Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction
Abstract
:1. Introduction
2. HFpEF Overview
3. HFpEF Epidemiology
4. Sex Differences in HFpEF
5. Cardiac Structure, Function, and Vasculature
6. Comorbidities
7. Immune Function and Inflammation
8. Sex Hormones
9. Sex-Specific SIRT Mechanisms
10. Pathogenesis of HFpEF
11. Application of Animal Models in HFpEF Research
12. Research Findings on SIRT Proteins in Animal Models
13. Limitations and Future Prospects
14. Studies of SIRTs and HFpEF
15. Biological Functions of SIRTs
15.1. Role of SIRTs in Inflammatory Responses
15.2. Role of SIRTs in Metabolism
15.3. Role of SIRTs in Oxidative Stress
15.4. Role of SIRTs in Apoptosis
15.5. SIRT1
15.6. SIRT3
15.7. SIRT6
15.8. SIRT2, SIRT4, SIRT5, and SIRT7
16. Research on Pseudomolecular Functions of SIRTs
17. Treatment of SIRTs and HFpEF
18. Resveratrol
19. Indole-3-Propionic Acid
20. Comparison of SIRTs Agonists and Traditional Drugs
21. Clinical Trial Data
22. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cui, Z.; Tian, G. Complete interpretation on 2021 ESC guideline for acute and chronic heart failure. Chin. J. Evid. Based Cardiovasc. Med. 2022, 14, 1281–1287. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 523. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart failure with preserved ejection fraction: JACC scientific statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Gottdiener, J.S.; Hetzel, S.J.; Mcmurray, J.J.; Komajda, M.; McKelvie, R.; Baicu, C.F.; Massie, B.M.; Carson, P.E. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011, 124, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Working Group on Heart Failure NCfCQIN. 2020 Clinical performance and quality measures for heart failure in China. Chin. Circ. J. 2021, 36, 221–238. [Google Scholar]
- Udelson, J.E.; Lewis, G.D.; Shah, S.J.; Zile, M.R.; Redfield, M.M.; Burnett, J.; Parker, J.; Seferovic, J.P.; Wilson, P.; Mittleman, R.S.; et al. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: The CAPACITY HFpEF randomized clinical trial. JAMA 2020, 324, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: The VITALITY-HFpEF randomized clinical trial. JAMA 2020, 324, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Heart failure with preserved and reduced ejection fraction: Different risk profiles for different diseases. Eur. Heart J. 2013, 34, 1393–1395. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Savarese, G.; Dahlström, U.; Lund, L.H.; Fu, M. Age-dependent differences in clinical phenotype and prognosis in heart failure with mid-range ejection compared with heart failure with reduced or preserved ejection fraction. Clin. Res. Cardiol. 2019, 108, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Kapłon-Cieślicka, A.; Benson, L.; Chioncel, O.; Crespo-Leiro, M.G.; Coats, A.J.; Anker, S.D.; Filippatos, G.; Ruschitzka, F.; Hage, C.; Drożdż, J.; et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction—Insights from the ESC-HFA EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2022, 24, 335–350. [Google Scholar] [CrossRef]
- Löfman, I.; Szummer, K.; Dahlström, U.; Jernberg, T.; Lund, L.H. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1606–1614. [Google Scholar] [CrossRef]
- Rosano, G.M.; Moura, B.; Metra, M.; Böhm, M.; Bauersachs, J.; Ben Gal, T.; Adamopoulos, S.; Abdelhamid, M.; Bistola, V.; Čelutkienė, J.; et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 872–881. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Jonsson, Å.; Hallberg, A.C.; Dahlström, U.; Edner, M.; Lund, L.H. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int. J. Cardiol. 2020, 298, 59–65. [Google Scholar] [CrossRef]
- Savarese, G.; Settergren, C.; Schrage, B.; Thorvaldsen, T.; Löfman, I.; Sartipy, U.; Mellbin, L.; Meyers, A.; Farsani, S.F.; Brueckmann, M.; et al. Comorbidities and cause-specific outcomes in heart failure across the ejection fraction spectrum: A blueprint for clinical trial design. Int. J. Cardiol. 2020, 313, 76–82. [Google Scholar] [CrossRef]
