Recent Progress in the Research on RNA-Binding Proteins in Bone Development and Diseases
Abstract
1. Introduction
2. RBPs Involved in the Differentiation of Bone-Marrow-Derived MSCs
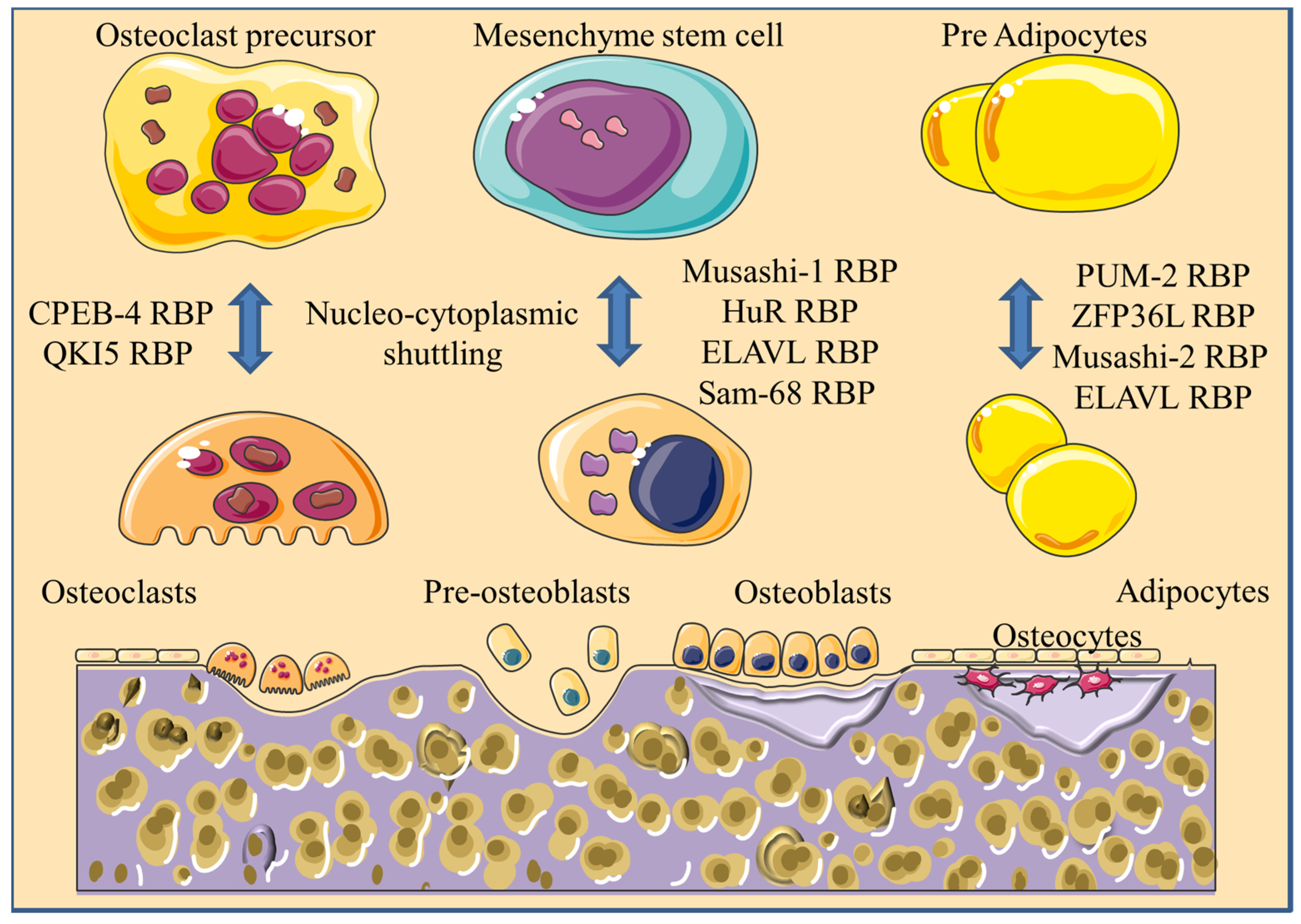
2.1. Musashi RBPs
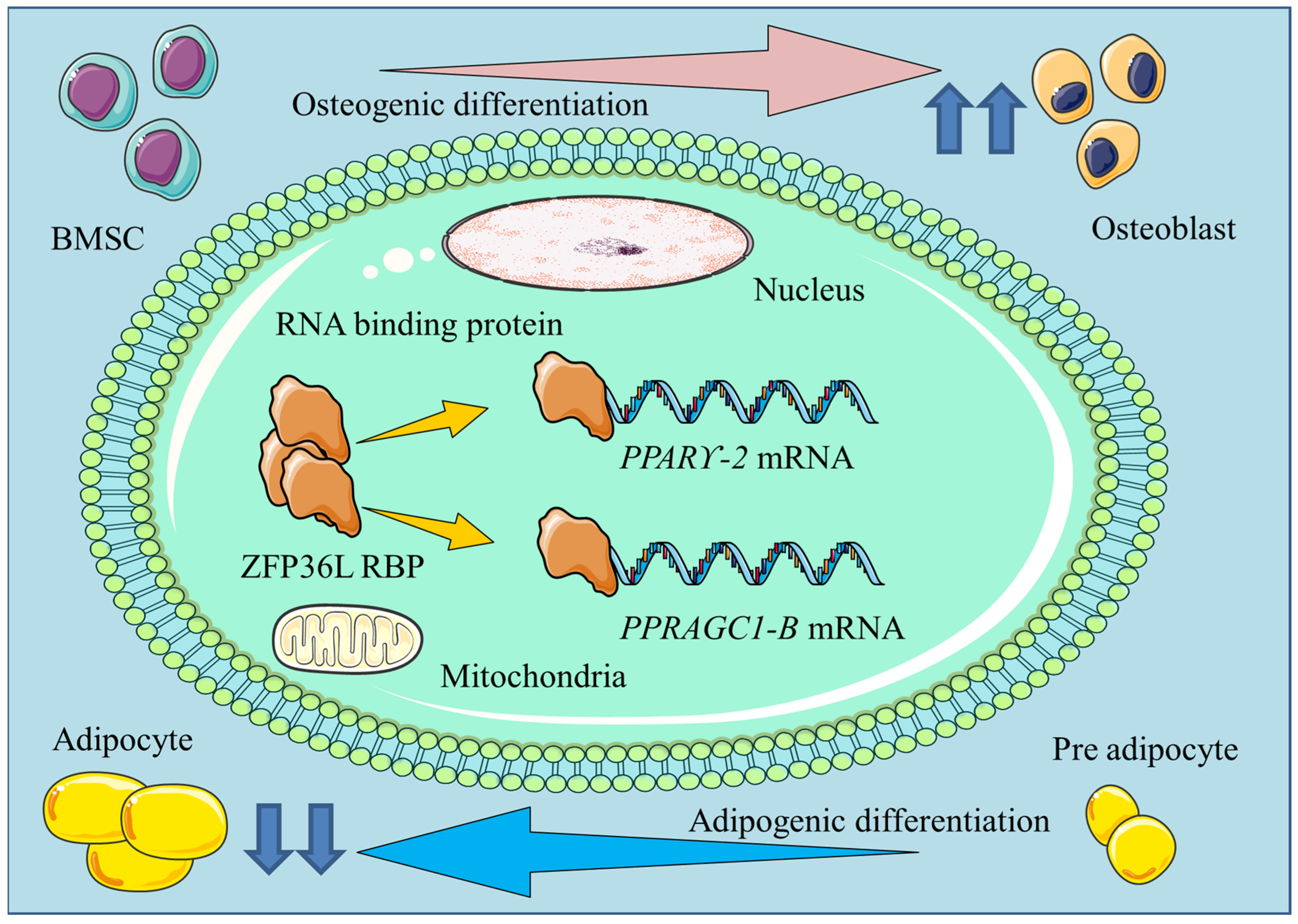
2.2. ZFP36L1
2.3. PUM RBPs
2.4. ELAVL1
3. RBPs Involved in Osteoblastogenesis
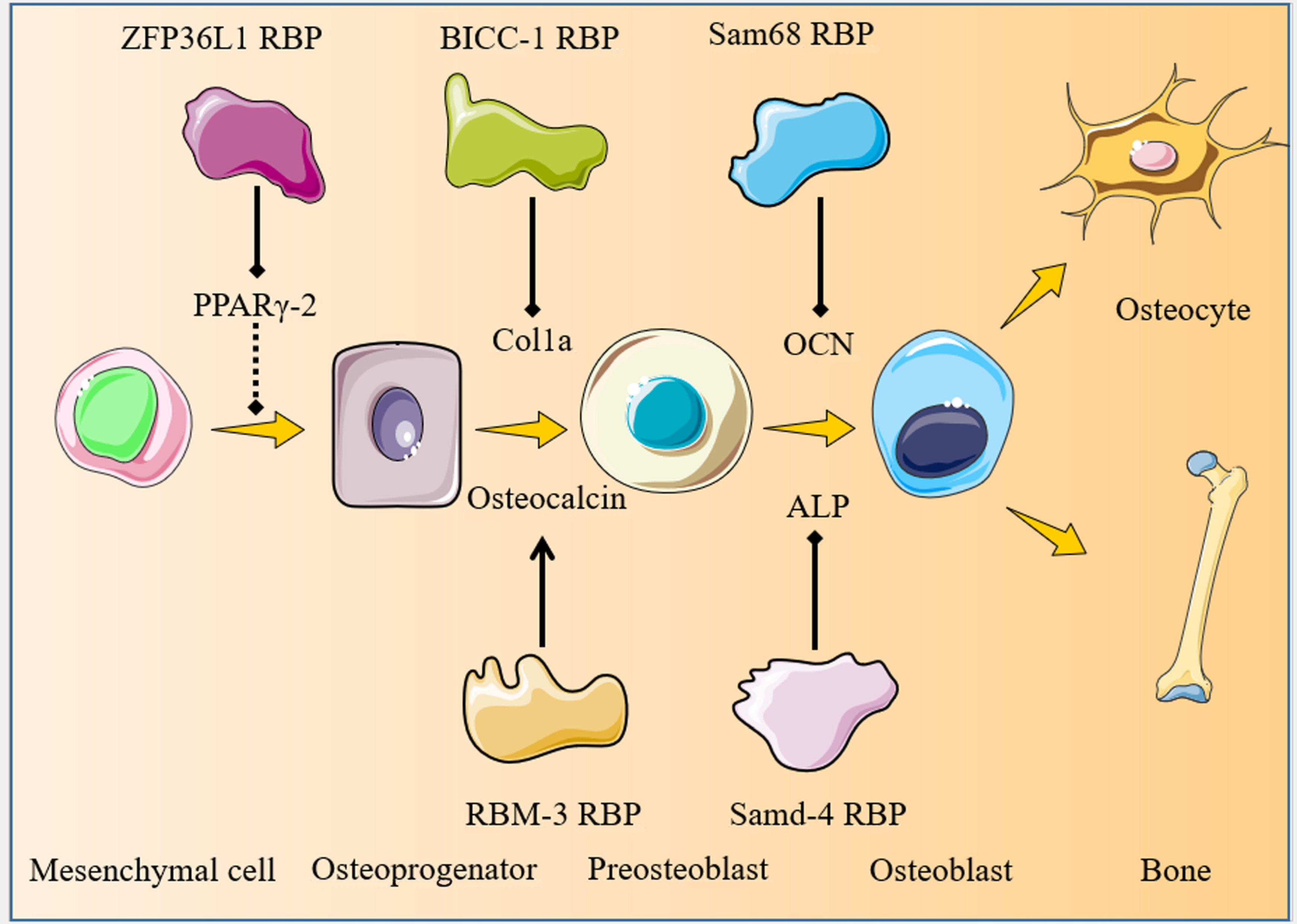
3.1. ZFP36L1
3.2. BICC1
3.3. RBM3
3.4. Sam68
3.5. SAMD4
4. RBPs Involved in Osteoclastogenesis
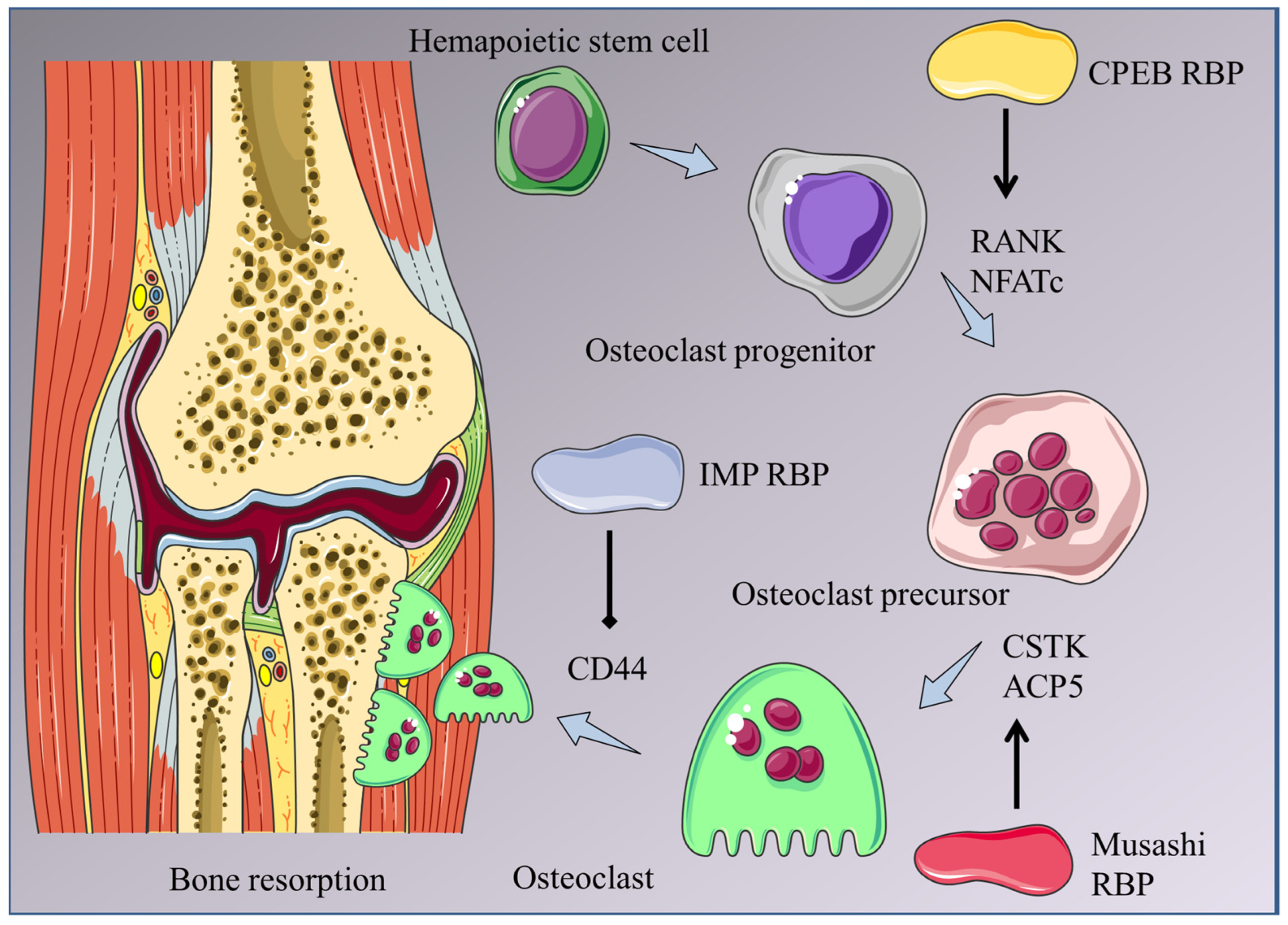
4.1. CPEB RBPs
4.2. Musashi2
4.3. Tristetraprolin
4.4. IMP2
4.5. Mex3B
4.6. QKI
5. RBPs Associated with Bone Diseases
5.1. RBPs Associated with Diabetic Osteoporosis
5.1.1. ELAVL1
5.1.2. PCBP1
5.2. RBPs Associated with Osteosarcoma
5.2.1. HuR
5.2.2. LIN28A
5.2.3. PTBP-1
5.3. RBPs Associated with Osteoarthritis
5.3.1. ZFP36L1
5.3.2. GNL3
5.3.3. TDP-43
5.3.4. SND1
5.4. RBPs Associated with Osteoporosis
5.4.1. PTBP1
5.4.2. HuR
5.5. RBPs Associated with Calcific Tendinopathy
RBFOX2
6. Summary and Perspectives
| RBP | Biological Functions in Bone Development and Diseases | References |
|---|---|---|
| Musashi-1 | Upregulated in bone-marrow-MSCs and bone cells during bone healing and correlates with osteogenic differentiation | [19,20,21] |
| Musashi-2 | Controls osteoblast-adipocyte lineage commitment by suppressing PPARγ | [22] |
| Promotes osteoclastogenesis and osteoclast survival through Notch and NF-κB | [39] | |
| ZFP36L1 | Suppresses bone-marrow-MSC adipogenic differentiation by directly targeting PPARGC1B and PPARγ-2 mRNAs | [23,29] |
| Upregulated in OA chondrocytes and promotes OA by directing targeting the HSP70 family mRNAs | [54] | |
| PUM2 | Enhances MSC adipogenesis and inhibits osteogenesis by directly targeting the JAK2 and RUNX2 mRNAs | [24] |
| PUM1 | Protects against chondrogenic loss in OA by directly targeting the TLR4 mRNA | [25] |
| ELAVL1 | Inhibits bone-marrow-MSC osteogenic differentiation by directly targeting ECM-related mRNAs | [26] |
| BICC1 | Promotes osteoblastogenesis and bone mineral deposition via PKD2 | [30] |
| RBM3 | Stimulates osteoblastogenesis via ERK | [31] |
| Sam68 | Inhibits osteogenesis and promotes adipogenesis | [32] |
| Promotes FLS inflammation, proliferation, migration, and invasion in RA through NF-κB P65 | [33] | |
| SAMD4 | Promotes osteogenesis and bone development by binding the MIG6 mRNA and inhibits MIG6 protein synthesis | [34] |
| CPEB4 | Promotes osteoclastogenesis downstream of RANKL | [36] |
| Promotes macrophage M2 polarization and OS progression | [37] | |
| CPEB1 | Promotes OS progression downstream of miR-377-3p | [38] |
