Analysis of the Skin and Brain Transcriptome of Normally Pigmented and Pseudo-Albino Southern Flounder (Paralichthys lethostigma) Juveniles to Study the Molecular Mechanisms of Hypopigmentation and Its Implications for Species Survival in the Natural Environment
Abstract
:1. Introduction
2. Results
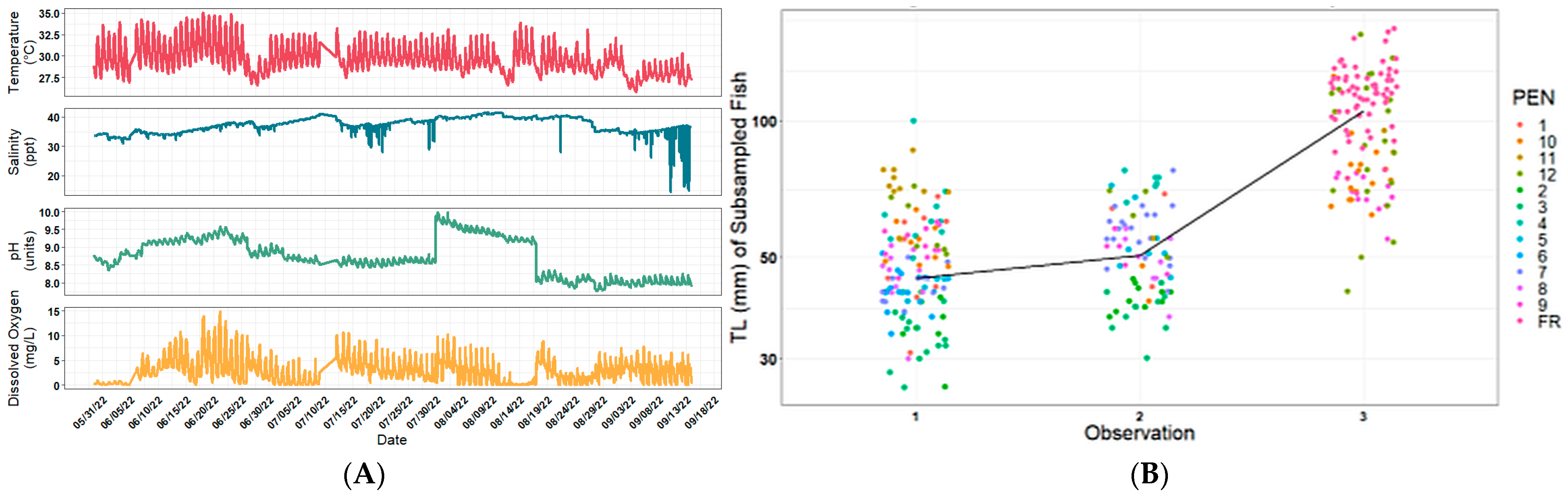
