Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy
Abstract
1. Introduction
| Main Classes | Graft-Type Examples | Source | Histology/ Bone Source | Bone Graft Form | General Features | Commercial Products | Ref. |
|---|---|---|---|---|---|---|---|
| Autografts | Intraoral: incisive fossa, coronoid process, zygomatic body, anterior maxillary sinus wall, nasal spine, ascending ramus, maxillary tuberosity, mandibular symphysis, palate, torus Extraoral: iliac crest, cranium, radius, tibia, rib, fibula | Patients themselves | Cortical, cancellous, corticocancellous | Blocks | Bearing osteoinductive, osteoconductive, and osteogenic potential, with limited graft volume, absence of immunogenic reaction, and disease transmission | - | [19] |
| Allografts | Freeze-dried bone, demineralized freeze-dried bone, fresh and/or frozen bone | Different donors of the same species or genetically non-identical members | Cortical, cancellous, corticocancellous, osteoarticular | Particulate bone | Bearing osteoinductive and osteoconductive potential; availability in large amounts with various particle sizes | MTF®-FDBA (Musculoskeletal Transplant Foundation: Edison, NJ, USA), OsteoSponge (Xtant medical: Belgrade, MT, USA), DynaBlast® (Keystone Dental Group: Burlington, MA, USA), Puros® (Zimmer Biomet: Warsaw, IN, USA), MinerOss (BioHorizons: Birmingham, AL, USA), Dynagraft® (Keystone Dental Group: Burlington, MA, USA), Grafton® (BioHorizons: Birmingham, AL, USA), MTF®-DFDBA (Musculoskeletal Transplant Foundation, Edison, NJ, USA), Raptos® (Citagenix: Laval, QC, Canada), DBX® Putty (Dentsply Sirona Inc., Charlotte, NC, USA), Opteform® (Exatech Inc.: Gainesville, FL, USA) | [20] |
| Xenografts | Bovine Hydroxyapatite, Coralline calcium carbonate, Porcine bone, Equine bone, Algae | Grafts from different species such as cats, dogs, rats, cows, bullfrogs, sheep, pigs, and chickens. | Cortical, cancellous, corticocancellous | Slurry | Osteoconductive, bone minerals with no or fewer organic elements, high availability, limited resorptive potential | FRIOS®Algipore® (Dentsply Sirona Inc., Charlotte, NC, USA), Bio-Oss® (Geistlich Pharma: Wolhusen, Switzerland), Cerabone® (Botiss biomaterials GmbH: Zossen, Germany), Gen-OS® (Tecnoss dental: Torino, Italy), Biocoral® (Inoteb, Saint-Gonnery, France), Osteobiol® (Tecnoss dental: Torino, Italy), Pro Osteon® (Zimmer Biomet: Warsaw, IN, USA), Interpore-200® (Interpore International: Irvine, CA, USA), OsteoGraf/N (Dentsply Sirona Inc.: Charlotte, NC, USA), MinerOssTM X (BioHorizons: Birmingham, AL, USA) | [21] |
| Alloplasts |
| Synthetic biomaterials | - | Powder, paste | Osteoconductive, bears similarity to bone mineral, degradable and non-degradable nature, low cost | Biogran® (Biomet 3i Innovations Inc.: Plam beach gardens, FL, USA), BonePlast® (Zimmer Biomet: Warsaw, IN, USA), Cortoss® (Stryker Corporation: Kalamazoo, MI, USA), Guidor® easy graft (Sunstar: Osaka, Japan), Hydroset®, IngeniOs® (Zimmer Biomet: Warsaw, IN, USA), B-Ostin® (Basic Healthcare: Himachal Pradesh, India), Perioglass® (Novabone: Jacksonville, FL, USA), Rhakoss® (Orthovita, Inc: Malvern, PA, USA), Vitoss® (Stryker Corporation: Kalamazoo, MI, USA) | [22] |
2. Search Methodology
3. Brief Introduction to Natural Bone Substitutes
3.1. Autografts
3.2. Allografts
3.3. Xenografts
4. Alloplastic Bone Graft Biomaterials
4.1. Calcium Phosphate Ceramics
4.1.1. Hydroxyapatite
4.1.2. Tricalcium Phosphate (β-TCP)
4.1.3. Biphasic Calcium Phosphate
4.2. Calcium Phosphate Cement
4.3. Calcium Sulfate
4.4. Bioactive Glass
4.5. PMMA
5. Barrier Membrane and Biomaterials
5.1. Biodegradable Barrier Membranes
5.1.1. Biodegradable Natural Polymers
5.1.2. Biodegradable Synthetic Polymers
5.2. Non-Biodegradable Barrier Membranes
5.3. Biomaterial Additive Membranes as Delivery Devices
6. Outlook
6.1. Treatment Plan Considerations by Dentists
6.2. Future Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PRP Treatment for Periodontal Disease, Prpmed.De. Available online: https://prpmed.de/en/blog/news/prp-treatment-for-periodontal-disease (accessed on 20 February 2024).
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Lang, N.P.; Buser, D.; Bragger, U. Bone regeneration adjacent to titanium dental implants using guided tissue regeneration: A report of two cases. Int. J. Oral Maxillofac. Implants 1990, 5, 9–14. [Google Scholar]
- Lee, H.-S.; Byun, S.-H.; Cho, S.-W.; Yang, B.-E. Past, Present, and Future of Regeneration Therapy in Oral and Periodontal Tissue: A Review. Appl. Sci. 2019, 9, 1046. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Ezzati, A.; Mehrandish, S.; Asare-Addo, K.; Nokhodchi, A. An overview of guided tissue regeneration (GTR) systems designed and developed as drug carriers for management of periodontitis. J. Drug Deliv. Sci. Technol. 2022, 71, 103341. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.-S.; Jiang, H.-B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Srivastava, R. Osteoinductive and Osteoconductive Biomaterials. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–395. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, M.P.; Saleh, K.J.; Einhorn, T.A. Osteoinductive growth factors in preclinical fracture and long bone defects models. Orthop. Clin. N. Am. 1999, 30, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Özcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Lenarda, R. Three-Dimensional Bone Substitutes for Oral and Maxillofacial Surgery: Biological and Structural Characterization. J. Funct. Biomater. 2018, 9, 62. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Material 2021, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Nicholls, F.; Kruse, A.; Zwahlen, R.A.; Weber, F.E. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin. Oral Implants Res. 2013, 24, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Iliac Transplants in Periodontal Therapy. Available online: https://pubmed.ncbi.nlm.nih.gov/4918578/ (accessed on 12 February 2024).
- Balaji, V.R.; Manikandan, D.; Ramsundar, A. Bone Grafts in Periodontics. Matrix Sci. Medica 2020, 4, 57. [Google Scholar] [CrossRef]
- Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Available online: https://pubmed.ncbi.nlm.nih.gov/29744432/ (accessed on 15 February 2024).
- Alloplastic Implants of Tricalcium Phosphate Ceramic in Human Periodontal Osseous Defects. Available online: https://pubmed.ncbi.nlm.nih.gov/6376756/ (accessed on 15 February 2024).
- A Clinical and Histological Evaluation of Autogenous Iliac Bone Grafts In Humans. I. Wound Healing 2 to 8 Months. Available online: https://pubmed.ncbi.nlm.nih.gov/4583377/ (accessed on 12 February 2024).
- Hiatt, W.H.; Schallhorn, R.G. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J. Periodontol. 1973, 44, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Garrett, S.; Shallhorn, R.G.; Egelberg, J. Healing after treatment of periodontal intraosseous defects. III. Effect of osseous grafting and citric acid conditioning. J. Clin. Periodontol. 1985, 12, 441–455. [Google Scholar] [CrossRef]
- Sepe, W.W.; Bowers, G.M.; Lawrence, J.J.; Friedlaender, G.E.; Koch, R.W. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects--part II. J. Periodontol. 1978, 49, 9–14. [Google Scholar] [CrossRef]
- Bright, R.W.; Friedlaender, G.E.; Sell, K.W. Tissue banking: The United States Navy Tissue Bank. Mil. Med. 1977, 142, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mellonig, J.T. Autogenous and Allogeneic Bone Grafts in Periodontal Therapy. Crit. Rev. Oral Biol. Med. 1992, 3, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.W.; Yukna, R.A.; Sepe, W.W. Freeze-dried bone allografts combined with tetracycline in the treatment of juvenile periodontitis. J. Periodontol. 1985, 56, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Libin, B.M.; Ward, H.L.; Fishman, L. Decalcified, lyophilized bone allografts for use in human periodontal defects. J. Periodontol. 1975, 46, 51–56. [Google Scholar] [CrossRef]
- Rummelhart, J.M.; Mellonig, J.T.; Gray, J.L.; Towle, H.J. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J. Periodontol. 1989, 60, 655–663. [Google Scholar] [CrossRef]
- Comparison of Bone Graft Materials: Part I. New Bone Formation with Autografts and Allografts Determined by Strontium-85—Mellonig—1981—Journal of Periodontology—Wiley Online Library. Available online: https://aap.onlinelibrary.wiley.com/doi/10.1902/jop.1981.52.6.291 (accessed on 12 February 2024).