| TTP | Inhibits inflammation and prevents periodontal bone loss | [40] |
| IMP2 | Regulates osteoclast activity and adhesion | [41] |
| Mex3B | Inhibits osteoclastogenesis by downregulating DCSTAMP | [42] |
| QKI | Promotes osteoblastogenesis, inhibits osteoclastogenesis, and protects against osteoporosis | [43] |
| QKI5 | Inhibits osteoclastogenesis by downregulating CSF-1R and RANK | [44] |
| PCBP1 | Protects osteoblasts and inhibits ferroptosis induced by high glucose in diabetic osteoporosis | [48] |
| HuR | Upregulated in OS tissues and cell lines and promotes OS progression by binding the AGO2, HMGA1, and YAP mRNAs | [49,50,51] |
| Promotes osteoblastic differentiation and protects against osteoporosis by binding and stabilizing the LRP6 mRNA to activate Wnt | [60] | |
| Promotes osteoblastic differentiation and protects against osteoporosis by translocation to the cytoplasm upon association with circStag1 to activate Wnt | [61] | |
| Lin28A | Promotes OS cell progression by binding LncRNA MALAT1 | [52] |
| Promotes osteogenic differentiation by interacting with LncRNA TUG1 | [64] | |
| PTBP1 | Promotes OS cell viability, proliferation, migration, and invasion by binding ZMIZ1-AS1 to stabilize the ZMIZ1 mRNA | [53] |
| Inhibits osteogenic differentiation, enhances adipogenic differentiation, and promotes osteoporosis by interacting with LncRNA SNHGI to upregulate DNMT1 | [59] | |
| GNL3 | Upregulated in human OA lesions and promotes OA by upregulating IL24 and PTN | [55,56] |
| TDP-43 | Downregulated in human OA lesions and alleviates cartilage degradation and bone remodeling in OA | [57] |
| SND1 | Enhances IL-1β-induced chondrocyte ferroptosis and promotes OA progression by binding and destabilizing the HSPA5 mRNA | [58] |
| RBFOX2 | Its cytoplasmic localization in calcific tendons may contribute to the development of calcific tendinopathy | [62] |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| RBPs | RNA-binding proteins |
| MSCs | Mesenchymal stem cells |
| RUNX2 | Runt-related transcription factor 2 |
| OSX | Osterix |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| POSTN | Periostin |
| PPARγ | Peroxisome proliferator activated receptor γ |
| AA | Aplastic anemia |
| NF-κB | Nuclear factor κB |
| TRL-4 | Toll-like receptor 4 |
| ELAVL-1 | Embryonic lethal abnormal version like 1 |
| ECM | Extracellular matrix |
| ChREBP | Carbohydrate responsive element binding protein |
| BICC1 | Bicaudal C homolog-1 |
| BMD | Bone mineral density |
| PKD2 | Polycystic kidney disease 2 |
| ERK | Extracellular-signal-regulated kinase |
| P38 | P38 kinase |