2.1. Environmental Parameters and Pseudo-Albino Phenotype Occurrence
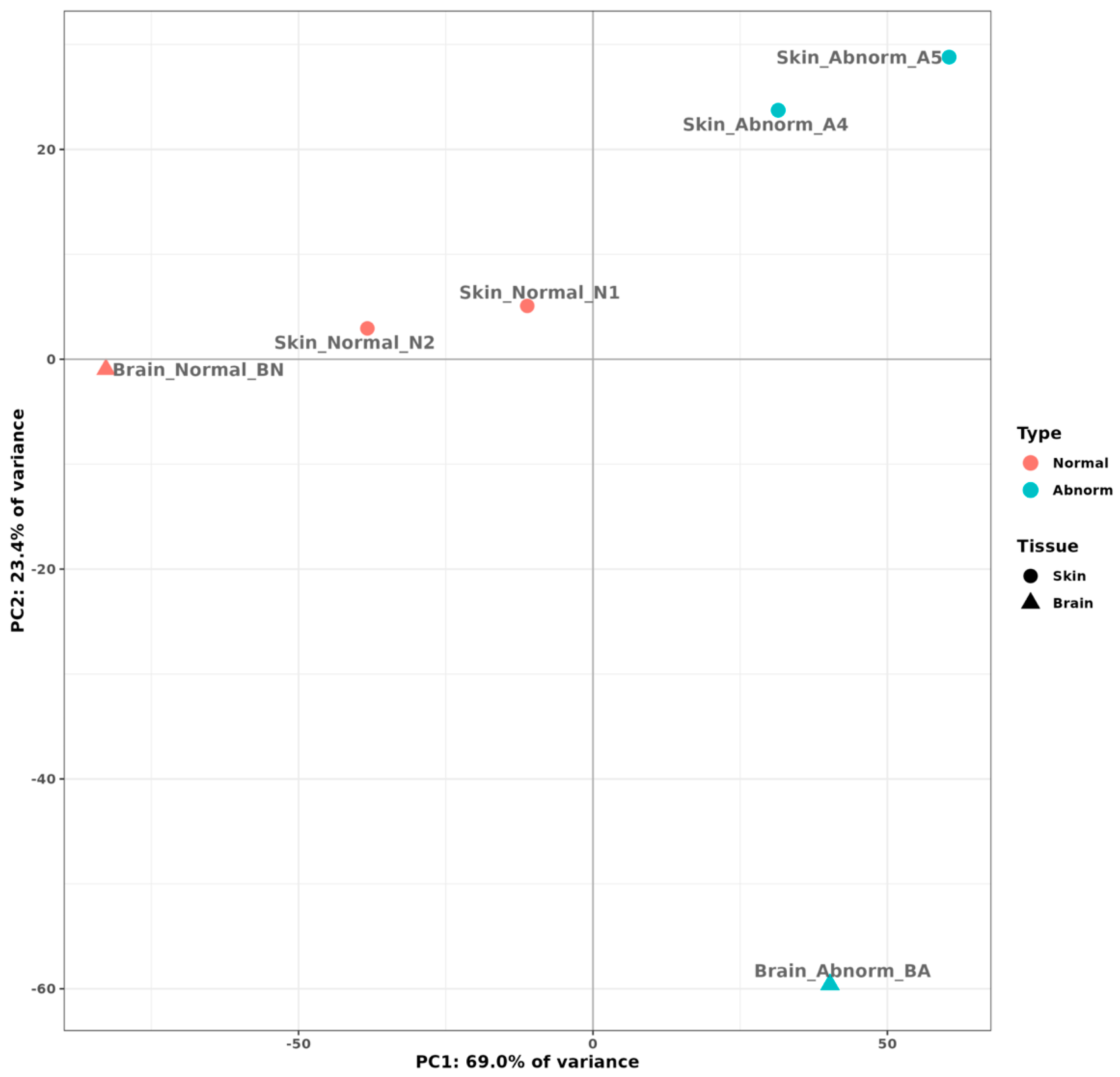
2.2. Transcriptome Comparison between Normally Pigmented and Pseudo-Albino Southern Flounders
2.2.1. Hallmark Pathways Enriched in Pseudo-Albino Southern Flounder
2.2.2. Enriched Pathways in the Pseudo-Albino Skin
2.2.3. Enriched Pathways in the Pseudo-Albino Brain
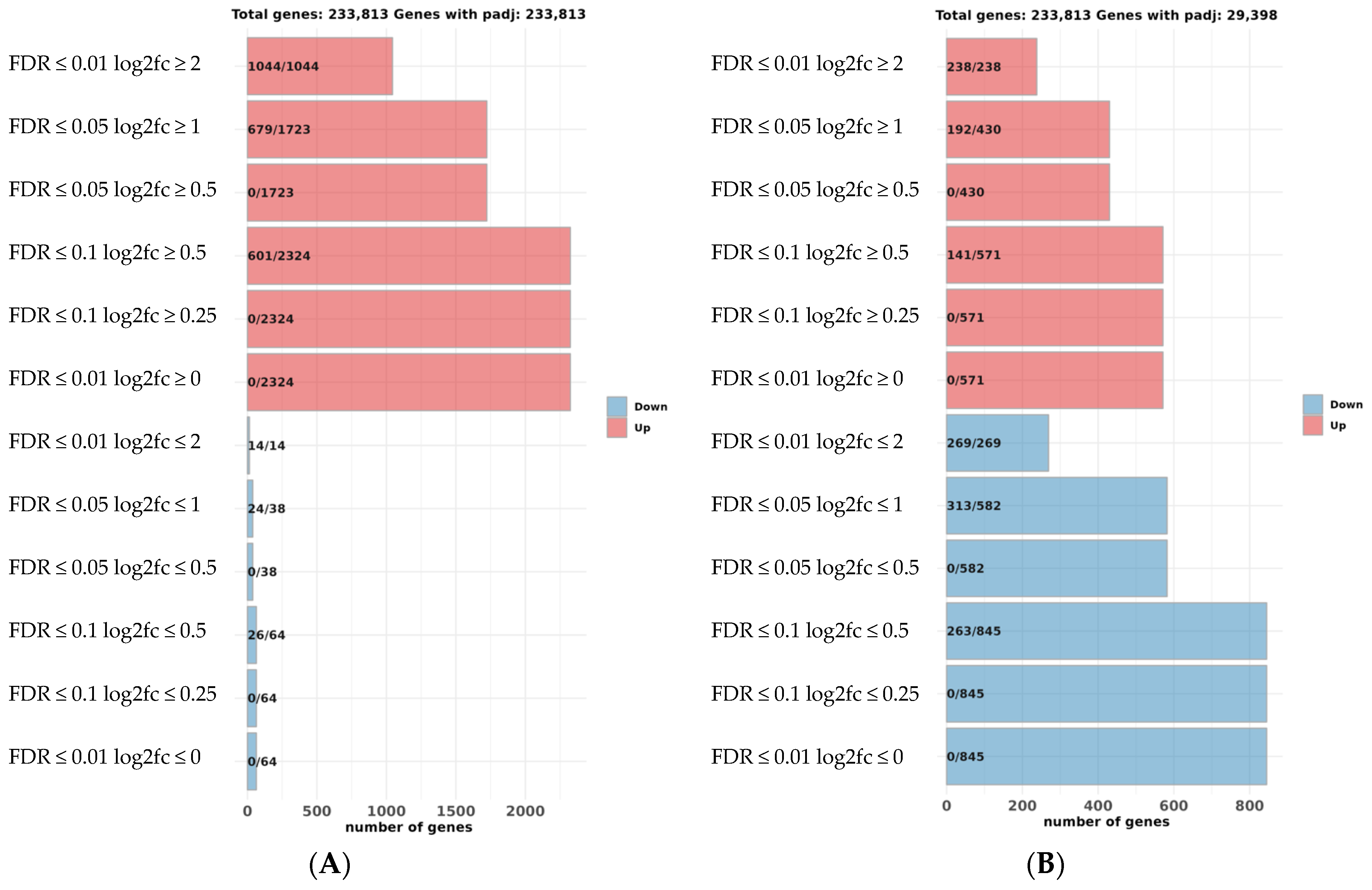