- Hamada, T.; Matsubara, H.; Hikichi, T.; Shimokawa, K.; Tsuchiya, H. Rat model of an autologous cancellous bone graft. Sci. Rep. 2021, 11, 18001. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Costa, C.A.; Deliberador, T.M.; Abuna, R.P.F.; Rodrigues, T.L.; de Souza, S.L.S.; Palioto, D.B. Mesenchymal stem cells surpass the capacity of bone marrow aspirate concentrate for periodontal regeneration. J. Appl. Oral Sci. 2022, 30, e20210359. [Google Scholar] [CrossRef]
- Does PRP Enhance Bone Integration with Grafts, Graft Substitutes, or Implants? A Systematic Review. Available online: https://pubmed.ncbi.nlm.nih.gov/24261343/ (accessed on 17 February 2024).
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- The Effects of Freeze-Drying and Rehydration on Cancellous Bone. Available online: https://pubmed.ncbi.nlm.nih.gov/8472461/ (accessed on 17 February 2024).
- The Biology of Allograft Incorporation. Available online: https://pubmed.ncbi.nlm.nih.gov/16893161/ (accessed on 17 February 2024).
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. Br. Vol. 2007, 89-B, 574–580. [Google Scholar] [CrossRef]
- Xu, G.; Hu, X.; Han, L.; Zhao, Y.; Li, Z. The construction of a novel xenograft bovine bone scaffold, (DSS)6-liposome/CKIP-1 siRNA/calcine bone and its osteogenesis evaluation on skull defect in rats. J. Orthop. Transl. 2021, 28, 74–82. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef]
- Marques, C.F.; Perera, F.H.; Marote, A.; Ferreira, S.; Vieira, S.I.; Olhero, S.; Miranda, P.; Ferreira, J.M.F. Biphasic calcium phosphate scaffolds fabricated by direct write assembly: Mechanical, anti-microbial and osteoblastic properties. J. Eur. Ceram. Soc. 2017, 37, 359–368. [Google Scholar] [CrossRef]
- Principles of Tissue Engineering—4th Edition | Elsevier Shop. Available online: https://shop.elsevier.com/books/principles-of-tissue-engineering/lanza/978-0-12-398358-9 (accessed on 17 February 2024).
- Lee, J.-T.; Cha, J.-K.; Kim, S.; Jung, U.-W.; Thoma, D.S.; Jung, R.E. Lateral onlay grafting using different combinations of soft-type synthetic block grafts and resorbable collagen membranes: An experimental in vivo study. Clin. Oral Implants Res. 2020, 31, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Healing of Artificially Created Gap Non-Union Using Autologous Cultured Osteoblasts Impregnated Over Three-Dimensional Biodegradable Scaffold: An Experimental Study (Rabbit) | Indian Journal of Orthopaedics. Available online: https://link.springer.com/article/10.1007/s43465-020-00288-z (accessed on 20 February 2024).
- Agarwal, A.; Yogendra Raj, R.; Shanker, M. Clinicoradiological outcomes following single-stage treatment using external fixator, copious bone grafting and high dose antibiotics for infected postosteomyelitic nonunion of femoral shaft. J. Pediatr. Orthop. B 2021, 30, 85. [Google Scholar] [CrossRef]
- Chen, I.-C.; Su, C.-Y.; Lai, C.-C.; Tsou, Y.-S.; Zheng, Y.; Fang, H.-W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Tham, D.Q.; Huynh, M.D.; Linh, N.T.D.; Van, D.T.C.; Cong, D.V.; Dung, N.T.K.; Trang, N.T.T.; Lam, P.V.; Hoang, T.; Lam, T.D. PMMA Bone Cements Modified with Silane-Treated and PMMA-Grafted Hydroxyapatite Nanocrystals: Preparation and Characterization. Polymers 2021, 13, 3860. [Google Scholar] [CrossRef] [PubMed]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Recombinant Human Platelet–Derived Growth Factor BB in Combination with a Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft—Timothy R. Daniels, John Anderson, Michael P. Swords, Greg Maislin, Rafe Donahue, Ellie Pinsker, Jovelyn D. Quiton. 2019. Available online: https://journals.sagepub.com/doi/full/10.1177/1071100719851468 (accessed on 20 February 2024).
- i-FactorTM Bone Graft vs Autograft in Anterior Cervical Discectomy and Fusion: 2-Year Follow-Up of the Randomized Single-Blinded Food and Drug Administration Investigational Device Exemption Study. Available online: https://pubmed.ncbi.nlm.nih.gov/28945914/ (accessed on 17 February 2024).
- Lee, H.-J.; Kim, B.; Padalhin, A.R.; Lee, B.-T. Incorporation of chitosan-alginate complex into injectable calcium phosphate cement system as a bone graft material. Mater. Sci. Eng. C 2019, 94, 385–392. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Bone Healing and Graft Resorption of Autograft, Anorganic Bovine Bone and β-tricalcium Phosphate. A Histologic and Histomorphometric Study in the Mandibles Of Minipigs—Jensen—2006—Clinical Oral Implants Research—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0501.2005.01257.x (accessed on 22 February 2024).
- Rivara, F.; Negri, M.; Lumetti, S.; Parisi, L.; Toffoli, A.; Calciolari, E.; Manfredi, E.; Macaluso, G.M. Maxillary Sinus Floor Augmentation Using an Equine-Derived Graft Material: Preliminary Results in 17 Patients. BioMed Res. Int. 2017, 2017, e9164156. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Hsu, C.-C.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Pan, Y.-H.; Chang, W.-J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef] [PubMed]
- Turhani, D.; Cvikl, B.; Watzinger, E.; Weißenböck, M.; Yerit, K.; Thurnher, D.; Lauer, G.; Ewers, R. In Vitro Growth and Differentiation of Osteoblast-Like Cells on Hydroxyapatite Ceramic Granule Calcified From Red Algae. J. Oral Maxillofac. Surg. 2005, 63, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Demers, C.; Hamdy, C.R.; Corsi, K.; Chellat, F.; Tabrizian, M.; Yahia, L. Natural coral exoskeleton as a bone graft substitute: A review. Bio-Med. Mater. Eng. 2002, 12, 15–35. [Google Scholar]
- Tricalcium Phosphate (-Containing) Biomaterials in the Treatment of Periodontal Infra-Bony Defects: A Systematic Review and Meta-Analysis. Available online: https://pubmed.ncbi.nlm.nih.gov/34530060/ (accessed on 15 February 2024).
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Application of Calcium Phosphate Materials in Dentistry. Available online: https://www.linkedin.com/pulse/application-calcium-phosphate-materials-dentistry-mohammed-alousaimi (accessed on 1 July 2024).
- Nery, E.B.; Lee, K.K.; Czajkowski, S.; Dooner, J.J.; Duggan, M.; Ellinger, R.F.; Henkin, J.M.; Hines, R.; Miller, M.; Olson, J.W. A Veterans Administration Cooperative Study of biphasic calcium phosphate ceramic in periodontal osseous defects. J. Periodontol. 1990, 61, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hydroxyapatite–Past, Present, and Future in Bone Regeneration—Vivekanand Sabanna Kattimani, Sudheer Kondaka, Krishna Prasad Lingamaneni. 2016. Available online: https://journals.sagepub.com/doi/full/10.4137/BTRI.S36138 (accessed on 15 February 2024).
- Resorption of Synthetic Porous Hydroxyapatite and Replacement by Newly Formed Bone—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S094926581533311X (accessed on 15 February 2024).
- Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1742706111001310?casa_token=eGddbm8F2cEAAAAA:d3lhg0FDsvL_Rm-VY4Q7lu4UfryOL52krJfHCqp8hLxEVrWGWvljDYXhHEJ52xbJNjtlwh7DjQ (accessed on 15 February 2024).
- Biomedicines | Free Full-Text | Design, In Vitro Evaluation and In Vivo Biocompatibility of Additive Manufacturing Three-Dimensional Printing of β-Tricalcium Phosphate Scaffolds for Bone Regeneration. Available online: https://www.mdpi.com/2227-9059/12/5/1049 (accessed on 1 July 2024).