| JNK | C-Jun N-terminal kinase |
| Sam68 | Src associated in mitosis 68 KDa |
| RA | Rheumatoid arthritis |
| FLS | Fibroblast like synoviocytes |
| SAMD4 | Sterile alpha motif domain containing protein 4 |
| MIG-6 | Mitogen inducible gene 6 |
| TTP | Tristetraprolin |
| TNFα | Tumor necrosis factor α |
| IMP2 | Insulin like growth factor 2 |
| HCMV | Human cyotomegalovirus |
| DM | Diabetes mellitus |
| DMT1 | Divalent metal transporter 1 |
| OS | Osteosarcoma |
| EMT | Epithelial–mesenchymal transition |
| ZMIZ1- | AS1 Zinc finger MIZ type containing 1 antisense |
| DNMT | DNA methyltransferase methylation |
| GNL3 | Nuclear GTP binding protein 3 |
| TDP-43 | TAR RNA binding protein 43 |
| SND-1 | Staphylococcal nuclease domain containing 1 |
| HSPA-5 | Heat shock protein family A member 5 |
| OVX | Ovariectomy |
| RBFOX2 | RNA binding protein Fox-1 homolog-2 |
| ALS | Amyotrophic lateral sclerosis |
| FTD | Frontotemporal dementia |
| CPEB | Cytoplasmic polyadenylation element binding protein |
References
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E587–E593. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The roles of osteocytes in alveolar bone destruction in periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Corley, M.; Burns, M.C.; Yeo, G.W. How RNA-Binding Proteins Interact with RNA: Molecules and Mechanisms. Mol. Cell 2020, 78, 9–29. [Google Scholar] [CrossRef]
- Van Nostrand, E.L.; Freese, P.; Pratt, G.A.; Wang, X.; Wei, X.; Xiao, R.; Blue, S.M.; Chen, J.Y.; Cody, N.A.L.; Dominguez, D.; et al. A large-scale binding and functional map of human RNA-binding proteins. Nature 2020, 583, 711–719. [Google Scholar] [CrossRef]
- Kelaini, S.; Chan, C.; Cornelius, V.A.; Margariti, A. RNA-Binding Proteins Hold Key Roles in Function, Dysfunction, and Disease. Biology 2021, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Jiang, Q. Effect of RNA-binding proteins on osteogenic differentiation of bone marrow mesenchymal stem cells. Mol. Cell. Biochem. 2024, 479, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, L.; Yang, K.; Xu, J.; Zhang, D.; Ling, J.; Xia, P.; Wu, Y.; Liu, X.; Liu, J.; et al. The role and mechanism of RNA-binding proteins in bone metabolism and osteoporosis. Ageing Res. Rev. 2024, 96, 102234. [Google Scholar] [CrossRef] [PubMed]
