Morphogenetic Designs, and Disease Models in Central Nervous System Organoids
Abstract
1. Introduction
2. Morphogenetic Design of CNS Organoids
2.1. Cortical Organoid
2.2. Forebrain Organoid
2.3. Midbrain Organoid
2.4. Cerebellar Organoid
2.5. Spinal Cord Organoid
| Author (Year) | Cell Source | Region Designed | Significance |
|---|---|---|---|
| Meinhardt et al. (2014) [67] | Mouse ESC | Neural tube | Spatial organization of neurons along the dorsal and ventral axis |
| Ranga et al. (2016) [68] | Mouse ESC | Neural tube | Focus on extracellular matrix and cytoskeleton during patterning |
| Lippmann et al. (2015) [70] | hiPSC | Neuromesoderm | Identified importance of HOX |
| Ogura et al. (2018) [71] | hiPSC | Dorsal and ventral spinal cord-like tissues | Dorsal and ventral tissues generated using BMP4 and Shh signaling |
| Andersen et al. (2020) [72] | hiPSC | Dorsal and ventral spinal cord-like tissues | A continuous dorsal and ventral tissue generated and connected muscle spheroids with neurons |
| Mouilleau et al. (2021) [73] | hESC/hiPSC | Rostro-caudal patterning | Differentiation of motor neurons through FGF and HOX expression |
| Karzbrun et al. (2021) [75] | hiPSC | Neural tube | Developed chip-based culture system for homogeneity |
| Lee et al. (2022) [27] | hiPSC | Spinal cord | Generated tubular structure with functionality |
| Shin et al. (2023) [76] | hPSC | Spinal cord | Necrotic core was reduced |
| Gribaudo et al. (2023) [28] | hiPSC | Spinal cord | Autonomously forming AP axis |
3. CNS Organoids as Disease Models and Drug Screening Platforms
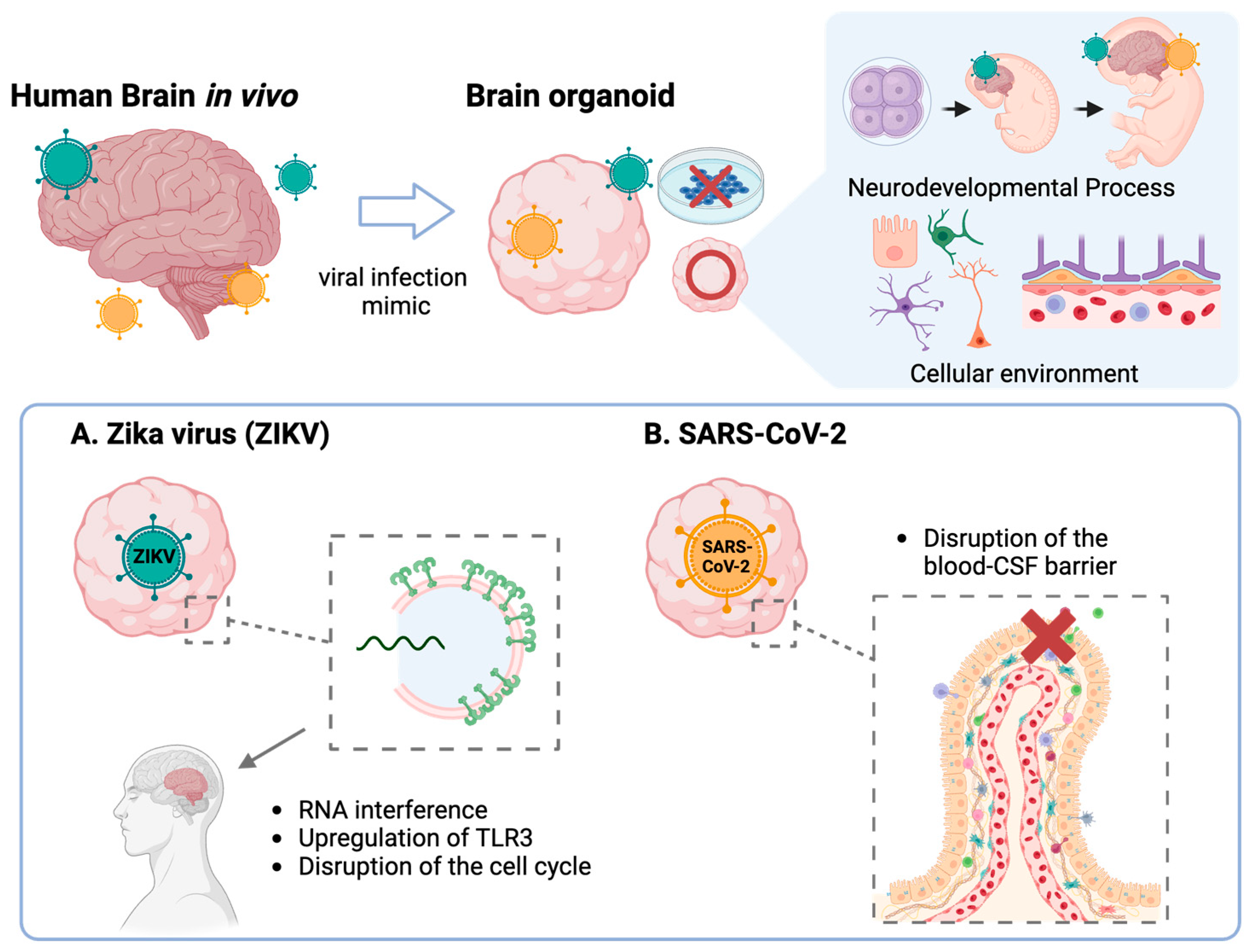
3.1. Viral Infections
3.1.1. Zika Virus (ZIKV)
3.1.2. Herpes Simplex Virus (HSV)
3.1.3. Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-CoV-2)
3.2. Neurodevelopmental Diseases
3.2.1. Microcephaly
3.2.2. Autism Spectrum Disorder (ASD)
3.2.3. Developmental and Epileptic Encephalopathy (DEE)
3.3. Neurodegenerative Diseases
3.3.1. Alzheimer’s Disease (AD)
3.3.2. Parkinson’s Disease (PD)
3.3.3. Huntington’s Disease (HD)
3.3.4. Motor Neuron Diseases
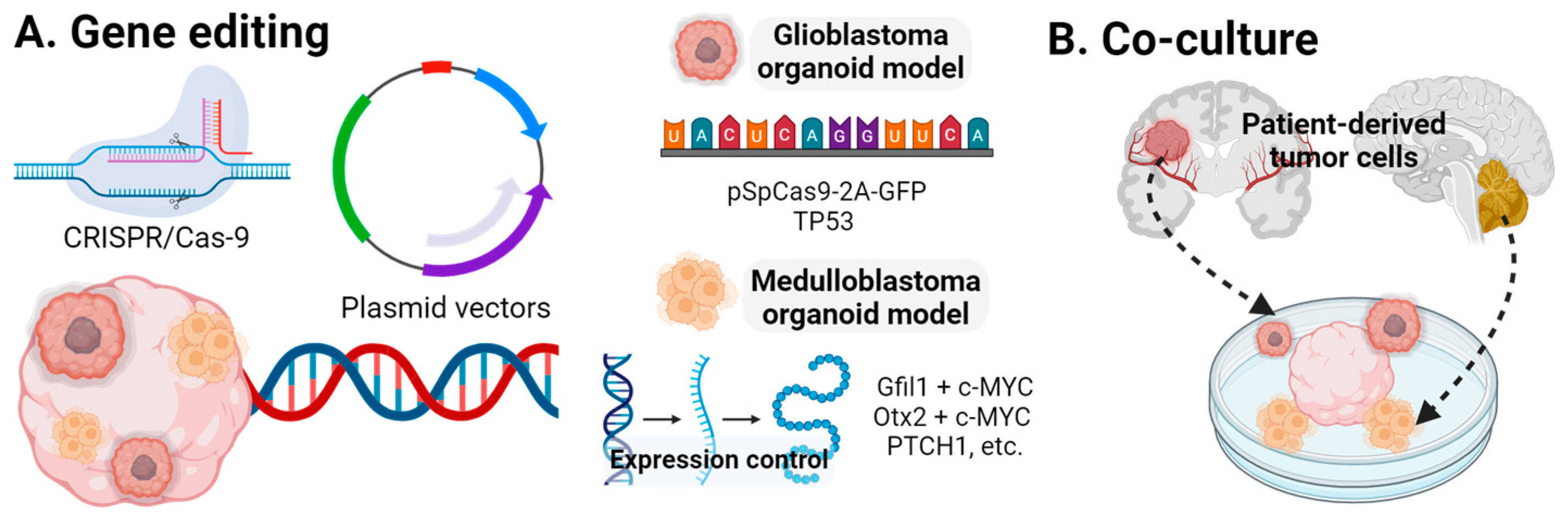
3.4. CNS Tumors
Glioblastoma
4. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Jang, S.Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.H.; Seo, J.; Choi, M.; et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials. JACC Basic. Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- van Strien, N.M.; Wideroe, M.; van de Berg, W.D.; Uylings, H.B. Imaging hippocampal subregions with in vivo MRI: Advances and limitations. Nat. Rev. Neurosci. 2011, 13, 70. [Google Scholar] [CrossRef]
- Chen, Y.; Bury, L.A.; Chen, F.; Aldinger, K.A.; Miranda, H.C.; Wynshaw-Boris, A. Generation of advanced cerebellar organoids for neurogenesis and neuronal network development. Hum. Mol. Genet. 2023, 32, 2832–2841. [Google Scholar] [CrossRef]
- Cowan, C.S.; Renner, M.; De Gennaro, M.; Gross-Scherf, B.; Goldblum, D.; Hou, Y.; Munz, M.; Rodrigues, T.M.; Krol, J.; Szikra, T. Cell types of the human retina and its organoids at single-cell resolution. Cell 2020, 182, 1623–1640.e34. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarro, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Vilar, E.; Tsai, S.Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Jee, J.; Park, J.H.; Im, J.H.; Kim, M.S.; Park, E.; Lim, T.; Choi, W.H.; Kim, J.H.; Kim, W.R.; Ko, J.S.; et al. Functional recovery by colon organoid transplantation in a mouse model of radiation proctitis. Biomaterials 2021, 275, 120925. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2016, 536, 238. [Google Scholar] [CrossRef] [PubMed]