- Albee, F.H. Studies in bone growth: Triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Poly Lactic-Co-Glycolic Acid-Coated β-tricalcium Phosphate for Alveolar Ridge Preservation: A Multicenter Randomized Controlled Trial. Available online: https://pubmed.ncbi.nlm.nih.gov/32996128/ (accessed on 15 February 2024).
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E. Ridge Preservation Using Composite Alloplastic Materials: A Randomized Control Clinical and Histological Study in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef]
- Kumar, C.Y.; Nalini, K.B.; Menon, J.; Patro, D.K.; Banerji, B.H. Calcium Sulfate as Bone Graft Substitute in the Treatment of Osseous Bone Defects, A Prospective Study. J. Clin. Diagn. Res. 2013, 7, 2926–2928. [Google Scholar] [CrossRef]
- Lee, J.-H.; An, H.; Im, J.-S.; Kim, W.-J.; Lee, D.-W.; Yun, J.-H. Evaluation of the clinical and radiographic effectiveness of treating peri-implant bone defects with a new biphasic calcium phosphate bone graft: A prospective, multicenter randomized controlled trial. J. Periodontal Implant Sci. 2023, 53, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Bielenstein, J.; Radenković, M.; Najman, S.; Liu, L.; Ren, Y.; Cai, B.; Beuer, F.; Rimashevskiy, D.; Schnettler, R.; Alkildani, S.; et al. In Vivo Analysis of the Regeneration Capacity and Immune Response to Xenogeneic and Synthetic Bone Substitute Materials. Int. J. Mol. Sci. 2022, 23, 10636. [Google Scholar] [CrossRef] [PubMed]
- Čandrlić, M.; Tomas, M.; Karl, M.; Malešić, L.; Včev, A.; Perić Kačarević, Ž.; Matijević, M. Comparison of Injectable Biphasic Calcium Phosphate and a Bovine Xenograft in Socket Preservation: Qualitative and Quantitative Histologic Study in Humans. Int. J. Mol. Sci. 2022, 23, 2539. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Karl, M.; Čandrlić, M.; Matijević, M.; Juzbašić, M.; Peloza, O.C.; Radetić, A.T.J.; Kuiš, D.; Vidaković, B.; Ivanišević, Z.; et al. A Histologic, Histomorphometric, and Immunohistochemical Evaluation of Anorganic Bovine Bone and Injectable Biphasic Calcium Phosphate in Humans: A Randomized Clinical Trial. Int. J. Mol. Sci. 2023, 24, 5539. [Google Scholar] [CrossRef] [PubMed]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1426–1435. [Google Scholar] [CrossRef]
- França, R.; Samani, T.D.; Bayade, G.; Yahia, L.; Sacher, E. Nanoscale surface characterization of biphasic calcium phosphate, with comparisons to calcium hydroxyapatite and β-tricalcium phosphate bioceramics. J. Colloid Interface Sci. 2014, 420, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Calcium Phosphate Cements For Bone Engineering and Their Biological Properties | Bone Research. Available online: https://www.nature.com/articles/boneres201756 (accessed on 15 February 2024).
- Chow, L.C. Development of Self-Setting Calcium Phosphate Cements. J. Ceram. Soc. Jpn. 1991, 99, 954–964. [Google Scholar] [CrossRef]
- Şahin, E. Calcium Phosphate Bone Cements. In Cement Based Materials; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Rujiraprasert, P.; Suriyasangpetch, S.; Srijunbarl, A.; Singthong, T.; Makornpan, C.; Nampuksa, K.; Osathanon, T.; Nantanapiboon, D.; Monmaturapoj, N. Calcium phosphate ceramic as a model for enamel substitute material in dental applications. BDJ Open 2023, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Development of Calcium Phosphate Cement Using Chitosan and Citric Acid for Bone Substitute Materials. Available online: https://pubmed.ncbi.nlm.nih.gov/11791912/ (accessed on 15 February 2024).
- Schickert, S.d.L.; Jansen, J.A.; Bronkhorst, E.M.; van den Beucken, J.J.; Leeuwenburgh, S.C. Stabilizing dental implants with a fiber-reinforced calcium phosphate cement: An in vitro and in vivo study. Acta Biomater. 2020, 110, 280–288. [Google Scholar] [CrossRef]
- The Use of Osteoconductive Bone Graft Substitutes in Orthopaedic Trauma. Available online: https://pubmed.ncbi.nlm.nih.gov/17761609/ (accessed on 15 February 2024).
- Välimäki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar] [CrossRef]
- Pandit, N.; Gupta, R.; Gupta, S. A comparative evaluation of biphasic calcium phosphate material and bioglass in the treatment of periodontal osseous defects: A clinical and radiological study. J. Contemp. Dent. Pract. 2010, 11, 025–032. [Google Scholar] [PubMed]
- Polymethyl Methacrylate (PMMA) | Britannica. Available online: https://www.britannica.com/science/polyacrylate (accessed on 28 February 2024).
- Charnley, J. Anchorage of the femoral head prosthesis to the shaft of the femur. J. Bone Jt. Surg. Br. 1960, 42-B, 28–30. [Google Scholar]
- [Infection Prevention and Surgical Management of Deep Insidious Infection in Total Endoprosthesis]. Available online: https://pubmed.ncbi.nlm.nih.gov/5084870/ (accessed on 14 March 2024).
- Blank, A.T.; Riesgo, A.M.; Gitelis, S.; Rapp, T.B. Bone Grafts, Substitutes, and Augments in Benign Orthopaedic Conditions: Current Concepts. Bull. NYU Hosp. Jt. Dis. 2017, 75, 119–127. [Google Scholar]
- Clinical Evaluation of HTR Polymer Bone Replacement Grafts in Human Mandibular Class II Molar Furcations. Available online: https://pubmed.ncbi.nlm.nih.gov/8195979/ (accessed on 27 February 2024).
- Stahl, S.S.; Froum, S.J.; Tarnow, D. Human clinical and histologic responses to the placement of HTR polymer particles in 11 intrabony lesions. J. Periodontol. 1990, 61, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, K.; Fu, S.; Cuiffo, M.; Simon, M.; Rafailovich, M.; Romanos, G.E. In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Materials 2022, 15, 1955. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zou, H.; Liao, X.; Xiong, Y.; Hu, X.; Cao, J.; Pan, J.; Li, C.; Zheng, Y. Construction of PCL-collagen@PCL@PCL-gelatin three-layer small diameter artificial vascular grafts by electrospinning. Biomed. Mater. 2022, 18, 015008. [Google Scholar] [CrossRef] [PubMed]
- Materials | Free Full-Text | Evaluation of 3D Templated Synthetic Vascular Graft Compared with Standard Graft in a Rat Model: Potential Use as an Artificial Vascular Graft in Cardiovascular Disease. Available online: https://www.mdpi.com/1996-1944/14/5/1239 (accessed on 2 July 2024).
- Singh, P.; Wadhawan, A.; Rana, M.N.; Kaushik, M. Demineralized freeze-dried bone allograft with Periocol® membrane versus Perioglas® with Periocol® membrane in the treatment of intrabony defects: A case report. Int. J. Appl. Dent. Sci. 2021, 7, 391–394. [Google Scholar] [CrossRef]
- Leventis, M.D.; Fairbairn, P.; Kakar, A.; Leventis, A.D.; Margaritis, V.; Lückerath, W.; Horowitz, R.A.; Rao, B.H.; Lindner, A.; Nagursky, H. Minimally Invasive Alveolar Ridge Preservation Utilizing an In Situ Hardening β-Tricalcium Phosphate Bone Substitute: A Multicenter Case Series. Int. J. Dent. 2016, 2016, e5406736. [Google Scholar] [CrossRef] [PubMed]
- Bhamb, N.; Kanim, L.E.A.; Drapeau, S.; Mohan, S.; Vasquez, E.; Shimko, D.; Mckay, W.; Bae, H.W. Comparative Efficacy of Commonly Available Human Bone Graft Substitutes as Tested for Posterolateral Fusion in an Athymic Rat Model. Int. J. Spine Surg. 2019, 13, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Gindraux, F.; Lepage, D.; Petite, H.; Garbuio, P.; Obert, L. Injectable synthetic bone substitute and distal radius surgery: Prospective continue study on comminutive fracture and extra articular malunion. Bone 2010, 47, S96–S97. [Google Scholar] [CrossRef]
- Cheo, F.Y.; Soeharno, H.; Woo, Y.L. Cost-effective office 3D printing process in orthopaedics and its benefits: A case presentation and literature review. Proc. Singap. Healthc. 2024, 33, 20101058241227336. [Google Scholar] [CrossRef]
- Yumpu.com Comparison Of A Synthetic And Bovine Derived...—Zimmer Dental. Available online: https://www.yumpu.com/en/document/view/8096758/comparison-of-a-synthetic-and-bovine-derived-zimmer-dental (accessed on 18 February 2024).