- Kokabu, S.; Lowery, J.W.; Jimi, E. Cell Fate and Differentiation of Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3753581. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; de Girolamo, L.; Bonasia, D.E.; Bruzzone, M.; Mattia, S.; Rossi, R.; Montaruli, A.; Dettoni, F.; Castoldi, F.; Peretti, G. Bone marrow derived stem cells in joint and bone diseases: A concise review. Int. Orthop. 2014, 38, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okano, H.; Blendy, J.A.; Montell, C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron 1994, 13, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.M.; Fraser, B.A.; Sobinoff, A.P.; Pye, V.J.; Davidson, T.L.; Siddall, N.A.; Koopman, P.; Hime, G.R.; McLaughlin, E.A. Developmental expression of Musashi-1 and Musashi-2 RNA-binding proteins during spermatogenesis: Analysis of the deleterious effects of dysregulated expression. Biol. Reprod. 2014, 90, 92. [Google Scholar] [CrossRef]
- Sutherland, J.M.; Sobinoff, A.P.; Fraser, B.A.; Redgrove, K.A.; Davidson, T.L.; Siddall, N.A.; Koopman, P.; Hime, G.R.; McLaughlin, E.A. RNA binding protein Musashi-1 directly targets Msi2 and Erh during early testis germ cell development and interacts with IPO5 upon translocation to the nucleus. FASEB J. 2015, 29, 2759–2768. [Google Scholar] [CrossRef]
- Padial-Molina, M.; Crespo-Lora, V.; Candido-Corral, C.; Martin-Morales, N.; Abril-Garcia, D.; Galindo-Moreno, P.; Hernandez-Cortes, P.; O’Valle, F. Expression of Musashi-1 Increases in Bone Healing. Int. J. Mol. Sci. 2021, 22, 3395. [Google Scholar] [CrossRef] [PubMed]
- O’Valle, F.; de Buitrago, J.G.; Hernández-Cortés, P.; Padial-Molina, M.; Crespo-Lora, V.; Cobo, M.; Aguilar, D.; Galindo-Moreno, P. Increased Expression of Musashi-1 Evidences Mesenchymal Repair in Maxillary Sinus Floor Elevation. Sci. Rep. 2018, 8, 12243. [Google Scholar] [CrossRef]
- Padial-Molina, M.; de Buitrago, J.G.; Sainz-Urruela, R.; Abril-Garcia, D.; Anderson, P.; O’Valle, F.; Galindo-Moreno, P. Expression of Musashi-1 During Osteogenic Differentiation of Oral MSC: An In Vitro Study. Int. J. Mol. Sci. 2019, 20, 2171. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zou, S.; Wang, J.; Han, Y.; Zhang, L.; Lv, C.; Jiang, B.; Ren, Q.; Chen, L.; Yang, L.; et al. The RNA-binding protein Musashi2 governs osteoblast-adipocyte lineage commitment by suppressing PPARγ signaling. Bone Res. 2022, 10, 31. [Google Scholar] [CrossRef]
- Liu, L.L.; Liu, L.; Liu, H.H.; Ren, S.S.; Dou, C.Y.; Cheng, P.P.; Wang, C.L.; Wang, L.N.; Chen, X.L.; Zhang, H.; et al. Levamisole suppresses adipogenesis of aplastic anaemia-derived bone marrow mesenchymal stem cells through ZFP36L1-PPARGC1B axis. J. Cell. Mol. Med. 2018, 22, 4496–4506. [Google Scholar] [CrossRef]
- Lee, M.H.; Wu, X.; Zhu, Y. RNA-binding protein PUM2 regulates mesenchymal stem cell fate via repression of JAK2 and RUNX2 mRNAs. J. Cell. Physiol. 2020, 235, 3874–3885. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, K.M.; Choi, Y.; Ko, E.A.; Lee, N.H.; Cho, S.; Park, K.H.; Lee, J.H.; Kim, H.W.; Lee, J.W. TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ. 2022, 29, 1364–1378. [Google Scholar] [CrossRef]