- Vanslambrouck, J.M.; Wilson, S.B.; Tan, K.S.; Groenewegen, E.; Rudraraju, R.; Neil, J.; Lawlor, K.T.; Mah, S.; Scurr, M.; Howden, S.E.; et al. Enhanced metanephric specification to functional proximal tubule enables toxicity screening and infectious disease modelling in kidney organoids. Nat. Commun. 2022, 13, 5943. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, H.; Joo, K.M.; Lee, S.H. Advances in Neural Stem Cell Therapy for Spinal Cord Injury: Safety, Efficacy, and Future Perspectives. Neurospine 2022, 19, 946–960. [Google Scholar] [CrossRef]
- Eiraku, M.; Sasai, Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 2012, 22, 768–777. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Challet Meylan, L.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 2019, 569, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Nikolaev, M.; Brown, T.E.; Mitrofanova, O.; Brandenberg, N.; DelRio, F.W.; Yavitt, F.M.; Liberali, P.; Anseth, K.S.; Lutolf, M.P. Tissue geometry drives deterministic organoid patterning. Science 2022, 375, eaaw9021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Song, H.; Ming, G.-L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, H.; Shaker, M.R.; Kim, H.J.; Park, S.H.; Kim, J.H.; Lee, N.; Kang, M.; Cho, S.; Kwak, T.H.; et al. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat. Biomed. Eng. 2022, 6, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Gribaudo, S.; Robert, R.; van Sambeek, B.; Mirdass, C.; Lyubimova, A.; Bouhali, K.; Ferent, J.; Morin, X.; van Oudenaarden, A.; Nedelec, S. Self-organizing models of human trunk organogenesis recapitulate spinal cord and spine co-morphogenesis. Nat. Biotechnol. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Yang, S.; Palmquist, K.H.; Nathan, L.; Pfeifer, C.R.; Schultheiss, P.J.; Sharma, A.; Kam, L.C.; Miller, P.W.; Shyer, A.E.; Rodrigues, A.R. Morphogens enable interacting supracellular phases that generate organ architecture. Science 2023, 382, eadg5579. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, S.C. Differentiation of neuroepithelia from human embryonic stem cells. Methods Mol. Biol. 2009, 549, 51–58. [Google Scholar] [CrossRef] [PubMed]
- LaMonica, B.E.; Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat. Commun. 2013, 4, 1665. [Google Scholar] [CrossRef] [PubMed]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L. Establishing cerebral organoids as models of human-specific brain evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Su, Y.; Adam, C.D.; Deutschmann, A.U.; Pather, S.R.; Goldberg, E.M.; Su, K.; Li, S.; Lu, L.; Jacob, F.; et al. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell 2020, 26, 766–781.e9. [Google Scholar] [CrossRef] [PubMed]
- Uzquiano, A.; Kedaigle, A.J.; Pigoni, M.; Paulsen, B.; Adiconis, X.; Kim, K.; Faits, T.; Nagaraja, S.; Anton-Bolanos, N.; Gerhardinger, C.; et al. Proper acquisition of cell class identity in organoids allows definition of fate specification programs of the human cerebral cortex. Cell 2022, 185, 3770–3788.e27. [Google Scholar] [CrossRef]
- Renner, M.; Lancaster, M.A.; Bian, S.; Choi, H.; Ku, T.; Peer, A.; Chung, K.; Knoblich, J.A. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 2017, 36, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Krefft, O.; Jabali, A.; Iefremova, V.; Koch, P.; Ladewig, J. Generation of Standardized and Reproducible Forebrain-type Cerebral Organoids from Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2018, 131, e56768. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Albanna, W.; Pasquini, G.; Ramani, A.; Josipovic, N.; Mariappan, A.; Riparbelli, M.G.; Callaini, G.; Karch, C.M.; Goureau, O.; et al. Generation of iPSC-derived human forebrain organoids assembling bilateral eye primordia. Nat. Protoc. 2023, 18, 1893–1929. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Wong, S.Z.H.; Pather, S.R.; Nguyen, P.T.; Zhang, F.; Zhang, D.Y.; Zhang, Z.; Lu, L.; Fang, W.; Chen, L. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 2021, 28, 1657–1670.e10. [Google Scholar] [CrossRef] [PubMed]