- Teng, T.K.; Tay, W.T.; Dahlstrom, U.; Benson, L.; Lam, C.S.P.; Lund, L.H. Different relationships between pulse pressure and mortality in heart failure with reduced, mid-range and preserved ejection fraction. Int. J. Cardiol. 2018, 254, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vedin, O.; Lam, C.S.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.; Savarese, G.; Dahlström, U.; Lund, L.H. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: A nationwide cohort study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: A report from 14,964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef]
- Wang, H.; Chai, K.; Du, M.; Wang, S.; Cai, J.-P.; Li, Y.; Zeng, P.; Zhu, W.; Zhan, S.; Yang, J. Prevalence and incidence of heart failure among urban patients in china: A national population-based analysis. Circ. Heart Fail. 2021, 14, e008406. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Desai, A.S.; Vaduganathan, M.; Cleland, J.G.; Claggett, B.L.; Barkoudah, E.; Finn, P.; McCausland, F.R.; Yilmaz, M.B.; Lefkowitz, M.; Shi, V.; et al. Mode of death in patients with heart failure and preserved ejection fraction: Insights from PARAGON-HF trial. Circ. Heart Fail. 2021, 14, e008597. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Gona, P.; Pencina, M.J.; Tu, J.V.; Austin, P.C.; Vasan, R.S.; Kannel, W.B.; D’Agostino, R.B.; Lee, D.S.; Levy, D. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur. Heart J. 2012, 33, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- A Masoudi, F.; Havranek, E.P.; Smith, G.; Fish, R.H.; Steiner, J.F.; Ordin, D.L.; Krumholz, H.M. Gender, age, and heart failure with preserved left ventricular systolic function. J. Am. Coll. Cardiol. 2003, 41, 217–223. [Google Scholar] [CrossRef]
- Meyer, S.; Brouwers, F.P.; Voors, A.A.; Hillege, H.L.; de Boer, R.A.; Gansevoort, R.T.; van der Harst, P.; Rienstra, M.; van Gelder, I.C.; van Veldhuisen, D.J.; et al. Sex differences in new-onset heart failure. Clin. Res. Cardiol. 2015, 104, 342–350. [Google Scholar] [CrossRef]
- Dalen, H.; Thorstensen, A.; Vatten, L.J.; Aase, S.A.; Stoylen, A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ. Cardiovasc. Imaging 2010, 3, 614–622. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Borlaug, B.A.; Rodeheffer, R.J.; Kass, D.A. Age- and gender-related ventricular-vascular stiffening:a community-based study. Circulation 2005, 112, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Takada, Y.; Yamabe, A.; Kubo, T.; Asawa, K.; Ozaki, T.; Yamagishi, H.; Toda, I.; Yoshiyama, M.; Yoshikawa, J.; et al. Age- and gender-specific changes in the left ventricular relaxation: A Doppler echocardiographic study in healthy individuals. Circ. Cardiovasc. Imaging 2009, 2, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Merz, C.N.B.; Beltrame, J.F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Camici, P.G. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: A paradigm shift. Eur. Heart J. 2017, 38, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Tofovic, D.; Chami, T.; Al-Kindi, S.G.; Oliveira, G.H. Subtypes of heart failure in autoimmune diseases. J. Card. Fail. 2017, 23, S22. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Kuch, B.; Muscholl, M.; Luchner, A.; Döring, A.; Riegger, G.; Schunkert, H.; Hense, H. Gender specific differences in left ventricular adaptation to obesity and hypertension. J. Hum. Hypertens. 1998, 12, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Subramanya, V.; Zhao, D.; Ouyang, P.; Lima, J.A.; Vaidya, D.; Ndumele, C.E.; Bluemke, D.A.; Shah, S.J.; Guallar, E.; Nwabuo, C.C.; et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018, 108, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Avşar, Ş.; Hayıroğlu, M.İ.; Keskin, T.; Börklü, E.B.; Kaya, A.; Uzun, A.O.; Akyol, B.; Güvenç, T.S.; Kozan, Ö. Relation of the Number of Parity to Left Ventricular Diastolic Function in Pregnancy. Am. J. Cardiol. 2017, 120, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, G.M.; Kam, K.W.; Liu, J.; Wu, S.; Wong, T.M. Altered Ca2+ handling by ryanodine receptor and Na+-Ca2+ exchange in the heart from ovariectomized rats: Role of protein kinase A. Am. J. Physiol. Cell Physiol. 2007, 292, C1625–C1635. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Brokat, S.; Tschope, C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog. Cardiovasc. Dis. 2007, 49, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Magerko, K.A.; Lawson, B.R.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011, 589 Pt 18, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Payavula, H.Y.; Jamadandu, D.; Velpula, S.; Digumarti, R.R.; Satti, V.; Annamaneni, S. VNTR Polymorphism in the Intron 5 of SIRT3 and Susceptibility to Breast Cancer. Asian Pac. J. Cancer Prev. 2023, 24, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Kolinko, L.; Shlykova, O.; Izmailova, O.; Vesnina, L.; Kaidashev, I. SIRT1 contributes to polarization of peripheral blood monocytes by increasing STAT6 expression in young people with overweight and low-risk obesity. Georgian Med. News 2021, 313, 102–112. [Google Scholar]