- Kota, S.K.; Lim, Z.W.; Kota, S.B. Elavl1 Impacts Osteogenic Differentiation and mRNA Levels of Genes Involved in ECM Organization. Front. Cell Dev. Biol. 2021, 9, 606971. [Google Scholar] [CrossRef]
- Kwan Tat, S.; Pelletier, J.P.; Lajeunesse, D.; Fahmi, H.; Lavigne, M.; Martel-Pelletier, J. The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin. Exp. Rheumatol. 2008, 26, 295–304. [Google Scholar] [PubMed]
- Tat, S.K.; Pelletier, J.P.; Lajeunesse, D.; Fahmi, H.; Duval, N.; Martel-Pelletier, J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: Influence of osteotropic factors. Bone 2008, 43, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.Y.; Chen, Y.H.; Lin, S. Zinc finger protein ZFP36L1 promotes osteoblastic differentiation but represses adipogenic differentiation of mouse multipotent cells. Oncotarget 2017, 8, 20588–20601. [Google Scholar] [CrossRef]
- Mesner, L.D.; Ray, B.; Hsu, Y.H.; Manichaikul, A.; Lum, E.; Bryda, E.C.; Rich, S.S.; Rosen, C.J.; Criqui, M.H.; Allison, M.; et al. Bicc1 is a genetic determinant of osteoblastogenesis and bone mineral density. J. Clin. Investig. 2014, 124, 2736–2749. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, K.M.; Kim, E.J.; Jang, W.G. Hypothermia-induced RNA-binding motif protein 3 (RBM3) stimulates osteoblast differentiation via the ERK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 498, 459–465. [Google Scholar] [CrossRef]
- Richard, S.; Torabi, N.; Franco, G.V.; Tremblay, G.A.; Chen, T.; Vogel, G.; Morel, M.; Cléroux, P.; Forget-Richard, A.; Komarova, S.; et al. Ablation of the Sam68 RNA binding protein protects mice from age-related bone loss. PLoS Genet. 2005, 1, e74. [Google Scholar] [CrossRef]
- Sun, W.; Qin, R.; Qin, R.; Wang, R.; Ding, D.; Yu, Z.; Liu, Y.; Hong, R.; Cheng, Z.; Wang, Y. Sam68 Promotes Invasion, Migration, and Proliferation of Fibroblast-like Synoviocytes by Enhancing the NF-κB/P65 Pathway in Rheumatoid Arthritis. Inflammation 2018, 41, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Xiang, J.F.; Yang, Q.; Wang, L.; Wei, Z.; Chen, L.L.; Yang, L.; Zou, W. RNA-binding protein SAMD4 regulates skeleton development through translational inhibition of Mig6 expression. Cell Discov. 2017, 3, 16050. [Google Scholar] [CrossRef]