- Blaess, S.; Szabo, N.; Haddad-Tovolli, R.; Zhou, X.; Alvarez-Bolado, G. Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 2014, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Corman, T.S.; Bergendahl, S.E.; Epstein, D.J. Distinct temporal requirements for Sonic hedgehog signaling in development of the tuberal hypothalamus. Development 2018, 145, dev167379. [Google Scholar] [CrossRef] [PubMed]
- Sarrafha, L.; Neavin, D.R.; Parfitt, G.M.; Kruglikov, I.A.; Whitney, K.; Reyes, R.; Coccia, E.; Kareva, T.; Goldman, C.; Tipon, R.; et al. Novel human pluripotent stem cell-derived hypothalamus organoids demonstrate cellular diversity. iScience 2023, 26, 107525. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tanaka, Y.; Cakir, B.; Patterson, B.; Kim, K.-Y.; Sun, P.; Kang, Y.-J.; Zhong, M.; Liu, X.; Patra, P. hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 2019, 24, 487–497.e7. [Google Scholar] [CrossRef]
- Kiral, F.R.; Cakir, B.; Tanaka, Y.; Kim, J.; Yang, W.S.; Wehbe, F.; Kang, Y.J.; Zhong, M.; Sancer, G.; Lee, S.H.; et al. Generation of ventralized human thalamic organoids with thalamic reticular nucleus. Cell Stem Cell 2023, 30, 677–688.e5. [Google Scholar] [CrossRef]
- Duzel, E.; Bunzeck, N.; Guitart-Masip, M.; Wittmann, B.; Schott, B.H.; Tobler, P.N. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Goke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Kwak, T.H.; Kang, J.H.; Hali, S.; Kim, J.; Kim, K.P.; Park, C.; Lee, J.H.; Ryu, H.K.; Na, J.E.; Jo, J.; et al. Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson’s disease modeling. Stem Cells 2020, 38, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Nickels, S.L.; Modamio, J.; Mendes-Pinheiro, B.; Monzel, A.S.; Betsou, F.; Schwamborn, J.C. Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson’s disease. Stem Cell Res. 2020, 46, 101870. [Google Scholar] [CrossRef]
- Berger, E.; Magliaro, C.; Paczia, N.; Monzel, A.S.; Antony, P.; Linster, C.L.; Bolognin, S.; Ahluwalia, A.; Schwamborn, J.C. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab. Chip 2018, 18, 3172–3183. [Google Scholar] [CrossRef]
- Renner, H.; Grabos, M.; Becker, K.J.; Kagermeier, T.E.; Wu, J.; Otto, M.; Peischard, S.; Zeuschner, D.; TsyTsyura, Y.; Disse, P.; et al. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. Elife 2020, 9, e52904. [Google Scholar] [CrossRef]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kamei, T.; Tamada, A.; Kimura, T.; Kakizuka, A.; Asai, A.; Muguruma, K. Survival and process outgrowth of human iPSC-derived cells expressing Purkinje cell markers in a mouse model for spinocerebellar degenerative disease. Exp. Neurol. 2023, 369, 114511. [Google Scholar] [CrossRef]
- Muguruma, K. Self-Organized Cerebellar Tissue from Human Pluripotent Stem Cells and Disease Modeling with Patient-Derived iPSCs. Cerebellum 2018, 17, 37–41. [Google Scholar] [CrossRef]
- Tamada, A.; Watanabe, S.; Muguruma, K. Investigating developmental and disease mechanisms of the cerebellum with pluripotent stem cells. Mol. Cell Neurosci. 2020, 107, 103530. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Andreu, A.; Mecklenburg, N.; Echevarria, D. Cellular and molecular basis of cerebellar development. Front. Neuroanat. 2013, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Fernandes, T.G.; Nogueira, D.E.S.; Rodrigues, C.A.V.; Bekman, E.P.; Hashimura, Y.; Jung, S.; Lee, B.; Carmo-Fonseca, M.; Cabral, J.M.S. Scalable Generation of Mature Cerebellar Organoids from Human Pluripotent Stem Cells and Characterization by Immunostaining. J. Vis. Exp. 2020, 160, e61143. [Google Scholar] [CrossRef]
- Nayler, S.; Agarwal, D.; Curion, F.; Bowden, R.; Becker, E.B.E. High-resolution transcriptional landscape of xeno-free human induced pluripotent stem cell-derived cerebellar organoids. Sci. Rep. 2021, 11, 12959. [Google Scholar] [CrossRef] [PubMed]