- Pektaş, M.B.; Sadi, G.; Akar, F. Long-Term Dietary Fructose Causes Gender-Different Metabolic and Vascular Dysfunction in Rats: Modulatory Effects of Resveratrol. Cell. Physiol. Biochem. 2015, 37, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.C.; Gjesdal, O.; Almeida, A.; Nacif, M.; Wu, C.; Bluemke, D.A.; Brumback, L.; Lima, J.A.C. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: The multi-ethnic study of atherosclerosis. Echocardiography 2014, 31, 12–20. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Brooks, A.; Xu, S.; Luo, J.; Steiner, R.; Mickelsen, D.M.; Moravec, C.S.; Alexis, J.D.; Small, E.M.; et al. SIRT6 Mitigates Heart Failure with Preserved Ejection Fraction in Diabetes. Circ. Res. 2022, 131, 926–943. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. The Function of PGC1αin Physiological and Pathological Cardiachypertrophy. Master’s Thesis, Nanchang University, Nanchang, China, 2024. [Google Scholar] [CrossRef]
- Lopes, E.C.P.; Paim, L.R.; Carvalho-Romano, L.F.R.S.; Marques, E.R.; Minin, E.O.Z.; Vegian, C.F.L.; Pio-Magalhães, J.A.; Velloso, L.A.; Coelho-Filho, O.R.; Sposito, A.C.; et al. Relationship Between Circulating MicroRNAs and Left Ventricular Hypertrophy in Hypertensive Patients. Front. Cardiovasc. Med. 2022, 9, 798954. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Katz, D.H.; Deo, R.C. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail. Clin. 2014, 10, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Q.; Chen, Y.; Yu, B.; Wang, X.; Qu, A. Recent advances in pathophysiology of heart failure with preserved ejectionfraction. Chin. J. Pathophysiol. 2023, 39, 1499–1508. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, J.; Liu, C.; Dong, G. Study on mechanism of heart failure related diseases and cardiac dysfunctionaffecting ejection fraction retentionl. Chin. Heart J. 2021, 33, 655–660, 665. [Google Scholar] [CrossRef]
- Dong, S.; Ma, W.; Hao, B.; Hu, F.; Yan, L.; Yan, X.; Wang, Y.; Chen, Z.; Wang, Z. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int. J. Clin. Exp. Pathol. 2014, 7, 565–574. [Google Scholar] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bretherton, R.; Bugg, D.; Olszewski, E.; Davis, J. Regulators of cardiac fibroblast cell state. Matrix Biol. 2020, 91–92, 117–135. [Google Scholar] [CrossRef]
- Brilla, C.G.; Maisch, B.; Zhou, G.; Weber, K.T. Hormonal regulation of cardiac fibroblast function. Eur. Heart J. 1995, 16 (Suppl. C), 45–50. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Van Linthout, S. New insights in (inter)cellular mechanisms by heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2014, 11, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Silljé, H.H.W.; Schouten, E.M.; Dokter, M.M.; A Voors, A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-Y.; Jiang, D.-L.; Jia, X.-T.; Fu, L.-Y.; Tian, H.; Liu, K.-L.; Qi, J.; Kang, Y.-M.; Yu, X.-J. Capsaicin improves hypertension and cardiac hypertrophy via SIRT1/NF-κB/MAPKs pathway in the hypothalamic paraventricular nucleus. Phytomedicine 2023, 118, 154951. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Chen, H.; Han, W.; Deng, J. miR-200a-3p overexpression alleviates diabetic cardiomyopathy injury in mice by regulating autophagy through the FOXO3/Mst1/Sirt3/AMPK axis. PeerJ 2023, 11, e15840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, W.; Song, B.; Ye, X.; Li, Z.; Lu, C. Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS)in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway. J. Nutr. Health Aging 2022, 26, 297–306. [Google Scholar] [CrossRef]