- Anil, A.; Sadasivan, A.; Koshi, E. Physicochemical Characterization of Five Different Bone Graft Substitutes Used in Periodontal Regeneration: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2020, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.d.A.Q.; Lehman, L.F.C.; Diniz, M.G.; Ferreira, A.J.; Silva, R.M.F.d.C.e.; Silva, T.A.; Mesquita, R.A.; Oliveira, R.F.d.; Noronha, M.S.; Leão, D.M.; et al. Biogran Grafting in Rat Tibia Defects—A Model of High Bone Metabolism Site. Braz. Arch. Biol. Technol. 2024, 67, e24230003. [Google Scholar] [CrossRef]
- Cochran, D.L. The scientific basis for and clinical experiences with Straumann implants including the ITI® Dental Implant System: A consensus report Note. Clin. Oral Implants Res. 2000, 11, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, M.; Mauceri, R.; Campisi, G.; Pizzo, G.; Alessandro, R.; Tomasello, L.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Growth and Osteogenic Differentiation of Discarded Gingiva-Derived Mesenchymal Stem Cells on a Commercial Scaffold. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, M.; Osorio, R. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Hydroxylapatite-Collagen Hybrid Scaffold Induces Human Adipose-Derived Mesenchymal Stem Cells to Osteogenic Differentiation In Vitro and Bone Regrowth in Patients | Stem Cells Translational Medicine | Oxford Academic. Available online: https://academic.oup.com/stcltm/article/9/3/377/6407168 (accessed on 18 February 2024).
- Effects of Bio-Oss® and Cerasorb® dental M on the Expression of Bone-Remodeling Mediators in Human Monocytes—De Assis Gonzaga—2017—Journal of Biomedical Materials Research Part B: Applied Biomaterials—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jbm.b.33747?casa_token=L4xQ6OHjaSIAAAAA:ul9W8fNHYRo6koL8B4winLFbID8OhmgNzA88zytk_hXkMy6TTipGeQep9lI7kqzXn2g8CCsQWDiuO-4 (accessed on 18 February 2024).
- Figliuzzi, M.M.; De Fazio, R.; Tiano, R.; De Franceschi, S.; Pacifico, D.; Mangano, F.; Fortunato, L. Histological evaluation of a biomimetic material in bone regeneration after one year from graft. Ann. Stomatol. (Roma) 2014, 5, 103–107. [Google Scholar] [CrossRef]
- Frasca, S.; Norol, F.; Le Visage, C.; Collombet, J.-M.; Letourneur, D.; Holy, X.; Sari Ali, E. Calcium-phosphate ceramics and polysaccharide-based hydrogel scaffolds combined with mesenchymal stem cell differently support bone repair in rats. J. Mater. Sci. Mater. Med. 2017, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, D.; Petersen, E.B.; Watson, N.; Grosland, N.; Gibson-Corley, K.; Smucker, J. Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model. Iowa Orthop. J. 2016, 36, 167–173. [Google Scholar] [PubMed]
- The Use of Stimulan in Bone and Joint Infections | Bone & Joint. Available online: https://boneandjoint.org.uk/article/10.1302/2633-1462.47.BJO-2023-0036.R1 (accessed on 18 February 2024).
- Cheng, L.; Yu, T.; Shi, Z. Osteoinduction Mechanism of Calcium Phosphate Biomaterials In Vivo: A Review. J. Biomater. Tissue Eng. 2017, 7, 911–918. [Google Scholar] [CrossRef]
- Kotagudda Ranganath, S.; Schlund, M.; Delattre, J.; Ferri, J.; Chai, F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater. Today Bio 2022, 14, 100267. [Google Scholar] [CrossRef]
- Brueckner, T.; Heilig, P.; Jordan, M.C.; Paul, M.M.; Blunk, T.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Biomechanical Evaluation of Promising Different Bone Substitutes in a Clinically Relevant Test Set-Up. Materials 2019, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Cellular Interactions and Bone Healing Responses to a Novel Porous Tricalcium Phosphate Bone Craft Material | Orthopedics. Available online: https://journals.healio.com/doi/abs/10.3928/0147-7447-20040102-18 (accessed on 11 March 2024).
- Sionek, A.; Czwojdziński, A.; Kowalczewski, J.; Okoń, T.; Marczak, D.; Sibiński, M.; Złotorowicz, M.; Czubak, J. Hip osteonecroses treated with calcium sulfate-calcium phosphate bone graft substitute have different results according to the cause of osteonecrosis: Alcohol abuse or corticosteroid-induced. Int. Orthop. 2018, 42, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Epstein, G.Z. The role of bioceramics in the management of osteomyelitic voids. SA Orthop. J. 2023, 22, 152–156. [Google Scholar] [CrossRef]
- Manhas, V.; Guyot, Y.; Kerckhofs, G.; Chai, Y.C.; Geris, L. Computational modelling of local calcium ions release from calcium phosphate-based scaffolds. Biomech. Model. Mechanobiol. 2017, 16, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Ohsaki, K.; Ii, K.; Ye, Q.; Nobuto, Y.; Tenshin, S.; Takano-Yamamoto, T. Long-term observation of subcutaneous tissue reaction to synthetic auditory ossicle (Apaceram®) in rats. J. Laryngol. Otol. 1997, 111, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontology 2000 2023, 93, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent. Clin. 2017, 61, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Döri, F.; Huszár, T.; Nikolidakis, D.; Arweiler, N.B.; Gera, I.; Sculean, A. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J. Clin. Periodontol. 2007, 34, 254–261. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, R.; Jiang, X.; Xie, J.; Cai, P.; Chen, H.; Zhan, X.; Lei, D.; Zhao, J.; Zheng, L. Electrospun PLGA/PCL/OCP nanofiber membranes promote osteogenic differentiation of mesenchymal stem cells (MSCs). Mater. Sci. Eng. C 2019, 104, 109796. [Google Scholar] [CrossRef]
- Materials | Free Full-Text | Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Available online: https://www.mdpi.com/1996-1944/13/3/786 (accessed on 13 February 2024).
- De Angelis, N.; Felice, P.; Pellegrino, G.; Camurati, A.; Gambino, P.; Esposito, M. Guided bone regeneration with and without a bone substitute at single post-extractive implants: 1-year post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral Implants 2011, 4, 313–325. [Google Scholar]
- Tri-Layered Functionally Graded Membrane for Potential Application in Periodontal Regeneration—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0928493119302085 (accessed on 14 February 2024).
- Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Available online: https://pubmed.ncbi.nlm.nih.gov/31509976/ (accessed on 14 February 2024).
- Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Available online: https://pubmed.ncbi.nlm.nih.gov/32326625/ (accessed on 14 February 2024).
- Polyglycerol Hyperbranched Polyesters: Synthesis, Properties and Pharmaceutical and Biomedical Applications. Available online: https://pubmed.ncbi.nlm.nih.gov/31835372/ (accessed on 14 February 2024).
- Fabrication of Novel Poly(lactic acid/caprolactone) Bilayer Membrane for GBR Application. Available online: https://pubmed.ncbi.nlm.nih.gov/32224061/ (accessed on 14 February 2024).
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, I.; Sasaki, J.-I.; Tsuboi, R.; Yamaguchi, S.; Kitagawa, H.; Imazato, S. Development of layered PLGA membranes for periodontal tissue regeneration. Dent. Mater. 2018, 34, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef] [PubMed]
- Blašković, M.; Butorac Prpić, I.; Blašković, D.; Rider, P.; Tomas, M.; Čandrlić, S.; Botond Hangyasi, D.; Čandrlić, M.; Perić Kačarević, Ž. Guided Bone Regeneration Using a Novel Magnesium Membrane: A Literature Review and a Report of Two Cases in Humans. J. Funct. Biomater. 2023, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.H.; Yukna, R.A.; Cambre, K.M.; Gardiner, D.L. Clinical regeneration with guided tissue barriers. Curr. Opin. Periodontol. 1997, 4, 75–81. [Google Scholar] [PubMed]
- The Effect of Membrane Exposure on the Outcome of Regenerative Procedures in Humans: A Meta-Analysis—Machtei—2001—Journal of Periodontology—Wiley Online Library. Available online: https://aap.onlinelibrary.wiley.com/doi/abs/10.1902/jop.2001.72.4.512 (accessed on 13 February 2024).
- New Attachment following Surgical Treatment of Human Periodontal Disease—Nyman—1982—Journal of Clinical Periodontology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-051X.1982.tb02095.x (accessed on 13 February 2024).