- Atamian, A.; Birtele, M.; Hosseini, N.; Nguyen, T.; Seth, A.; Del Dosso, A.; Paul, S.; Tedeschi, N.; Taylor, R.; Coba, M.P.; et al. Human cerebellar organoids with functional Purkinje cells. Cell Stem Cell 2024, 31, 39–51.e6. [Google Scholar] [CrossRef] [PubMed]
- Gadot, R.; Smith, D.N.; Prablek, M.; Grochmal, J.K.; Fuentes, A.; Ropper, A.E. Established and Emerging Therapies in Acute Spinal Cord Injury. Neurospine 2022, 19, 283–296. [Google Scholar] [CrossRef]
- Meinhardt, A.; Eberle, D.; Tazaki, A.; Ranga, A.; Niesche, M.; Wilsch-Brauninger, M.; Stec, A.; Schackert, G.; Lutolf, M.; Tanaka, E.M. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. 2014, 3, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Girgin, M.; Meinhardt, A.; Eberle, D.; Caiazzo, M.; Tanaka, E.M.; Lutolf, M.P. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl. Acad. Sci. USA 2016, 113, E6831–E6839. [Google Scholar] [CrossRef]
- Zhuo, Y.; Ai, K.; He, K.; Wu, B.; Peng, J.; Xiang, J.; Zhang, G.; Jiao, Z.; Zhou, R.; Zhang, H. Global Research Trends of Exosomes in the Central Nervous System: A Bibliometric and Visualized Analysis. Neurospine 2023, 20, 507–524. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Williams, C.E.; Ruhl, D.A.; Estevez-Silva, M.C.; Chapman, E.R.; Coon, J.J.; Ashton, R.S. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep. 2015, 4, 632–644. [Google Scholar] [CrossRef]
- Ogura, T.; Sakaguchi, H.; Miyamoto, S.; Takahashi, J. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 2018, 145, dev162214. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e26. [Google Scholar] [CrossRef] [PubMed]
- Mouilleau, V.; Vaslin, C.; Robert, R.; Gribaudo, S.; Nicolas, N.; Jarrige, M.; Terray, A.; Lesueur, L.; Mathis, M.W.; Croft, G.; et al. Dynamic extrinsic pacing of the HOX clock in human axial progenitors controls motor neuron subtype specification. Development 2021, 148, dev194514. [Google Scholar] [CrossRef]
- Musso, D.; Baud, D.; Gubler, D.J. Zika virus: What do we know? Clin. Microbiol. Infect. 2016, 22, 494–496. [Google Scholar] [CrossRef]
- Karzbrun, E.; Khankhel, A.H.; Megale, H.C.; Glasauer, S.M.K.; Wyle, Y.; Britton, G.; Warmflash, A.; Kosik, K.S.; Siggia, E.D.; Shraiman, B.I.; et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 2021, 599, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Ryu, J.R.; Kim, B.G.; Sun, W. Establishment and Validation of a Model for Fetal Neural Ischemia Using Necrotic Core-Free Human Spinal Cord Organoids. Stem Cells Transl. Med. 2023, 13, 268–277. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19, 258–265. [Google Scholar] [CrossRef]
- Xu, Y.P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.R.; et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Bloom, D.C.; Naciri, J.N.; Smith, A.; Edwards, T.G.; McClain, L.; Callio, J.A.; Jessup, M.; Wood, J.; Chowdari, K.; et al. Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Krenn, V.; Bosone, C.; Burkard, T.R.; Spanier, J.; Kalinke, U.; Calistri, A.; Salata, C.; Rilo Christoff, R.; Pestana Garcez, P.; Mirazimi, A.; et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 2021, 28, 1362–1379.e7. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Wu, C.Y.; Lin, Y.H.; Huang, Y.C.; Yang, W.C.; Chen, T.W.; Ma, W.L.; Lin, W.H.; Hsu, F.M.; Xiao, F.; et al. Delay of Surgery for Spinal Metastasis due to the COVID-19 Outbreak Affected Patient Outcomes. Neurospine 2023, 20, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Z.; Chu, H.; Han, S.; Shuai, H.; Deng, J.; Hu, Y.F.; Gong, H.R.; Lee, A.C.; Zou, Z.; Yau, T.; et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020, 30, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Fair, S.R.; Schwind, W.; Julian, D.L.; Biel, A.; Guo, G.; Rutherford, R.; Ramadesikan, S.; Westfall, J.; Miller, K.E.; Kararoudi, M.N.; et al. Cerebral organoids containing an AUTS2 missense variant model microcephaly. Brain 2023, 146, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Ramani, A.; Altinisik, N.; Gopalakrishnan, J. Human Brain Organoids to Decode Mechanisms of Microcephaly. Front. Cell Neurosci. 2020, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Sievert, D.; Smith, D.E.C.; Mendes, M.I.; Chen, D.Y.; Stanley, V.; Ghosh, S.; Wang, Y.; Kara, M.; et al. Loss of NARS1 impairs progenitor proliferation in cortical brain organoids and leads to microcephaly. Nat. Commun. 2020, 11, 4038. [Google Scholar] [CrossRef]