- Ivshina, M.; Lasko, P.; Richter, J.D. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu. Rev. Cell Dev. Biol. 2014, 30, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Arasaki, Y.; Li, M.; Akiya, T.; Nozawa, I.; Ezura, Y.; Hayata, T. The RNA-binding protein Cpeb4 is a novel positive regulator of osteoclast differentiation. Biochem. Biophys. Res. Commun. 2020, 528, 621–627. [Google Scholar] [CrossRef]
- Yang, D.; Liu, K.; Fan, L.; Liang, W.; Xu, T.; Jiang, W.; Lu, H.; Jiang, J.; Wang, C.; Li, G.; et al. LncRNA RP11-361F15.2 promotes osteosarcoma tumorigenesis by inhibiting M2-Like polarization of tumor-associated macrophages of CPEB4. Cancer Lett. 2020, 473, 33–49. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Li, Z.; Wang, L. Circ_0003732 promotes osteosarcoma progression through regulating miR-377-3p/CPEB1 axis and Wnt/β-catenin signaling pathway. Anti-Cancer Drugs 2022, 33, e299–e310. [Google Scholar] [CrossRef]
- Fujiwara, T.; Zhou, J.; Ye, S.; Zhao, H. RNA-binding protein Musashi2 induced by RANKL is critical for osteoclast survival. Cell Death Dis. 2016, 7, e2300. [Google Scholar] [CrossRef]
- Steinkamp, H.M.; Hathaway-Schrader, J.D.; Chavez, M.B.; Aartun, J.D.; Zhang, L.; Jensen, T.; Shojaee Bakhtiari, A.; Helke, K.L.; Stumpo, D.J.; Alekseyenko, A.V.; et al. Tristetraprolin Is Required for Alveolar Bone Homeostasis. J. Dent. Res. 2018, 97, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, D.; Liu, S.; Liu, Z.; Li, M. Histochemical evidence of IGF2 mRNA-binding protein 2-mediated regulation of osteoclast function and adhesive ability. Histochem. Cell Biol. 2018, 149, 343–351. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.Y.; Li, Z.Q.; Wu, H.N. Mex3B inhibits DC-STAMP mRNA level and osteoclastogenesis. Cell Insight 2022, 1, 100002. [Google Scholar] [CrossRef]
- Du, T.; Yan, Z.; Zhu, S.; Chen, G.; Wang, L.; Ye, Z.; Wang, W.; Zhu, Q.; Lu, Z.; Cao, X. QKI deficiency leads to osteoporosis by promoting RANKL-induced osteoclastogenesis and disrupting bone metabolism. Cell Death Dis. 2020, 11, 330. [Google Scholar] [CrossRef]
- Rauwel, B.; Degboé, Y.; Diallo, K.; Sayegh, S.; Baron, M.; Boyer, J.F.; Constantin, A.; Cantagrel, A.; Davignon, J.L. Inhibition of Osteoclastogenesis by the RNA-Binding Protein QKI5: A Novel Approach to Protect from Bone Resorption. J. Bone Miner. Res. 2020, 35, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Inaba, M.; Terada, M.; Koyama, H.; Yoshida, O.; Ishimura, E.; Kawagishi, T.; Okuno, Y.; Nishizawa, Y.; Otani, S.; Morii, H. Influence of high glucose on 1,25-dihydroxyvitamin D3-induced effect on human osteoblast-like MG-63 cells. J. Bone Miner. Res. 1995, 10, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, M.; Wang, X.; Xu, B.; Xu, Z.; Su, B. ELAV-like RNA binding protein 1 regulates osteogenesis in diabetic osteoporosis: Involvement of divalent metal transporter 1. Mol. Cell Endocrinol. 