- Peng, S.; Lu, X.-F.; Qi, Y.-D.; Li, J.; Xu, J.; Yuan, T.-Y.; Wu, X.-Y.; Ding, Y.; Li, W.-H.; Zhou, G.-Q.; et al. LCZ696 Ameliorates Oxidative Stress and Pressure Overload-Induced Pathological Cardiac Remodeling by Regulating the Sirt3/MnSOD Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 9815039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, M.; Wang, D.; Hu, Y.; Wang, R.; Diao, H.; Shao, X.; Li, Y.; Li, X.; Leng, M.; et al. FTZ protects against cardiac hypertrophy and oxidative injury via microRNA-214 / SIRT3 signaling pathway. Biomed. Pharmacother. 2022, 148, 112696. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Song, X.; Zheng, X.; Yi, J.; Liu, D.; Wang, S.; Chu, C.; Yang, J. Exogenous Hydrogen Sulfide Ameliorates Diabetic Myocardial Fibrosis by Inhibiting Cell Aging Through SIRT6/AMPK Autophagy. Front. Pharmacol. 2020, 11, 1150. [Google Scholar] [CrossRef]
- Sinclair, D.A.; Guarente, L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell 1997, 91, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Frydzińska, Z.; Owczarek, A.; Winiarska, K. Sirtuins and their role in metabolism regulation. Postep. Biochem. 2019, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Ding, H.; Li, D.; Shen, W.; Zhang, X. The current state of research on sirtuin-mediated autophagy in cardiovascular diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A com-prehensive review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, G.; Guo, C.; Zhao, X.; Shen, D.; Yang, N. MiR-128-3p mediates TNF-α-induced inflammatory responses by regulating Sirt1 expression in bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 521, 98–105. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Lee, S.Y.; Han, M.K.; Kim, D.H.; Kim, W. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem. Biophys. Res. Commun. 2012, 419, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Anoopkumar-Dukie, S.; Mallik, S.B.; Davey, A.K. SIRT1 and SIRT2 modulators reduce LPS-induced inflammation in HAPI microglial cells and protect SH-SY5Y neuronal cells in vitro. J. Neural Transm. 2021, 128, 631–644. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Cao, W.; Wei, X.; Chen, J.; Ying, W. SIRT2 Plays Significant Roles in Lipopolysaccharides-Induced Neuroinflammation and Brain Injury in Mice. Neurochem. Res. 2016, 41, 2490–2500. [Google Scholar] [CrossRef]
- Kurundkar, D.; Kurundkar, A.R.; Bone, N.B.; Becker, E.J.; Liu, W.; Chacko, B.; Darley-Usmar, V.; Zmijewski, J.W.; Thannickal, V.J. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 2019, 4, e120722. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef]
- Palomer, X.; Román-Azcona, M.S.; Pizarro-Delgado, J.; Planavila, A.; Villarroya, F.; Valenzuela-Alcaraz, B.; Crispi, F.; Sepúlveda-Martínez, Á.; Miguel-Escalada, I.; Ferrer, J.; et al. SIRT3-mediated inhibition of FOS through histone H3 deacetylation prevents cardiac fibrosis and inflammation. Signal Transduct. Target. Ther. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Rideout, D.A.; Rakita, S.S.; Gower, W.R., Jr.; You, M.; Murr, M.M. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass in obese rats? J. Gastrointest. Surg. 2010, 14, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Gu, M.; Peng, Y. lncRNA TUG1 promotes the brown remodeling of white adipose tissue by regulating miR-204-targeted SIRT1 in diabetic mice. Int. J. Mol. Med. 2020, 46, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Gouranton, E.; Romier, B.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Landrier, J.-F. Visfatin is involved in TNFα-mediated insulin resistance via an NAD(+)/SIRT1/PTP1B pathway in 3T3-L1 adipocytes. Adipocyte 2014, 3, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.; Viollet, B.; Caton, P.; Leclerc, J.; Sakakibara, I.; Foretz, M.; Holness, M.; Sugden, M. The AMPK-SIRT signaling network regulates glucose tolerance under calorie restriction conditions. Life Sci. 2014, 100, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Reznick, J.; Wright, L.E.; Sinclair, D.A.; Cooney, G.J.; Turner, N. Liver-specific overexpression of SIRT3 enhances oxidative metabolism, but does not impact metabolic defects induced by high fat feeding in mice. Biochem. Biophys. Res. Commun. 2022, 607, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Sarikhani, M.; Dasgupta, S.; Maniyadath, B.; Pandit, A.S.; Mishra, S.; Ahamed, F.; Dubey, A.; Fathma, N.; Atreya, H.S.; et al. SIRT6 deacetylase transcriptionally regulates glucose metabolism in heart. J. Cell. Physiol. 2018, 233, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Wang, Y.; Terkeltaub, R.; Liu-Bryan, R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthr. Cartil. 2018, 26, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Karthikeyan, A.; Lu, J.; Ling, E.A.; Dheen, S.T. Sirtuin 3 regulates Foxo3a-mediated antioxidant pathway in microglia. Neuroscience 2015, 311, 398–414. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef]