- Li, R.; Sun, L.; Fang, A.; Li, P.; Wu, Q.; Wang, X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell 2017, 8, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, S.L.; Yang, M.; Herrlinger, S.; Shao, Q.; Collar, J.L.; Fierro, E.; Shi, Y.; Liu, A.; Lu, H.; et al. Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors. Nat. Commun. 2019, 10, 2612. [Google Scholar] [CrossRef]
- Dhaliwal, N.; Choi, W.W.Y.; Muffat, J.; Li, Y. Modeling PTEN overexpression-induced microcephaly in human brain organoids. Mol. Brain 2021, 14, 131. [Google Scholar] [CrossRef]
- Quadrato, G.; Brown, J.; Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 2016, 22, 1220–1228. [Google Scholar] [CrossRef]
- Urresti, J.; Zhang, P.; Moran-Losada, P.; Yu, N.K.; Negraes, P.D.; Trujillo, C.A.; Antaki, D.; Amar, M.; Chau, K.; Pramod, A.B.; et al. Cortical organoids model early brain development disrupted by 16p11.2 copy number variants in autism. Mol. Psychiatry 2021, 26, 7560–7580. [Google Scholar] [CrossRef]
- de Jong, J.O.; Llapashtica, C.; Genestine, M.; Strauss, K.; Provenzano, F.; Sun, Y.; Zhu, H.; Cortese, G.P.; Brundu, F.; Brigatti, K.W.; et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder. Nat. Commun. 2021, 12, 4087. [Google Scholar] [CrossRef]
- Jourdon, A.; Wu, F.; Mariani, J.; Capauto, D.; Norton, S.; Tomasini, L.; Amiri, A.; Suvakov, M.; Schreiner, J.D.; Jang, Y.; et al. Modeling idiopathic autism in forebrain organoids reveals an imbalance of excitatory cortical neuron subtypes during early neurogenesis. Nat. Neurosci. 2023, 26, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Hengel, H.; Bosso-Lefevre, C.; Grady, G.; Szenker-Ravi, E.; Li, H.; Pierce, S.; Lebigot, E.; Tan, T.T.; Eio, M.Y.; Narayanan, G.; et al. Loss-of-function mutations in UDP-Glucose 6-Dehydrogenase cause recessive developmental epileptic encephalopathy. Nat. Commun. 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Borghi, R.; Magliocca, V.; Petrini, S.; Conti, L.A.; Moreno, S.; Bertini, E.; Tartaglia, M.; Compagnucci, C. Dissecting the Role of PCDH19 in Clustering Epilepsy by Exploiting Patient-Specific Models of Neurogenesis. J. Clin. Med. 2021, 10, 2754. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.J.; Repudi, S.; Saleem, A.; Kustanovich, I.; Viukov, S.; Abudiab, B.; Banne, E.; Mahajnah, M.; Hanna, J.H.; Stern, S.; et al. Modeling genetic epileptic encephalopathies using brain organoids. EMBO Mol. Med. 2021, 13, e13610. [Google Scholar] [CrossRef] [PubMed]
- Negraes, P.D.; Trujillo, C.A.; Yu, N.K.; Wu, W.; Yao, H.; Liang, N.; Lautz, J.D.; Kwok, E.; McClatchy, D.; Diedrich, J.; et al. Altered network and rescue of human neurons derived from individuals with early-onset genetic epilepsy. Mol. Psychiatry 2021, 26, 7047–7068. [Google Scholar] [CrossRef]
- Vieira de Sa, R.; Canizares Luna, M.; Pasterkamp, R.J. Advances in Central Nervous System Organoids: A Focus on Organoid-Based Models for Motor Neuron Disease. Tissue Eng. Part. C Methods 2021, 27, 213–224. [Google Scholar] [CrossRef]