- IJMS | Free Full-Text | In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Available online: https://www.mdpi.com/1422-0067/19/10/2952 (accessed on 13 February 2024).
- Expanded vs. Dense Polytetrafluoroethylene Membranes in Vertical Ridge Augmentation around Dental Implants: A Prospective Randomized Controlled Clinical Trial—Ronda—2014—Clinical Oral Implants Research—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/clr.12157 (accessed on 13 February 2024).
- Ottria, L.; Lauritano, D.; Andreasi Bassi, M.; Palmieri, A.; Candotto, V.; Tagliabue, A.; Tettamanti, L. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J. Biol. Regul. Homeost. Agents 2018, 32, 81–90. [Google Scholar] [PubMed]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Hasegawa, H.; Masui, S.; Ishihata, H. New microperforated pure titanium membrane created by laser processing for guided regeneration of bone. Br. J. Oral Maxillofac. Surg. 2018, 56, 642–643. [Google Scholar] [CrossRef]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/β-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Nakahira, A.; Sasaki, J.; Egusa, H.; Sohmura, T. Modification of apatite materials for bone tissue engineering and drug delivery carriers. Curr. Med. Chem. 2007, 14, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Niu, L.; Li, J.; Du, J.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W.; Wang, K. Reinforced chitosan membranes by microspheres for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2018, 81, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Fabrication of Blended Polycaprolactone/poly(lactic-co-glycolic acid)/β-tricalcium Phosphate Thin Membrane Using Solid Freeform Fabrication Technology for Guided Bone Regeneration. Available online: https://pubmed.ncbi.nlm.nih.gov/22934667/ (accessed on 16 February 2024).
- Basile, M.A.; d’Ayala, G.G.; Malinconico, M.; Laurienzo, P.; Coudane, J.; Nottelet, B.; Ragione, F.D.; Oliva, A. Functionalized PCL/HA nanocomposites as microporous membranes for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Growth Factors in Periodontal Regeneration—Raja—2009—International Journal of Dental Hygiene—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1601-5037.2009.00380.x (accessed on 12 March 2024).
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2021, 28, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of the Regenerative Effect of a 25% Doxycycline-Loaded Biodegradable Membrane for Guided Tissue Regeneration—Chang—2000—Journal of Periodontology—Wiley Online Library. Available online: https://aap.onlinelibrary.wiley.com/doi/abs/10.1902/jop.2000.71.7.1086 (accessed on 12 March 2024).
- Guided Bone Regeneration Produced by New Mineralized and Reticulated Collagen Membranes in Critical-Sized rat Calvarial Defects. Available online: https://pubmed.ncbi.nlm.nih.gov/25245073/ (accessed on 16 February 2024).
- Sasaki, J.-I.; Kiba, W.; Abe, G.L.; Katata, C.; Hashimoto, M.; Kitagawa, H.; Imazato, S. Fabrication of strontium-releasable inorganic cement by incorporation of bioactive glass. Dent. Mater. 2019, 35, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Kim, E.-C.; Bang, S.-H.; Chung, C.-H.; Lee, Y.I.; Hyun, J.K.; Lee, H.-H.; Jang, J.-H.; Kim, T.-I.; Kim, H.-W. Bone regeneration by bioactive hybrid membrane containing FGF2 within rat calvarium. J. Biomed. Mater. Res. Part A 2010, 94A, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Schorn, L.; HANDSCHEL, J.; Lommen, J.; BECK, F.; DEPPRICH, R.; Kübler, N.; Holtmann, H. Evaluation of Biocompatibility of Different Membrane Surfaces Using Unrestricted Somatic Stem Cells. In Vivo 2019, 33, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef]
- Tay, J.R.H.; Ng, E.; Lu, X.J.; Lai, W.M.C. Healing complications and their detrimental effects on bone gain in vertical-guided bone regeneration: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 43–71. [Google Scholar] [CrossRef]
- Decco, O.; Cura, A.; Beltrán, V.; Lezcano, M.; Engelke, W. Bone augmentation in rabbit tibia using microfixed cobalt-chromium membranes with whole blood, tricalcium phosphate and bone marrow cells. Int. J. Clin. Exp. Med. 2015, 8, 135–144. [Google Scholar]
- Lin, W.-C.; Yao, C.; Huang, T.-Y.; Cheng, S.-J.; Tang, C.-M. Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite composite for bone tissue engineering. Dent. Mater. 2019, 35, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Grevstad, H.J.; Leknes, K.N. Ultrastructure of plaque associated with polytetrafluoroethylene (PTFE) membranes used for guided tissue regeneration. J. Clin. Periodontol. 1993, 20, 193–198. [Google Scholar] [CrossRef] [PubMed]
- In Vitro Evaluation of Barrier Function against Oral Bacteria of Dense and Expanded Polytetrafluoroethylene (PTFE) Membranes for Guided Bone Regeneration—Trobos—2018—Clinical Implant Dentistry and Related Research—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/cid.12629 (accessed on 16 February 2024).
- Zarkesh, N.; Nowzari, H.; Morrison, J.L.; Slots, J. Tetracycline-Coated Polytetrafluoroethylene Barrier Membranes in the Treatment of Intraosseous Periodontal Lesions. J. Periodontol. 1999, 70, 1008–1016. [Google Scholar] [CrossRef]
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin. Oral Investig. 2021, 25, 2969–2980. [Google Scholar] [CrossRef]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.; et al. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-inflammatory Tissue Response to a Newly Developed Pericardium-based Barrier Membrane for Guided Bone Regeneration. In Vivo 2020, 34, 985–1000. [Google Scholar] [CrossRef]
- Materials | Free Full-Text | Comparing Properties of Variable Pore-Sized 3D-Printed PLA Membrane with Conventional PLA Membrane for Guided Bone/Tissue Regeneration. Available online: https://www.mdpi.com/1996-1944/12/10/1718 (accessed on 16 February 2024).
- Wadhawan, A.; Gowda, T.M.; Mehta, D.S. Gore-tex® versus resolut adapt® GTR membranes with perioglas® in periodontal regeneration. Contemp. Clin. Dent. 2012, 3, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.; Nemoto, A.; Nakajima, K.; Kokubun, K.; Murakami, S.; Inoue, T.; Matsuzaka, K. The effect of fibroblast growth factor 7 on human dental pulp stem cells for differentiation to AQP5-positive and αSMA-positive cells in vitro and in vivo. Clin. Exp. Dent. Res. 2021, 7, 344–353. [Google Scholar] [CrossRef]
- Reininger, D.; Cobo-Vázquez, C.; Rosenberg, B.; López-Quiles, J. Alternative intraoral donor sites to the chin and mandibular body-ramus. J. Clin. Exp. Dent. 2017, 9, e1474–e1481. [Google Scholar] [CrossRef]
- Steijvers, E.; Ghei, A.; Xia, Z. Manufacturing artificial bone allografts: A perspective. Biomater. Transl. 2022, 3, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Amid, R.; Kheiri, A.; Kheiri, L.; Kadkhodazadeh, M.; Ekhlasmandkermani, M. Structural and chemical features of xenograft bone substitutes: A systematic review of in vitro studies. Biotechnol. Appl. Biochem. 2021, 68, 1432–1452. [Google Scholar] [CrossRef]
- Mahanty, A.; Shikha, D. Changes in the morphology, mechanical strength and biocompatibility of polymer and metal/polymer fabricated hydroxyapatite for orthopaedic implants: A review. J. Polym. Eng. 2022, 42, 298–322. [Google Scholar] [CrossRef]
- Bone Graft/Sinus Lifts. Available online: https://www.mcgill.ca/omfs/patient-info/bone-graftsinus-lifts (accessed on 11 March 2024).
- Guided Bone and Tissue Regeneration | Mark Forrest, D.M.D. Available online: https://www.dr4est.com/guided-bone-and-tissue-regeneration.php (accessed on 25 February 2024).