- Yu, S.-L.; Lee, S.-I.; Park, H.-W.; Lee, S.K.; Kim, T.-H.; Kang, J.; Park, S.-R. SIRT1 suppresses in vitro decidualization of human endometrial stromal cells through the downregulation of forkhead box O1 expression. Reprod. Biol. 2022, 22, 100672. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.C.; Divecha, N.; Lemieux, M.; Kamel, C.; Chen, D.; Gu, W.; Bultsma, Y.; McBurney, M.; Guarente, L. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004, 116, 551–563. [Google Scholar] [CrossRef]
- She, D.T.; Wong, L.J.; Baik, S.H.; Arumugam, T.V. SIRT2 Inhibition Confers Neuroprotection by Downregulation of FOXO3a and MAPK Signaling Pathways in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 9188–9203. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, P.; Wang, H.; Li, L.; Li, Q. SIRT3 promotion reduces resistance to cisplatin in lung cancer by modulating the FOXO3/CDT1 axis. Cancer Med. 2021, 10, 1394–1404. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Packer, M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021, 9, 535–549. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac rehabilitation increases SIRT1 activity and β-hydroxybutyrate levels and decreases oxidative stress in patients with HF with preserved ejection fraction. Oxid. Med. Cell. Longev. 2019, 2019, 7049237. [Google Scholar] [CrossRef]
- He, L.; Ma, S.; Zuo, Q.; Zhang, G.; Wang, Z.; Zhang, T.; Zhai, J.; Guo, Y. An effective sodium-dependent glucose transporter 2 inhibition, canagliflozin, prevents development of hypertensive heart failure in dahl salt-sensitive rats. Front. Pharmacol. 2022, 13, 856386. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Alrob, O.A.; Zhang, L.; Jaswal, J.S.; Wagg, C.S.; Fukushima, A.; Padwal, R.S.; Johnstone, D.E.; Sharma, A.M.; Lopaschuk, G.D. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: Implications for the obesity paradox. Diabetes 2015, 64, 1643–1657. [Google Scholar] [CrossRef]
- Costantino, S.; Mengozzi, A.; Velagapudi, S.; Mohammed, S.A.; Gorica, E.; Akhmedov, A.; Mongelli, A.; Pugliese, N.R.; Masi, S.; Virdis, A.; et al. Treatment with recombinant SIRT1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy. Cardiovasc. Diabetol. 2023, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Polito, M.V.; Ciccarelli, M.; Manzo, V.; Torsiello, M.; De Bellis, E.; D’auria, F.; Vitulano, G.; Piscione, F.; et al. SIRT1 activity in PBMCs as a biomarker of different heart failure phenotypes. Biomolecules 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Cantrell, A.C.; Chen, J.X.; Gu, W.; Zeng, H. SIRT3 deficiency enhances ferroptosis and promotes cardiac fibrosis via p53 acetylation. Cells 2023, 12, 1428. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, H.; Chen, S.T.; Roman, R.J.; Aschner, J.L.; Didion, S.; Chen, J.-X. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J. Mol. Cell. Cardiol. 2017, 112, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Methawasin, M.; Strom, J.G.; Slater, R.E.; Fernandez, V.; Saripalli, C.; Granzier, H. Experimentally increasing the compliance of titin through RNA binding motif-20 (RBM20) inhibition improves diastolic function in a mouse model of heart failure with preserved ejection fraction. Circulation 2016, 134, 1085–1099. [Google Scholar] [CrossRef]
- Franssen, C.; González Miqueo, A. The role of titin and extracellular matrix remodelling in heart failure with preserved ejection fraction. Neth. Heart J. 2016, 24, 259–267. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Franssen, C.P.; Hamdani, N.; Verheugt, F.W.; Somsen, G.A.; Paulus, W.J. Molecular and cellular basis for diastolic dysfunction. Curr. Heart Fail. Rep. 2012, 9, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sickinghe, A.A.; Korporaal, S.J.A.; den Ruijter, H.M.; Kessler, E.L. Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction. Front. Endocrinol. 2019, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Redfield, M.M. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011, 123, 2006–2013; discussion 2014. [Google Scholar] [CrossRef]