- Raja, W.K.; Mungenast, A.E.; Lin, Y.T.; Ko, T.; Abdurrob, F.; Seo, J.; Tsai, L.H. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS ONE 2016, 11, e0161969. [Google Scholar] [CrossRef]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. Elife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Alic, I.; Goh, P.A.; Murray, A.; Portelius, E.; Gkanatsiou, E.; Gough, G.; Mok, K.Y.; Koschut, D.; Brunmeir, R.; Yeap, Y.J.; et al. Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol. Psychiatry 2021, 26, 5766–5788. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021, 8, e2101462. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Mo, H.; Kim, J.; Kim, J.W.; Nam, Y.; Rim, Y.A.; Ju, J.H. Mitigating Effect of Estrogen in Alzheimer’s Disease-Mimicking Cerebral Organoid. Front. Neurosci. 2022, 16, 816174. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, K.; Stopperan, J.A.; Hauger, B.M.; Troutwine, B.R.; Lysaker, C.R.; Strope, T.A.; Csikos Drummond, V.; Gilmore, C.A.; Swerdlow, N.A.; Draper, J.M.; et al. Cell type and sex specific mitochondrial phenotypes in iPSC derived models of Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 1201015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Oh, J.K.; Kim, T.Y.; Kim, E.H.; Lee, H.S.; Yu, N.G.; Choi, G.H.; Yi, S.; Ha, Y.; Yoon, D.H.; et al. Surgical Outcomes of Spine Disorders in Patients with Parkinson’s Disease. Korean J. Spine 2011, 8, 208–214. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Park. Dis. 2019, 5, 5. [Google Scholar] [CrossRef]
- Wulansari, N.; Darsono, W.H.W.; Woo, H.J.; Chang, M.Y.; Kim, J.; Bae, E.J.; Sun, W.; Lee, J.H.; Cho, I.J.; Shin, H.; et al. Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease-linked DNAJC6 mutations. Sci. Adv. 2021, 7, eabb1540. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Mukherjee, A.; Ramirez, S.; Sepulveda, S.; Sinha, T.; Al-Lahham, R.; De Gregorio, N.; Gherardelli, C.; Soto, C. Lewy Body-like Pathology and Loss of Dopaminergic Neurons in Midbrain Organoids Derived from Familial Parkinson’s Disease Patient. Cells 2023, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ooi, J.; Utami, K.H.; Langley, S.R.; Aning, O.A.; Park, D.S.; Renner, M.; Ma, S.; Cheok, C.F.; Knoblich, J.A.; et al. Expanded huntingtin CAG repeats disrupt the balance between neural progenitor expansion and differentiation in human cerebral organoids. bioRxiv 2019, 850586. [Google Scholar] [CrossRef]
- Lisowski, P.; Lickfett, S.; Rybak-Wolf, A.; Le, S.; Dykstra, W.; Mlody, B.; Menacho, C.; Roth, P.; Richter, Y.; Kulka, L.A.M.; et al. Mutant Huntingtin impairs neurodevelopment in human brain organoids through CHCHD2-mediated neurometabolic failure. bioRxiv 2023. [Google Scholar] [CrossRef]
- Metzger, J.J.; Pereda, C.; Adhikari, A.; Haremaki, T.; Galgoczi, S.; Siggia, E.D.; Brivanlou, A.H.; Etoc, F. Deep-learning analysis of micropattern-based organoids enables high-throughput drug screening of Huntington’s disease models. Cell Rep. Methods 2022, 2, 100297. [Google Scholar] [CrossRef] [PubMed]
- Buchner, F.; Dokuzluoglu, Z.; Grass, T.; Rodriguez-Muela, N. Spinal Cord Organoids to Study Motor Neuron Development and Disease. Life 2023, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Oh, S.H.; Jeong, Y.H.; Park, S.M.; Jeon, H.S.; Kim, H.C.; An, S.B.; Shin, D.A.; Yi, S.; Kim, K.N.; et al. Surgical Strategies for Cervical Deformities Associated With Neuromuscular Disorders. Neurospine 2020, 17, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.D.; DuBreuil, D.M.; Devlin, A.C.; Held, A.; Sapir, Y.; Berezovski, E.; Hawrot, J.; Dorfman, K.; Chander, V.; Wainger, B.J. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat. Commun. 2021, 12, 4744. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Ross, J.P.; Alipour, P.; Castonguay, C.E.; Li, B.; Catoire, H.; Rochefort, D.; Urushitani, M.; Takahashi, R.; Sonnen, J.A.; et al. Spinal cord extracts of amyotrophic lateral sclerosis spread TDP-43 pathology in cerebral organoids. PLoS Genet. 2023, 19, e1010606. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Srivastava, A.; Srivastava, A.K.; Pandeya, A.; Vatsa, P.; Ansari, U.A.; Pant, A.B. Proteome architecture of human-induced pluripotent stem cell-derived three-dimensional organoids as a tool for early diagnosis of neuronal disorders. Indian J. Pharmacol. 2023, 55, 108–118. [Google Scholar] [CrossRef]
