):7729. https://doi.org/10.3390/ijms25147729
Chicago/Turabian StyleBotelho, Marco Antonio, and Dinalva Brito Queiroz. 2024. "Testosterone Nanoemulsion Prevents Prostate Cancer: PC-3 and LNCaP Cell Viability In Vitro" International Journal of Molecular Sciences 25, no. 14: 7729. https://doi.org/10.3390/ijms25147729
APA StyleBotelho, M. A., & Queiroz, D. B. (2024). Testosterone Nanoemulsion Prevents Prostate Cancer: PC-3 and LNCaP Cell Viability In Vitro. International Journal of Molecular Sciences, 25(14), 7729. https://doi.org/10.3390/ijms25147729