- Duca, F.; Zotter-Tufaro, C.; Kammerlander, A.A.; Aschauer, S.; Binder, C.; Mascherbauer, J.; Bonderman, D. Gender-related differences in heart failure with preserved ejection fraction. Sci. Rep. 2018, 8, 1080. [Google Scholar] [CrossRef]
- Zeng, H.; He, X.; Chen, J.X. A sex-specific role of endothelial sirtuin 3 on blood pressure and diastolic dysfunction in female mice. Int. J. Mol. Sci. 2020, 21, 9744. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Pozdniakova, S.; Kühl, A.A.; Baczko, I.; Ladilov, Y.; Regitz-Zagrosek, V. Sex differences in the aging human heart: Decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging 2019, 11, 1918–1933. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, S.-S.; Chen, S.-R.; Pi, R.-B.; Gao, S.; Li, H.; Ye, J.-T.; Liu, P.-Q. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett. 2012, 586, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Maksin-Matveev, A.; Kanfi, Y.; Hochhauser, E.; Isak, A.; Cohen, H.Y.; Shainberg, A. Sirtuin 6 protects the heart from hypoxic damage. Exp. Cell Res. 2015, 330, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; You, S.; Qian, H.; Wu, S.; Lu, S.; Zhang, Y.; Sun, Y.; Zhang, N. The role of SIRT2 in vascular-related and heart-related diseases: A review. J. Cell. Mol. Med. 2021, 25, 6470–6478. [Google Scholar] [CrossRef] [PubMed]
- Katare, P.B.; Nizami, H.L.; Paramesha, B.; Dinda, A.K.; Banerjee, S.K. Activation of toll like receptor 4 (TLR4) promotes cardiomyocyte apoptosis through SIRT2 dependent p53 deacetylation. Sci. Rep. 2020, 10, 19232. [Google Scholar] [CrossRef]
- Nasrin, N.; Wu, X.; Fortier, E.; Feng, Y.; Bare’, O.C.; Chen, S.; Ren, X.; Wu, Z.; Streeper, R.S.; Bordone, L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J. Biol. Chem. 2010, 285, 31995–32002. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Nakamura, Y.; Tanaka, D.; Zhuang, X.; Fujita, Y.; Obara, A.; Hamasaki, A.; Hosokawa, M.; Inagaki, N. Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem. Biophys. Res. Commun. 2010, 393, 73–78. [Google Scholar] [CrossRef]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef]
- Rifaï, K.; Judes, G.; Idrissou, M.; Daures, M.; Bignon, Y.-J.; Penault-Llorca, F.; Bernard-Gallon, D. SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer. Oncotarget 2018, 9, 30661–30678. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Yang, Y.; Guo, Y.; Fan, Y.; Zhang, M.; Man, W.; Gao, E.; Hu, W.; Reiter, R.J.; et al. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J. Pineal Res. 2017, 62, e12368. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-L.; Tang, H.; Ran, L.-K.; Ko, B.C.B.; Zhang, Z.-Z.; Chen, X.; Ren, J.-H.; Tao, N.-N.; Li, W.-Y.; Huang, A.-L.; et al. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3β/BCL2-associated X protein-dependent apoptotic pathway. Oncogene 2016, 35, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-X.; Tang, X.; An, X.-Z.; Xie, X.-M.; Chen, X.-F.; Zhao, X.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2017, 38, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, X.; Wang, W.; Liu, F.; Hao, Z.; Yang, Y.; Zhao, J.; Yin, W.; Xu, L.; Zhao, R.; et al. Cardioprotective Effects of SIRT6 in a Mouse Model of Transverse Aortic Constriction-Induced Heart Failure. Front. Physiol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. N. Y. Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Rössler, O.G. Resveratrol regulates gene transcription via activation of stimulus-responsive transcription factors. Pharmacol. Res. 2017, 117, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, S.; Ruiz, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Veksler, V.; Garnier, A.; Ventura-Clapier, R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS ONE 2011, 6, e26391. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tong, Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol. Biol. Cell 2009, 20, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, L.; Su, Q.; Qin, S.; Zhong, J.; Ni, Y.; Yang, J. Resveratrol inhibits insulin-induced vascular smooth muscle cell proliferation and migration by activating SIRT1. Evid. Based Complement. Altern. Med. 2022, 2022, 8537881. [Google Scholar] [CrossRef]