- Chow, S.Y.A.; Nakanishi, Y.; Kaneda, S.; Ikeuchi, Y. Modeling Axonal Degeneration Using Motor Nerve Organoids. Methods Mol. Biol. 2022, 2515, 89–97. [Google Scholar] [CrossRef]
- Abou-Mrad, Z.; Bou Gharios, J.; Moubarak, M.M.; Chalhoub, A.; Moussalem, C.; Bahmad, H.F.; Abou-Kheir, W. Central nervous system tumors and three-dimensional cell biology: Current and future perspectives in modeling. World J. Stem Cells 2021, 13, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Duan, W.; Guan, J.; Wang, K.; Liu, Z.; Wang, X.; Wang, Z.; Wu, H.; Chen, Z.; Jian, F. Detection of Glioma-Related Hotspot Mutations Through Sequencing of Cerebrospinal Fluid (CSF)-Derived Circulating Tumor DNA: A Pilot Study on CSF-Based Liquid Biopsy for Primary Spinal Cord Astrocytoma. Neurospine 2023, 20, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211.e5. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.; Mathew, R.K.; Polson, E.S.; Williams, J.; Wurdak, H. Spontaneous Glioblastoma Spheroid Infiltration of Early-Stage Cerebral Organoids Models Brain Tumor Invasion. SLAS Discov. 2018, 23, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Krieger, T.G.; Tirier, S.M.; Park, J.; Jechow, K.; Eisemann, T.; Peterziel, H.; Angel, P.; Eils, R.; Conrad, C. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro Oncol. 2020, 22, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.J.; Shakya, S.; Barnett, A.; Wallace, L.C.; Jeon, H.; Sloan, A.; Recinos, V.; Hubert, C.G. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol. 2022, 15, 101251. [Google Scholar] [CrossRef] [PubMed]
- Lago, C.; Gianesello, M.; Santomaso, L.; Leva, G.; Ballabio, C.; Anderle, M.; Antonica, F.; Tiberi, L. Medulloblastoma and high-grade glioma organoids for drug screening, lineage tracing, co-culture and in vivo assay. Nat. Protoc. 2023, 18, 2143–2180. [Google Scholar] [CrossRef]
- Ballabio, C.; Anderle, M.; Gianesello, M.; Lago, C.; Miele, E.; Cardano, M.; Aiello, G.; Piazza, S.; Caron, D.; Gianno, F.; et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat. Commun. 2020, 11, 583. [Google Scholar] [CrossRef]
- van Essen, M.J.; Apsley, E.J.; Riepsaame, J.; Xu, R.; Northcott, P.A.; Cowley, S.A.; Jacob, J.; Becker, E.B.E. PTCH1-mutant human cerebellar organoids exhibit altered neural development and recapitulate early medulloblastoma tumorigenesis. Dis. Model. Mech. 2024, 17, dmm050323. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Todhunter, M.E.; LaBarge, M.A.; Gartner, Z.J. Opportunities for organoids as new models of aging. J. Cell Biol. 2018, 217, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hyun, I.; Scharf-Deering, J.C.; Lunshof, J.E. Ethical issues related to brain organoid research. Brain Res. 2020, 1732, 146653. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Cell Source | Region Designed | Significance |
|---|---|---|---|
| Lancaster et al. (2013, 2014) [20,31] | hPSC | Heterogenous | -First organoid -Designed microcephaly |
| LaMonica et al. (2013) [33] | Fetal brain tissue | Neocortex, radial glia | Discovery of radial glia division and the involved pathways |
| Quadrato et al. (2017) [34] | hPSC | Cerebral cortex | Prolonged culture of cerebral organoids led to presented diverse cells |
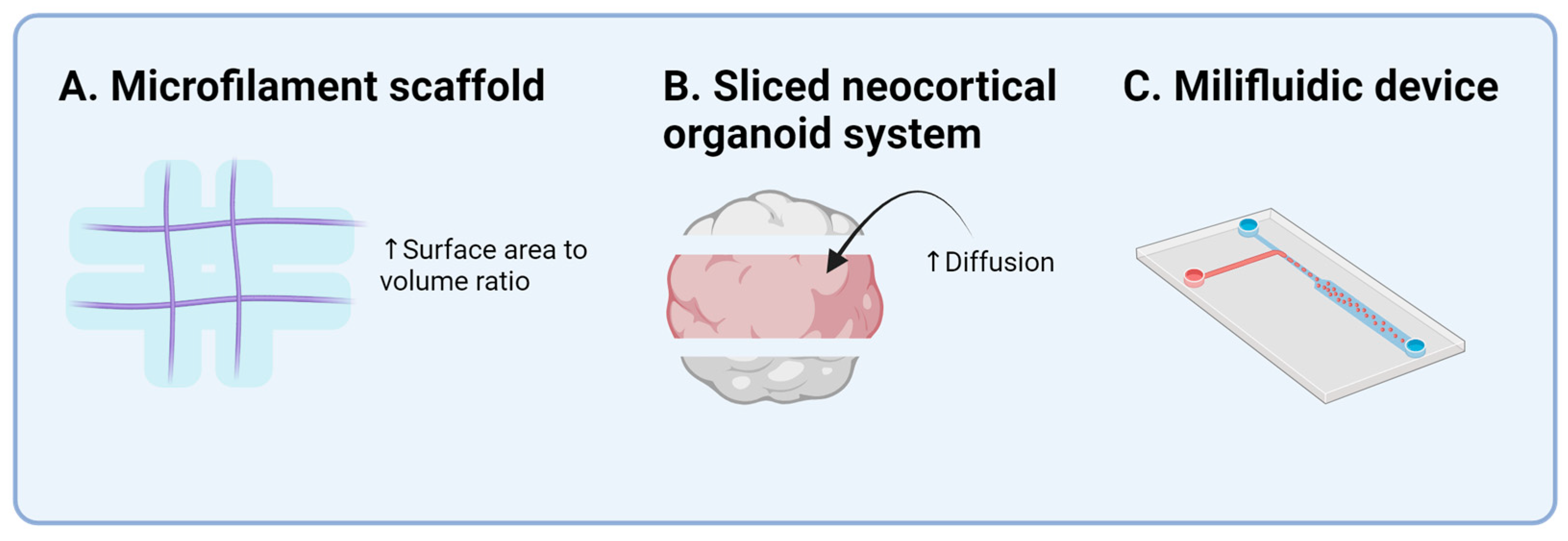
| Lancaster et al. (2017) [35] | hiPSC/hESC | Cerebral cortex | Reproducible cerebral organoids with microfilament device |
| Velasco et al. (2019) [36] | hiPSC | Cerebral cortex | Developed consistent ways to generate organoids |
| Pollen et al. (2019) [37] | hiPSC/Chimpanzee-induced PSC | Cerebral cortex | Investigation of organoids among species to analyze differences |
| Qian et al. (2020) [38] | hiPSC | Cerebral cortex | Developed sliced neocortical organoid system |
| Uzquiano et al. (2022) [39] | hESC/hiPSC | Cerebral cortex | Single cell analysis of multiple organoids derived from multiple cell sources |