- Wen, Y.; Xun, S.; Haoye, M.; Baichuan, S.; Peng, C.; Xuejian, L.; Kaihong, Z.; Xuan, Y.; Jiang, P.; Shibi, L. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Tae Young, A.; Kang, J.H.; Kang, D.J.; Venkatesan, J.; Chang, H.K.; Bhatnagar, I.; Chang, K.-Y.; Hwang, J.-H.; Salameh, Z.; Kim, S.-K.; et al. Interaction of stem cells with nano hydroxyapatite-fucoidan bionanocomposites for bone tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk implants for the healing of critical size bone defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Canadas, R.F.; Jiménez, G.; Perán, M.; Marchal, J.A.; Reis, R.L.; Oliveira, J.M. Biofunctional Ionic-Doped Calcium Phosphates: Silk Fibroin Composites for Bone Tissue Engineering Scaffolding. Cells Tissues Organs 2017, 204, 150–163. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Abdallah, M.-N.; Glogauer, M.; Grynpas, M. 15—Natural and synthetic bone replacement graft materials for dental and maxillofacial applications. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 347–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhou, W.; Fu, H.; Qian, L.; Miron, R.J. Addition of a Synthetically Fabricated Osteoinductive Biphasic Calcium Phosphate Bone Graft to BMP2 Improves New Bone Formation. Clin. Implant Dent. Relat. Res. 2016, 18, 1238–1247. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Osteogenic Hydrogels: Gelatin Templated Polypeptide Co-Cross-Linked Hydrogel for Bone Regeneration (Adv. Healthcare Mater. 1/2020). Adv. Healthc. Mater. 2020, 9, 2070001. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Yan, X.-Z. Periodontal Guided Tissue Regeneration Membranes: Limitations and Possible Solutions for the Bottleneck Analysis. Tissue Eng. Part B Rev. 2023, 29, 532–544. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Ku, J.-K. Extraction socket preservation. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 435–439. [Google Scholar] [CrossRef] [PubMed]
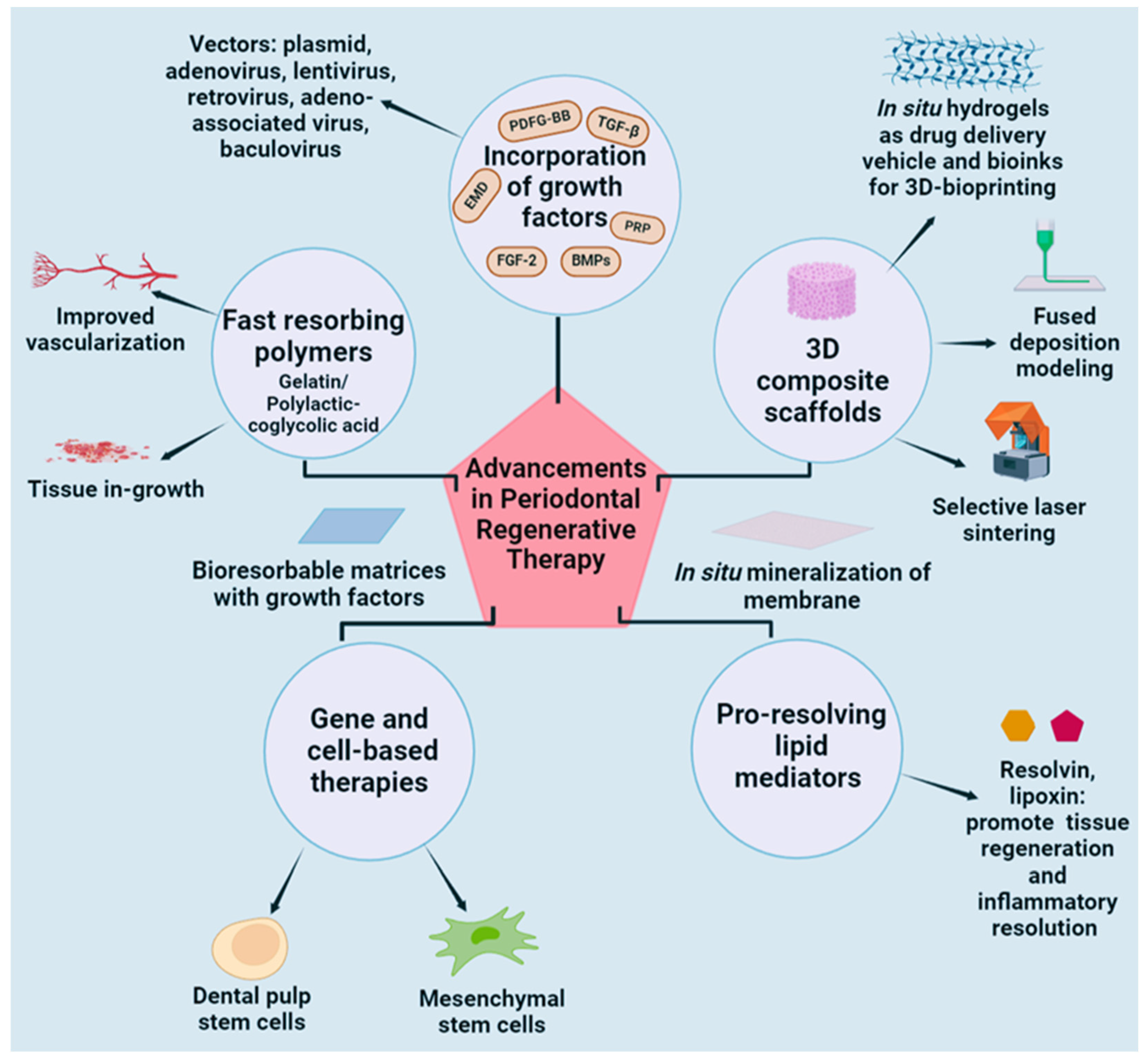
| Graft Type | Merits | Demerits | Mechanism and Level | Market Products | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Osteoconduction | Osteoinduction | Osteogenesis | Osteointegration | |||||
| Autograft The gold standard for bone grafting, but only a limited amount of graft material is available. Unpredictable and rapid resorption are possible drawbacks. The additional surgical site increases the risk of infection [21]. | ||||||||
| Cancellous | Revascularization due to a large surface area. | Poor mechanical strength. | Present (high) | Present (high) | Present (high) | Present (high) | [33] | |
| Cortical | Provides structural support and mechanical stability. | Takes longer to remodel than a cancellous graft. | Present (low) | Present (low) | Present (low) | Present (low) | - | [21] |
| Vascularized cortical | Provides structural support and rapid healing, osteoprogenitors, osteocytes, and cells preserved in the graft. | Implantation and harvesting are challenging. | Present (medium) | Present (low) | Present (medium) | - | - | [34] |
| Bone marrow aspirate | Harvesting is minimally invasive. | Presence of fewer stem cells in the graft. | Present (low) | Present (medium) | Present (high) | - | - | [35] |
| Platelet-rich plasma (PRP) | Easy to acquire, affordable, elicits the migration of MSCs to target site, smaller amounts of harvested bone needed. | Complexity of the procedure, variation in preparation method. | - | Present (high) | - | - | - | [36] |
| Allografts Second surgical site is avoided with no additional suffering or pain, minimal risk of infection, and unfavorable immunological response [37]. | ||||||||
| Cancellous | Low residual moisture due to freeze-drying, shelf-life of 4–5 years. | Mechanically weak grafts, difficulty to incorporate as grafts in fibrous tissue are incorporated by the body, immunogenicity. | Present (high) | Present (with fresh allografts) | - | Present (medium) | Cancellous chips/freeze-dried (Musculoskeletal Transplant Foundation: Edison, NJ, USA), Puros® (Zimmer Biomet: Warsaw, IN, USA), Raptos® (Citagenix: Laval, QC, Canada) | [38] |
| Cortical | Mechanically strong and can support the load. | Inflammatory response delays healing, immunogenicity. | Present (high) | Present (with fresh allografts) | - | Present (low) | Raptos® (Citagenix: Laval, QC, Canada) | [39] |
| Demineralized bone matrix (DBM) | Holds growth factors, low immunogenicity. | Variations in the content of growth factors amongst different batches, poor mechanical strength. | Present (low) | Present (low) | - | Present (medium) | Grafton® (BioHorizons: Birmingham, AL, USA), Opteform® (Exatech Inc.: Gainesville, FL, USA), OsteoSponge (Xtant medical: Belgrade, MT, USA), DynaBlast® (Keystone Dental Group: Burlington, MA, USA) | [40] |
| Xenografts Good mechanical strength, ample supply, and low degradation rate [41]. | ||||||||
| Xenografts | Similarity to human bone a calcium/phosphate ratio of 1.67 identical to human bone composition, low immunogenicity, and biomechanical properties same as the bone. | Ethical and religious issues, risks of disease transmission. | Present (high) | Present (low) | - | Present (low) | Gen-Os® (Cortico-cancellous heterologous bone mix) (Tecnoss dental, Torino, Italy), Cerabone® (Bovine cancellous bone grafting) (Straumann: Basel, Switzerland), Straumann® XenoFlex (biphasic composition: collagen and hydroxyapatite (xenogenic), (Straumann: Basel, Switzerland), Bio-Gen (equine bone) (Bioteck: Arcugnano, Vicenza (VI), Italy), InterOss® (bovine bone) (Sigmagraft Inc. biomaterials: Fullerton, CA, USA), NuOss (bovine bone) (ACE Surgical Supply Company, Inc: Brockton, MA, USA) | [42] |
| Alloplastic Various options for graft materials, custom scaffolds (3D printed, injectable), lack of growth factors [43]. | ||||||||
| Calcium phosphate ceramics/ biphasic calcium phosphate ceramics | Similar composition to bone, has excellent intraparticle cohesivity, resorbability of 85% β-TCP, and osteoconduction of 15% hydroxyapatite. | Compromised mechanical strength, difficult to mold. | Present (medium) | - | - | Present (medium) | Teebone® (Medibrex: Zalqa, Lebanon), Mastergraft™ (Medtronic Sofamor Danek, Memphis, TN, USA), Ossfinity® (Leader Biomedical: Amsterdam, The Netherlands), Maxresorb® (Botiss biomaterials GmbH: Zossen, Germany) | [44,45] |
| Beta tricalcium phosphate | Bears the most similarity in composition to bone and is called the ‘gold standard’ of synthetic grafts. | Unpredictable degradation, rapid resorption. | Present (low) | - | - | - | Vitoss® (Stryker Corporation: Kalamazoo, MI, USA) | [46] |
| Hydroxyapatite | Biocompatible and comparatively possessing high tensile strength. | Slow resorption. | Present (low) | - | - | - | Collagraft® (Zimmer Biomet: Warsaw, IN, USA), Healos® (DePuy Synthes Spine: Raynham, MA, USA) | [47] |
| Calcium phosphate cement | Easy to mold. | Poor mechanical strength. | Present (medium) | - | - | - | CopiOs® (ZimVie: Westminster, CO, USA), NorianTM (Synthes GmbH: Eimattstrasse, Oberdorf, Switzerland), ChronOS injectTM (DePuy Synthes Spine: Raynham, MA, USA), HydrosetTM (Stryker Corporation: Kalamazoo, MI, USA) | [48] |