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Chen, Z.Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, J.; Hu, Z.; Sun, B. Resveratrol protects against atherosclerosis by downregulating the PI3K/AKT/mTOR signaling pathway in atherosclerosis model mice. Exp. Ther. Med. 2022, 23, 414. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H424–H434. [Google Scholar] [CrossRef]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, e12419. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef]
- Mai, A.; Valente, S.; Meade, S.; Carafa, V.; Tardugno, M.; Nebbioso, A.; Galmozzi, A.; Mitro, N.; De Fabiani, E.; Altucci, L.; et al. Study of 1,4-dihydropyridine structural scaffold: Discovery of novel sirtuin activators and inhibitors. J. Med. Chem. 2009, 52, 5496–5504. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chang, J.; Wang, Y.; Pan, G. Indole-3-propionic acid, a product of intestinal flora, inhibits the HDAC6/NOX2 signaling and relieves doxorubicin-induced cardiomyocyte damage. Folia Morphol. 2023, 83, 382–390. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; Croix, C.M.S.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P.; et al. SIRT3–AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Koay, Y.C.; Pan, C.; Zhou, Z.; Tang, W.; Wilcox, J.; Li, X.S.; Zagouras, A.; Marques, F.; Allayee, H.; et al. Indole-3-propionic acid protects against heart failure with preserved ejection fraction. Circ. Res. 2024, 134, 371–389. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef]
- Hoffmann, E.; Wald, J.; Lavu, S.; Roberts, J.; Beaumont, C.; Haddad, J.; Elliott, P.; Westphal, C.; Jacobson, E. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br. J. Clin. Pharmacol. 2013, 75, 186–196. [Google Scholar] [CrossRef]
- Libri, V.; Brown, A.P.; Gambarota, G.; Haddad, J.; Shields, G.S.; Dawes, H.; Pinato, D.J.; Hoffman, E.; Elliot, P.J.; Vlasuk, G.P.; et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS ONE 2012, 7, e51395. [Google Scholar] [CrossRef]
- Venkatasubramanian, S.; Noh, R.M.; Daga, S.; Langrish, J.P.; Joshi, N.V.; Mills, N.L.; Hoffmann, E.; Jacobson, E.W.; Vlasuk, G.P.; Waterhouse, B.R.; et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J. Am. Heart Assoc. 2013, 2, e000042. [Google Scholar] [CrossRef]
- Baksi, A.; Kraydashenko, O.; Zalevkaya, A.; Stets, R.; Elliott, P.; Haddad, J.; Hoffmann, E.; Vlasuk, G.P.; Jacobson, E.W. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 2014, 78, 69–77. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Scicluna, B.P.; Moerland, P.D.; Lin, J.; Jacobson, E.W.; Vlasuk, G.P.; van der Poll, T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit. Care Med. 2015, 43, e199–e202. [Google Scholar] [CrossRef]
- Krueger, J.G.; Suárez-Fariñas, M.; Cueto, I.; Khacherian, A.; Matheson, R.; Parish, L.C.; Leonardi, C.; Shortino, D.; Gupta, A.; Haddad, J.; et al. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS ONE 2015, 10, e0142081. [Google Scholar] [CrossRef]
- Sands, B.E.; Joshi, S.; Haddad, J.; Freudenberg, J.M.; Oommen, D.E.; Hoffmann, E.; McCallum, S.W.; Jacobson, E. Assessing Colonic Exposure, Safety, and Clinical Activity of SRT2104, a Novel Oral SIRT1 Activator, in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 607–614. [Google Scholar] [CrossRef]
- Xia, H.-T.; Lu, C.-H.; Yang, K.; Sun, Q.-N.; Wang, K. Correlation Analysis of Serum SIRT1 Level with Inflammatory Factors and Oxidative Stress in Patients with Heart Failure with Preserved Ejection Fraction and its Influence Study on Prognosis. Prog. Mod. Biomed. 2023, 23, 356–360+383. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule SIRT1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]
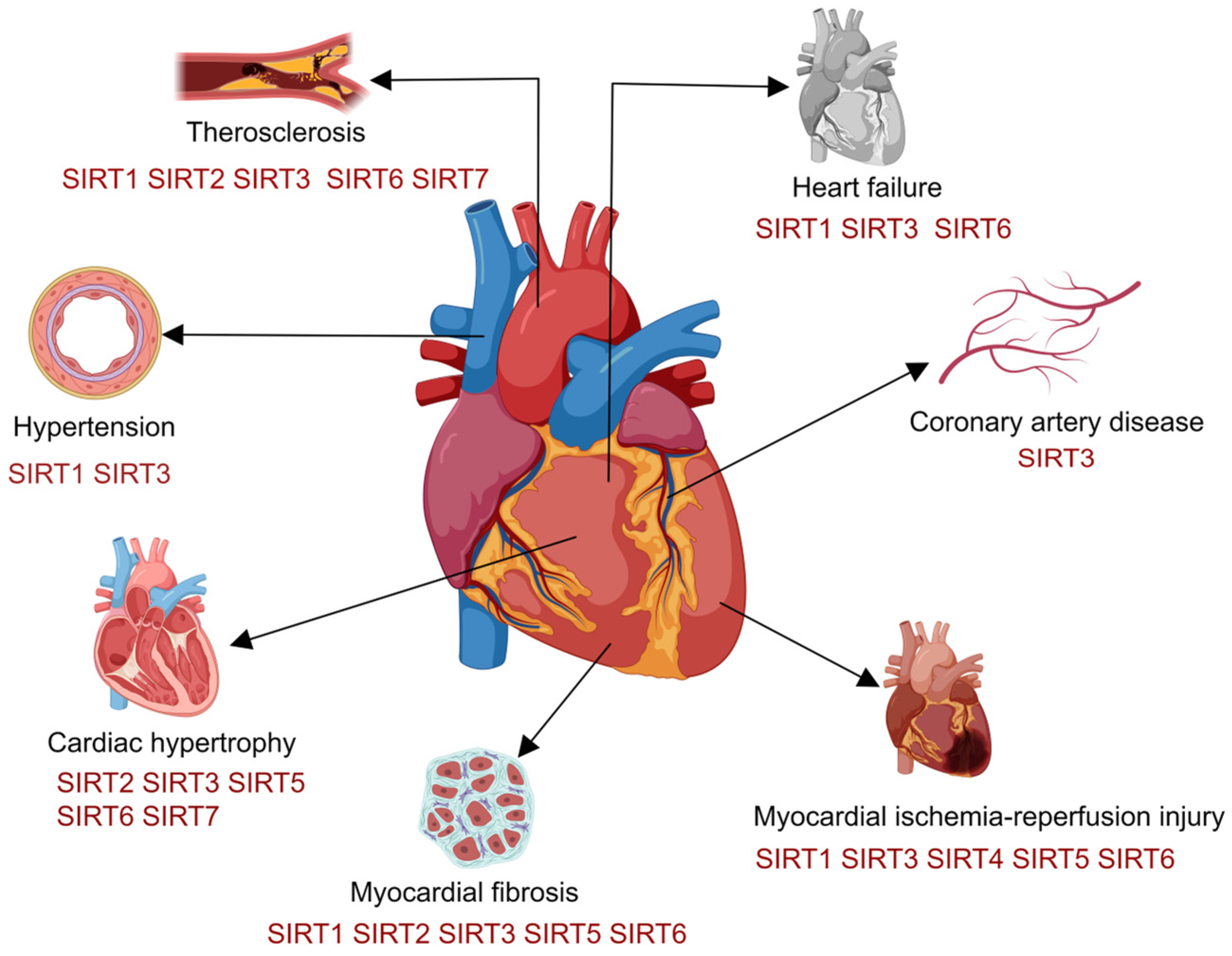
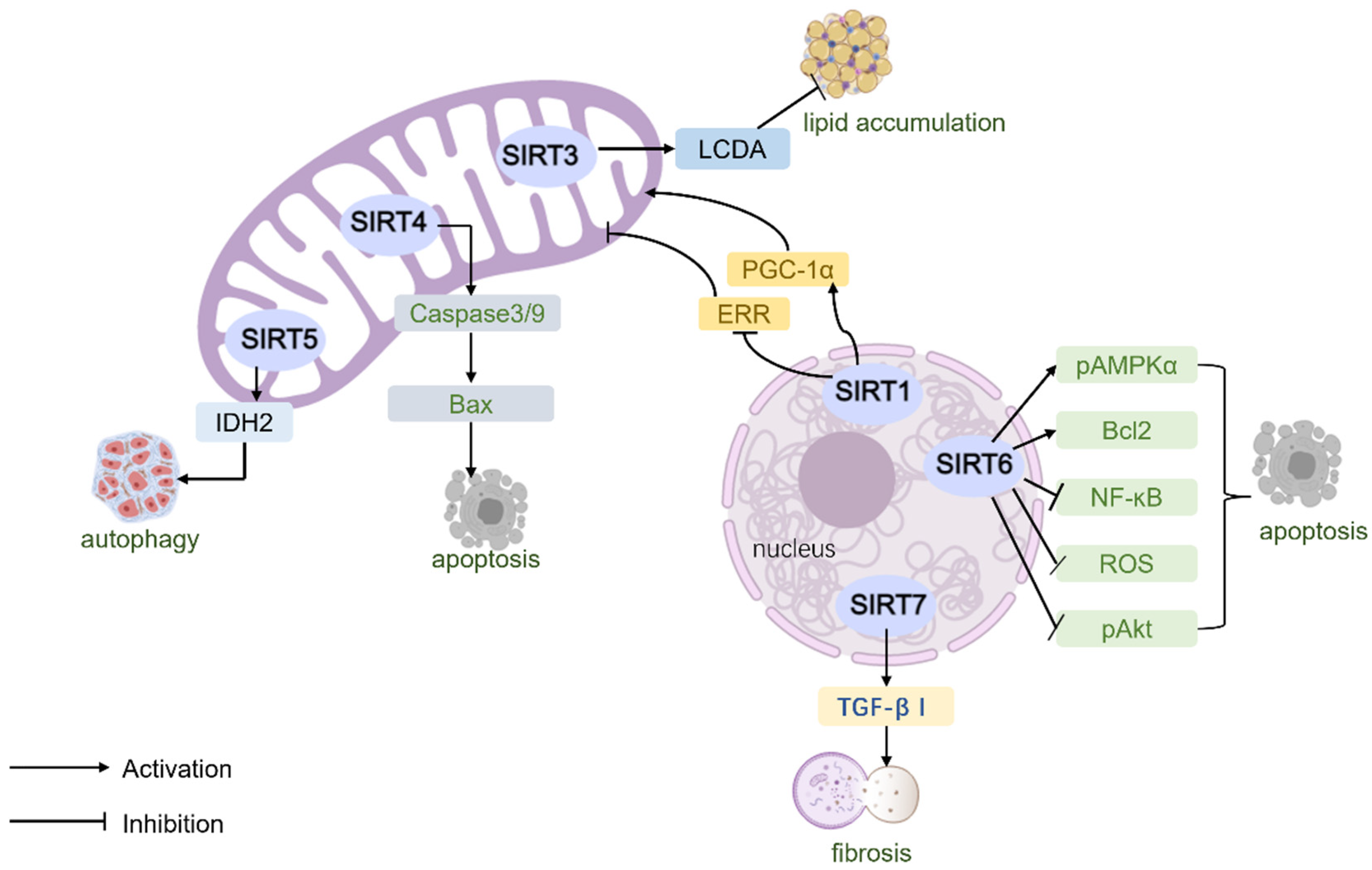
| Category | Animal Models | Changes in Cardiac Function | Pathological Changes |
|---|---|---|---|
| Hypertension | Aortic constriction model | Preserved LVEF Increased SBP Increased E/A Increased E/E′ Increased LVEDP | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; interstitial and perivascular fibrosis |