2022, 546, 111559. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.D.; Shi, L.; Li, H.T.; Wang, X.D.; Yang, M.W. Polycytosine RNA-binding protein 1 regulates osteoblast function via a ferroptosis pathway in type 2 diabetic osteoporosis. World J. Diabetes 2024, 15, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhang, J.; Ma, J.; Xu, X.; Wang, Y.; Zhou, Z.; Jiang, D.; Shen, S.; Ding, Y.; et al. Silencing of HuR Inhibits Osteosarcoma Cell Epithelial-Mesenchymal Transition via AGO2 in Association With Long Non-Coding RNA XIST. Front. Oncol. 2021, 11, 601982. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Pang, J.; Ji, B.; Wang, Z.; Liu, C.; Cheng, Y.; Zhang, L. RNA binding protein HuR promotes osteosarcoma cell progression via suppressing the miR-142-3p/HMGA1 axis. Oncol. Lett. 2018, 16, 1475–1482. [Google Scholar] [CrossRef]
- Xu, W.; Chen, C.; Xu, R.; Li, Y.; Hu, R.; Li, Z.; Zhu, X. Knockdown of HuR represses osteosarcoma cells migration, invasion and stemness through inhibition of YAP activation and increases susceptibility to chemotherapeutic agents. Biomed. Pharmacother. 2018, 102, 587–593. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, J.; Ji, B.; Zhang, S.; Cheng, Y.; Yu, L.; Pan, W. RNA binding protein Lin28A promotes osteocarcinoma cells progression by associating with the long noncoding RNA MALAT1. Biotechnol. Lett. 2018, 40, 493–500. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Q.; Chang, J.; Zhao, Z.; Sun, C. Long non-coding RNA ZMIZ1-AS1 promotes osteosarcoma progression by stabilization of ZMIZ1. Cell Biol. Toxicol. 2022, 38, 1013–1026. [Google Scholar] [CrossRef]
- Son, Y.O.; Kim, H.E.; Choi, W.S.; Chun, C.H.; Chun, J.S. RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat. Commun. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Louka, M.L.; Zakaria, Z.M.; Nagaty, M.M.; Elsebaie, M.A.; Nabil, L.M. Expression of nucleostemin gene in primary osteoarthritis. Gene 2016, 587, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, J.; Manandhar, U.; Yao, X.; Bian, Y.; Zhang, B. RNA binding protein GNL3 up-regulates IL24 and PTN to promote the development of osteoarthritis. Life Sci. 2021, 267, 118926. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Liu, A.; Xu, J.; Xu, X.; Dai, J.; Wu, R.; Yan, W.; Wang, R.; Sun, Z.; Ikegawa, S.; et al. TDP-43 maintains chondrocyte homeostasis and alleviates cartilage degradation in osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1036–1047. [Google Scholar] [CrossRef]