| Calcium sulfate | High biocompatibility, self-setting strength, and resorption with minimal inflammation, low cost, easy to prepare. | Risks of wound drainage, poor mechanical strength. Resorbs faster than the dissolution of bone itself. | Present (low) | - | - | Present (medium) | OsteoSet® (Wright Medical Group: Memphis, TN, USA) | [49] |
| Bioactive glass | Possesses antibacterial activity, formation of bonds between tissues and bones, stimulates osteogenesis, good biocompatibility | Low toughness and mechanical strength | Present (low) | - | - | - | BioGran® (Biomet 3i Innovations Inc.: Plam beach gardens, FL, USA) | [50] |
| PMMA bone cement | Secures implants in place, treats bone defects. | Poor adhesion, heat sensitivity, and the reaction to such a foreign body can result in a loosening of the implant and a risk of bone cement implantation disorder. | - | - | - | - | C-ment® (Leader Biomedical: Amsterdam, The Netherlands) | [51] |
| BMP-2, BMP-7 | Better bone regeneration reported in smokers. | Off-label use, swelling issues, risk of ectopic bone formation. | Present (with collagen carriers) | Present (low) | Present (low) | - | BMP-7/OP-1 (United States Biologicals: Swampscott, MA, USA), InfuseTM Bone Graft (Medtronic: Dublin, Ireland) | [52] |
| Recombinant human platelet-derived growth factor-BB homodimer (rhPDGF-BB) and ß-TCP | Less painful procedure as compared to natural grafts, treats intrabony, furcation periodontal defects. | Expensive, may require additional surgery, swelling, pain, bleeding. | Present (high) | - | - | - | GEM 21S® (Geistlich Pharma, Wolhusen, Switzerland) | [53] |
| iFactor (P-15) | Outcomes like in the case of autografts. | Dysphagia post-treatment. | Present (in combination with other synthetic grafts) | - | - | - | i-FACTOR Flex bone graft, (Cerapedics: Broomfield, CO, USA), p-15™ (Cerapedics: Broomfield, CO, USA) | [54] |
| Polymers (chitosan) | Present low immunological rejection. | Poor resorption. | Present (low) | - | - | - | Commercially provided by manufacturers | [55] |
| Synthetic Biomaterials | Features Related to the Product | Available Brand | Available Form | Manufacturers | Comments by Manufacturers | Ref. |
|---|---|---|---|---|---|---|
| Bioactive glass | Osteostimulation, osteoconduction, anti-inflammatory, and antibacterial activities (local, transient) | PerioGlas® | Cups, syringes | NovaBone (Jacksonville, FL, USA) | When the material is implanted in living cells, its surface undergoes a time-dependent kinetic alteration. | [98] |
| BCP (60% HA/40% β-TCP) | 100% degradable and bioresobable. | Guidor® easy-graft | Injectable/sterile powder | Sunstar (Osaka, Japan) | Repairs and fills the defects left by apicoectomy, autologous bone, and cyst removal. | [99] |
| Porous β-TCP | Osteoconduction, osteoinduction, resembling human cancellous bone. | Vitoss® | Moldable packs, malleable strips, and morsels. | Stryker Corporation (Kalamazoo, MI, USA) | When mixed or hydrated with bone marrow aspirate (BMA), it exhibits the same components of bone healing as the gold standard (iliac crest bone graft). | [100] |
| Phosphocalcium Cement (a mixture of calcium phosphate salts) Liquid phase: solution of Na2HPO4 (pH 8.7) to accelerate bone substitution setup | Osteoconduction and surface osseointegration, slow resorption. | Eurobone® 2std | Injectable synthetic cement | FH Ortho (Mulhouse, France) | Within 24 h of implantation, it is able to achieve maximum strength. | [101] |
| β-TCP | Osteoconduction, osteointegration, and biocompatible improved mechanical strength. | HydroSet XT | Injectable | Stryker Corporation (Kalamazoo, MI, USA) | Hydroset offers rigidity to encourage new bone formation. | [102] |
| Silicated β-TCP | Resorbable, mechanically stable, minimizing micro-movement. | IngeniOs® | Particles 1–2 mm | Zimmer Biomet (Warsaw, IN, USA) | Combination with biologic drivers in autologous PRP, bone marrow, or stem cells. | [103] |
| HA | Biocompatible, osteoconduction, non-immunogenicity. | B-OstIN® | Granules, rods, and blocks | Basic Healthcare (Himachal Pradesh, India) | The most active component among ceramics, non-toxic, promotes bone fusion within three months with no known side effects. | [104] |
| Bioactive glass | Highly resorbable due to small size. | BioGran® | Granules | Biomet 3i Innovations Inc. (Plam beach gardens, FL, USA) | Offers the special environment of protection required for osteogenesis. | [105] |
| 60% HA, 40% (β-TCP) | Reproducibility, biocompatibility, and osteoconduction facilitate osteoblast migration and vascularization. | BoneCeramic™ | Granules | Straumann (Basel, Switzerland) | The maximum space for the formation of new bone is provided by minimal amounts of a highly porous substance. | [106] |
| nano-HA | Osteoconduction, partially resorbable. | Fisiograft bone granular | Granules 250–1000 microns | Ghimas (Bologna, Italy) | Strongly interconnected porosity. | [107] |
| Calcium phosphate, lyophilized type I bovine collagen | Identical to human cancellous bone, promotes bone regrowth, resorbable. | CopiOs® | Paste, sponge | ZimVie (Westminster, CO, USA) | Enhance the solubility of bone morphogenetic proteins (BMPs) and other osteoinductive growth factors, a second surgical harvest procedure is not required. | [108] |
| Coralline HA | Similar to human cancellous bone, resorbable. | Pro Osteon® | Granules | Zimmer Biomet (Warsaw, IN, USA) | Residual material stays for years in ceramic form. | [109] |
| Calcium sulfate, calcium phosphate, demineralized bovine bone | Osteoconduction, osteoinduction, bioresorbable. | Pro-Stim® | Injectable inductive graft | Wright Medical Group (Memphis, TN, USA) | Do not supplement the graft with other drugs or substances; using additional chemicals or solutions may change the safety profile of the mixture. New bone formation occurs in 13 and 26 weeks. | [49] |
| β-TCP (>99%) | Resorbable, non-allergenic, and without systemic toxicity. | Cerasorb® | Granules (size: 150–2000 microns) | Curasan (Frankfurt am Main, Germany) | Promotes bone formation due to sufficient space among particles. | [110] |
| Porous HA | Long-lasting osseous integration, osteoconduction | ENGIpore® | - | Finceramica (Faenza RA, Italy) | Able to withstand the compressive forces associated with real bone. | [111] |
| β-TCP +HA | Osteoconduction, resorbable. | Calciresorb® C35 | Granules | Ceraver (Roissy Cdg Cedex - France) | - | [112] |
| Silicate-substituted calcium phosphate | Osteoconduction, osteostimulation, no risk of disease transmission, accelerated bone growth. | Actifuse® ABX | Granules | Baxter (Deerfield, IL, USA) | Bony regeneration may be hampered by a patient’s metabolism. | [113] |
| Calcium sulfate, antibiotics | Resorbable | Stimulan® | Beads | Biocomposites Ltd. (Keele, UK) | Can be mixed with tobramycin. vancomycin, gentamicin, it resorbs faster than bone. | [114] |
| 60% HA, 40% β-TCP | Structurally and chemically resembles and mimics cancellous bone, resorbable, osteoconductive, 70% porous | OpteMx® | Particles, sticks, wedges, cylinders of various sizes | Exatech (Gainesville, FL, USA) | Quite high compressive strength (2.6 MPa). It is advised to use rigid fixation techniques until OpteMx is reabsorbed. | [115] |