| Angiotensin II model | Preserved LVEF Increased SBP Decreased E/A Increased E/E′ Increased LVEDP | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; reduced myocardial capillary density; interstitial and perivascular fibrosis | |
| Dahl salt-sensitive rat model | Preserved LVEF Increased SBP Decreased E/A Increased E/E′ Increased LVEDP | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; decreased myocardial capillary density; interstitial and perivascular fibrosis | |
| Spontaneously hypertensive rat model | Preserved LVEF Increased SBP Increased E/A Increased E/E′ | Cardiomyocyte hypertrophy and ventricular hypertrophy; decreased myocardial capillary density; interstitial and perivascular fibrosis | |
| Obesity and diabetes model | STZ rat model | Preserved LVEF Decreased E/A | Cardiomyocyte hypertrophy; interstitial and perivascular fibrosis; decreased myocardial capillary density |
| “Multiple-Hit” model | ZSF1-obese rat model | Preserved LVEF Increased SBP Increased E/E′ | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; interstitial and perivascular fibrosis; decreased myocardial capillary density; pulmonary congestion |
| “2-hit” model | Preserved LVEF Increased SBP Decreased E/A Increased E/E′ Increased LVEDP | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; interstitial and perivascular fibrosis; decreased myocardial capillary density | |
| “3-hit” model | Preserved LVEF Increased SBP | Cardiomyocyte hypertrophy and ventricular hypertrophy; pulmonary congestion; interstitial and perivascular fibrosis; decreased myocardial capillary density; pulmonary congestion |
| SIRTs | Subcellular Localization | Cell Localization | Physiological Activity | Participate in Pathophysiological Processes |
|---|---|---|---|---|
| SIRT1 | Nucleus | Glucose metabolism, DNA repair, fatty acid metabolism, cell differentiation | Deacetylase | Myocardial hypertrophy, apoptosis, myocardial ischemia-reperfusion, heart failure |
| SIRT2 | Cytoplasm | Cell cycle, fat metabolism | Deacetylase | Myocardial hypertrophy and myocardial ischemia-reperfusion |
| SIRT3 | Nucleus, Cytoplasm | Mitochondrial autophagy, ATP synthesis, urea cycle, oxidative stress | Deacetylase | Myocardial hypertrophy, heart failure, apoptosis |
| SIRT4 | Mitochondria | Insulin secretion, fatty acid oxidation, DNA repair | Depolymerizing enzyme, de-glutarylation | Myocardial infarction, heart failure |
| SIRT5 | Mitochondria | Urea cycle | Desglutaryl enzyme, desmalonidase | Cell apoptosis |
| SIRT6 | Nucleus | DNA repair | Deacetylase | Heart failure, myocardial hypertrophy, myocardial ischemia-reperfusion |
| SIRT7 | Nucleus | Cell cycle | Deacetylase | Cell apoptosis |
| SIRT1 | SIRT2 | SIRT3 | SIRT4 | SIRT5 | SIRT6 | SIRT7 | |
|---|---|---|---|---|---|---|---|
| Inhibitor | Nicotinamide Leucine Oxidized paeoniflorin Suramin AGK2 HR73 MC2141 | Nicotinamide Quercetin Suramin AK-7 AGK2 EX-527 MC2141 MC2494 | Nicotinamide Quercetin 3-TYP AGK2 EX-527 | Quercetin EX-527 | Nicotinamide Quercetin Basalazine Peptides and amino acid inhibitors Suramin EX-527 | Nicotinamide Quinazolinedione compound EX527 | |
| Agonist | Resveratrol Quercetin 1pyr4-dihydropyridine small molecules Purple riveting Isoglycyrrhizic acid SRT172 SRT2104 | Resveratrol SRT1460 SRT1720 SRT2183 | Resveratrol aconitine polydatin magnolol ginseng polyphenols Chrysophanol SRT1460 SRT2183 | Resveratrol MC3138 | Anthocyanins Fucoidan Fluvastatin UBCS039 | Quercetin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Li, Y.; Xie, Y.; Bu, J.; Yuan, R.; Zhang, X. Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2024, 25, 7740. https://doi.org/10.3390/ijms25147740
Lu Y, Li Y, Xie Y, Bu J, Yuan R, Zhang X. Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences. 2024; 25(14):7740. https://doi.org/10.3390/ijms25147740
Chicago/Turabian StyleLu, Ying, Yongnan Li, Yixin Xie, Jiale Bu, Ruowen Yuan, and Xiaowei Zhang. 2024. "Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction" International Journal of Molecular Sciences 25, no. 14: 7740. https://doi.org/10.3390/ijms25147740
APA StyleLu, Y., Li, Y., Xie, Y., Bu, J., Yuan, R., & Zhang, X. (2024). Exploring Sirtuins: New Frontiers in Managing Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences, 25(14), 7740. https://doi.org/10.3390/ijms25147740