| Author (Year) | Cell Source | Region Designed | Significance |
|---|---|---|---|
| Krefft et al. (2018) [41] | hiPSC | Early dorsal telencephalic tissues | First forebrain organoid |
| Bagley et al. (2017) [42] | hiPSC | Fusion of dorsal and ventral forebrain organoid | -Production of dorsal and ventral forebrain organoid -Ability to study neurite dynamics |
| Cederquist et al. (2019) [43] | hPSC | Anterior forebrain | Positional identity defined |
| Gabriel et al. (2023) [44] | hiPSC | Forebrain with eye primordium | Study of multi-organ association |
| Huang et al. (2021) [45] | hiPSC | “Arcuate organoids” | First efforts to create diencephalon-type forebrain organoid |
| Blaess et al. (2014) [46] | hiPSC | Hypothalamus | Hypothalamus development |
| Corman et al. (2018) [47] | Mice ESC | Hypothalamus | Elucidated Shh signaling and its effects with time |
| Sarrafha et al. (2023) [48] | hPSC | Hypothalamus | Exploration of dopaminergic neurons |
| Xiang et al. (2019) [49] | hESC | Thalamus | Fusion of cortex with thalamus organoid |
| Kiral et al. (2023) [50] | hESC | Thalamus and Thalamic reticular nucleus | Generation of thalamic reticular nucleus |
| Author (Year) | Cell Source | Region Designed | Significance |
|---|---|---|---|
| Jo et al. (2016) [52] | hPSC | Midbrain | Developed midbrain organoids with dopamine neurons that form synapses and display electrophysiological properties |
| Monzel et al. (2009) [53] | Neuroepithelial stem cells | Midbrain | Glial cell differentiation |
| Kwak et al. (2020) [54] | hESC | Midbrain | Defining protocols to produce homogenous midbrain organoids |
| Nickels et al. (2020) [55] | hiPSC | Midbrain | Further tested protocols to generate organoids and reduce necrotic cores |
| Berger et al. (2018) [56] | Neuroepithelial stem cells | Midbrain | Introduced milifluidic device to reduce necrotic regions in long term culture |
| Renner et al. (2020) [57] | hPSC | Midbrain | Generated automated system for seeding, imaging, and analysis |
| Author (Year) | Cell Source | Region Designed | Significance |
|---|---|---|---|
| Kamei et al. (2023) [59] | hiPSC | Cerebellum | Investigated cerebellar progenitors through cell transplantation |
| Muguruma et al. (2015, 2018. 2020) [58,60,61] | hiPSC | Cerebellum, Purkinje cells | Ability to model cerebellar diseases |
| Silva et al. (2020) [63] | hiPSC | Cerebellum | Use of a single bioreactor to mass-produce homogenous cerebellar organoids |
| Nayler et al. (2021) [64] | hiPSC | Cerebellum | Air–liquid interface culture without SDF1 and FGF19 |
| Chen et al. (2023) [8] | hiPSC | Cerebellum | Long-term culture of cerebellar organoids |
| Atamian et al. (2024) [65] | hiPSC | Cerebellum | Analysis of cellular composition and spatial characteristics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, M.; Hong, S.J.; Zhang, S.; Yu, Y.; Lee, S.; Shin, H.; Choi, B.H.; Han, I. Morphogenetic Designs, and Disease Models in Central Nervous System Organoids. Int. J. Mol. Sci. 2024, 25, 7750. https://doi.org/10.3390/ijms25147750
Bock M, Hong SJ, Zhang S, Yu Y, Lee S, Shin H, Choi BH, Han I. Morphogenetic Designs, and Disease Models in Central Nervous System Organoids. International Journal of Molecular Sciences. 2024; 25(14):7750. https://doi.org/10.3390/ijms25147750
Chicago/Turabian StyleBock, Minsung, Sung Jun Hong, Songzi Zhang, Yerin Yu, Somin Lee, Haeeun Shin, Byung Hyune Choi, and Inbo Han. 2024. "Morphogenetic Designs, and Disease Models in Central Nervous System Organoids" International Journal of Molecular Sciences 25, no. 14: 7750. https://doi.org/10.3390/ijms25147750
APA StyleBock, M., Hong, S. J., Zhang, S., Yu, Y., Lee, S., Shin, H., Choi, B. H., & Han, I. (2024). Morphogenetic Designs, and Disease Models in Central Nervous System Organoids. International Journal of Molecular Sciences, 25(14), 7750. https://doi.org/10.3390/ijms25147750