| 85% β-TCP, 15% HA | Osteoconductive, resorbable | Mastergraft® | Granules, putty | Medtronic (Dublin, Ireland) | Mastergraft is offered as separate grains or in combination with collagen to produce a putty-like substance with cohesive and moldable properties. | [116] |
| Calcium phosphate and polymers | Biocompatible, moldable, and resorbable due to the addition of polylactide/glycolide copolymer fibers. | Norian Drillable Bone Void Filler | Injectable, sterile powder | Synthes GmbH (Eimattstrasse, Oberdorf, Switzerland) | Achieves ideal bone defect filling and a compressive strength of 35 MPa in 24 h. | [117] |
| Biphasic calcium phosphate | Rapidly absorbed; nano-HA particles provide an extremely large surface area for cellular interactions | Maxresorb® | Injectable paste, granules | Botiss biomaterials GmbH (Zossen, Germany) | 100% synthetic, almost the risk of infection, ensures a high degree of reproducibility and material safety. | [76] |
| β-TCP | Osteoconductive, resorbable, and mimics the structure of cancellous bone. | Cellplex® TCP | Granules | Wright Medical Group (Memphis, TN, USA) | Highly porous, interconnected structure, excellent carrier of BMA, packed in Marrow Infusion Chamber INFILTRATE® to give surgeons an easy way to combine BMA with CELLPLEX® TCP. | [118] |
| Calcium sulfate | Resorbable | MIIG® X3 | Injectable, paste | Wright Medical Group (Memphis, TN, USA) | It is essentially the same as other available bone void fillers. | [46] |
| Calcium sulfate, Calcium phosphate, β-TCP | Slow-resorbing, mechanically strong, and more dense bone | ProDense® | Injectable | Wright Medical Group (Memphis, TN, USA) | Regenerates new bone that bears nearly six times more compressive strength than autograft bone. | [119] |
| Calcium sulfate | Osteoconduction, resorbable | OsteoSet® | Beads | Wright Medical Group (Memphis, TN, USA) | Works as a passive osteoconductive scaffold. | [120] |
| β-TCP (80), type I bovine collagen (20) | Osteoconduction | Integra MozaikTM | Putty | Integra LifeSciences Corp. (Princeton, NJ, USA) | Mimics the original composition of bone, bends to fit uneven surfaces, and retains bioactive fluids within the scaffold to aid in protein binding. | [121] |
| Synthetic HA | Osteoconduction, biologically safe and biocompatible | Apaceram® | Block, granules | HOYA Technosurgical Corporation (Shinjuku-ku, Tokyo, Japan) | Available in different porosity ranges and mechanical strengths for intended usage. | [122] |
| Biomaterials for Barrier Membrane | Resorbable/Non-Resorbable | Advantages | Disadvantages | Commercial Products | Ref. |
|---|---|---|---|---|---|
| Non-biodegradable | |||||
| Metal: Titanium, titanium alloy | Non-resorbable | Improved mechanical strength, stability, durability, high biocompatibility, and barrier function | Expensive second surgery is required for membrane removal | GDT Titanium Mesh Membranes (GDT dental implant: Beer Sheva Israel), OSS Builder (Osstem Implant: Auckland, New Zealand), Titanium Mesh (Stanford Advance Materials: Lake Forest, CA, USA) | [146,160] |
| Cobalt, cobalt alloy | Non-resorbable | Affordable price, improved space-making, and mechanical strength | Less biocompatible | n!ce® Cobalt-Chromium (Straumann: Basel, Switzerland) | [161,162] |
| Polytetrafluoroethylene (PTFE) expanded PTFE (e-PTFE) | Non-resorbable | High biocompatibility and stability, stiffness and space maintainer, bacterial resistance | Membrane exposure, second surgery is required | Gore-Tex® (W. L. Gore & Associates: Newark, DE, USA), Cytoflex® (Unicare Biomedical: Laguna Hills, CA, USA), Cytoflex®Tef-Guard® (Unicare Biomedical: Laguna Hills, CA, USA) | [163,164,165] |
| Titanium-reinforced polytetrafluoroethylene (PTFE) | Non-resorbable | Hard tissue reconstruction, both vertically and horizontally, remains stable with minimal membrane exposure. | Ridge augmentation and additional surgeries are required. | Gore-Tex-TI (GORE-TEX: Newark, DE, USA), CytoplastTM Ti-Enforced® ePTFE ® (BioHorizons, Birmingham, AL, USA), NeoGen® Ti-reinforced (Straumann: Basel, Switzerland), OsseoGuard® PTFE (ZimVie: Westminster, CO, USA), Cytoflex® Ti-reinforced (Unicare Biomedical: Laguna Hills, CA, USA) | [166] |
| High-density PTFE (d-PTFE) | Non-resorbable | Improved bacterial resistance, prevents infections, highly stable, and offers better intracellular penetration due to pore size. | Chances for second surgery, has lower porosity compared with PTFE, not a fully inert material. | Cytoplast®TXT-200 ® (BioHorizons, Birmingham, AL, USA), NeoGen® (Neoss: Zurich, Switzerland), Permamem® (Straumann: Basel, Switzerland), OsseoGuard® (Zimmer Biomet: Warsaw, IN, USA) | [142] |
| Biodegradable (natural polymers) | |||||
| Alginate, Chitosan, Agarose | Resorbable | Prevents the need for surgical removal of the membrane and is highly biocompatible. | Suspicious barrier function, few documented trials are available, rapid resorption with weak mechanical strength. | - | [130,149] |
| Collagen | Resorbable | Avoids surgical removal, is biocompatible and suitable for wound healing and barrier functions. | Risk of disease transmission, uncontrolled biodegradability, and low mechanical strength | Cytoplast® RTM collagen (Osteogenics: Lubbock, TX, USA), AlloDerm® SELECTTM RTM (BioHorizons, Birmingham, AL, USA), BioMend® and BioMend ExtendTM (ZimVie: Westminster, CO, USA), Ossix Plus®(Dentsply Sirona Inc, Charlotte, NC, USA), Geistlich Bio-Gid® (Geistlich Pharma, Wolhusen, Switzerland) | [128,167] |
| Biodegradable Synthetic Polymer | |||||
| Aliphatic polyesters (PLA, PGA, and PCL), and copolymers | Resorbable | Biocompatible and reproducible, with a controlled mechanism and improved barrier functionality. | Mechanically weak, produces cytotoxic byproducts | Resolut Adapt® (W. L. Gore & Associates: Newark, DE, USA), Vicryl periodontal mesh (Ethicon, Inc: Raritan, NJ, USA), Atrisorb® (TOLMAR Inc: Fort Collins, CO, USA) | [123,136,168,169] |
| Cellulose acetate | Resorbable | Inert, biocompatible, cost-effective, stable; its durable renewability exhibits higher chlorine resistance. | Unstable at elevated temperatures, very thin and asymmetric structure, biodegradable, prone to rejection with acid or basic hydrolysis. | Millipore® (Merck KGaA, Darmstadt, Germany) | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. Int. J. Mol. Sci. 2024, 25, 7746. https://doi.org/10.3390/ijms25147746
Ashfaq R, Kovács A, Berkó S, Budai-Szűcs M. Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. International Journal of Molecular Sciences. 2024; 25(14):7746. https://doi.org/10.3390/ijms25147746
Chicago/Turabian StyleAshfaq, Rabia, Anita Kovács, Szilvia Berkó, and Mária Budai-Szűcs. 2024. "Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy" International Journal of Molecular Sciences 25, no. 14: 7746. https://doi.org/10.3390/ijms25147746
APA StyleAshfaq, R., Kovács, A., Berkó, S., & Budai-Szűcs, M. (2024). Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. International Journal of Molecular Sciences, 25(14), 7746. https://doi.org/10.3390/ijms25147746