- Lv, M.; Cai, Y.; Hou, W.; Peng, K.; Xu, K.; Lu, C.; Yu, W.; Zhang, W.; Liu, L. The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. Inflamm. Res. 2022, 71, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Song, M.S.; Rong, P.Z.; Chen, X.J.; Shi, L.; Wang, C.H.; Pang, Q.J. LncRNA SNHG1 modulates adipogenic differentiation of BMSCs by promoting DNMT1 mediated Opg hypermethylation via interacting with PTBP1. J. Cell. Mol. Med. 2022, 26, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, B.; Hu, H.; Li, X.; Zhang, X. Potential of RNA-binding protein human antigen R as a driver of osteogenic differentiation in osteoporosis. J. Orthop. Surg. Res. 2022, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Long, C.; Wang, S.; Wang, Z.; Chen, X.; Tang, W.; He, X.; Bao, Z.; Tan, B.; Zhao, J.; et al. Circular RNA circStag1 promotes bone regeneration by interacting with HuR. Bone Res. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Kim, J.O.; Lee, S.; Choi, S.; Kim, J.; Ko, M.S.; Park, S.J.; Ji, J.H.; Kim, K.K. Alternative splicing induces cytoplasmic localization of RBFOX2 protein in calcific tendinopathy. Exp. Mol. Pathol. 2019, 109, 36–41. [Google Scholar] [CrossRef]
- Mann, J.R.; Donnelly, C.J. RNA modulates physiological and neuropathological protein phase transitions. Neuron 2021, 109, 2663–2681. [Google Scholar] [CrossRef]
- He, Q.; Yang, S.; Gu, X.; Li, M.; Wang, C.; Wei, F. Long noncoding RNA TUG1 facilitates osteogenic differentiation of periodontal ligament stem cells via interacting with Lin28A. Cell Death Dis. 2018, 9, 455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, H.M.U.; Yang, L.; Cao, M.; Chen, Z.; Qian, A.; Dang, K. Recent Progress in the Research on RNA-Binding Proteins in Bone Development and Diseases. Int. J. Mol. Sci. 2024, 25, 7735. https://doi.org/10.3390/ijms25147735
Farooq HMU, Yang L, Cao M, Chen Z, Qian A, Dang K. Recent Progress in the Research on RNA-Binding Proteins in Bone Development and Diseases. International Journal of Molecular Sciences. 2024; 25(14):7735. https://doi.org/10.3390/ijms25147735
Chicago/Turabian StyleFarooq, Hafiz Muhammad Umer, Lihuizi Yang, Mengru Cao, Zhihao Chen, Airong Qian, and Kai Dang. 2024. "Recent Progress in the Research on RNA-Binding Proteins in Bone Development and Diseases" International Journal of Molecular Sciences 25, no. 14: 7735. https://doi.org/10.3390/ijms25147735
APA StyleFarooq, H. M. U., Yang, L., Cao, M., Chen, Z., Qian, A., & Dang, K. (2024). Recent Progress in the Research on RNA-Binding Proteins in Bone Development and Diseases. International Journal of Molecular Sciences, 25(14), 7735. https://doi.org/10.3390/ijms25147735