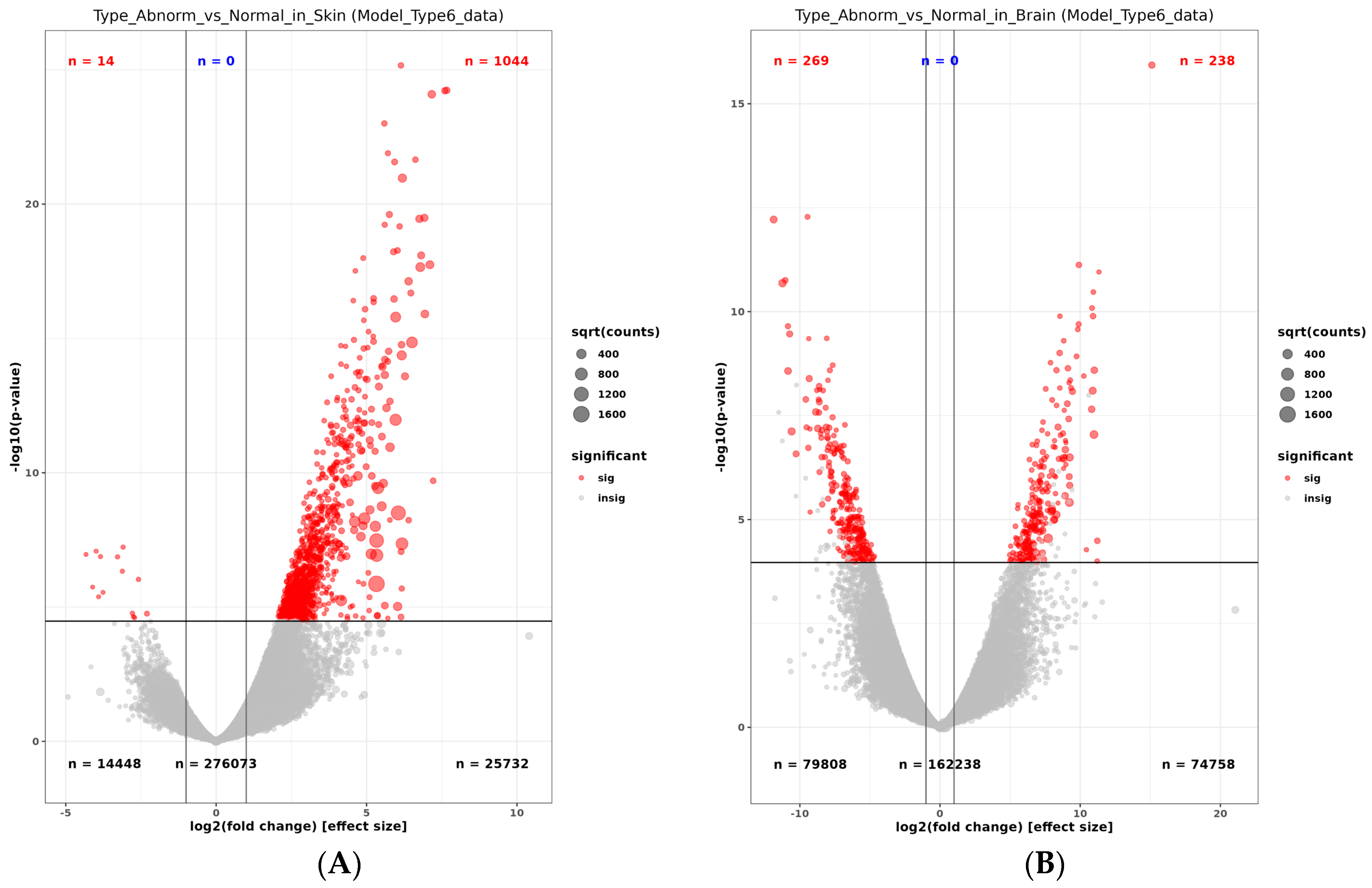
2.2.4. Differently Expressed Genes in the Pseudo-Albino Skin
2.2.5. Differently Expressed Genes in the Pseudo-Albino Brain
3. Discussion
3.1. Molecular Deregulation in the Pseudo-Albino Skin
3.2. Molecular Deregulation in the Pseudo-Albino Brain
4. Materials and Methods
4.1. Flounder Husbandry
4.2. Tissue Sampling and Transcriptome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugimoto, M.; Yuki, M.; Miyakoshi, T.; Maruko, K. The influence of long-term chromatic adaptation on pigment cells and striped pigment patterns in the skin of the zebrafish, Danio rerio. J. Exp. Zool. Comp. Exp. Biol. 2005, 303, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zheng, D.; Yang, Z.; Shi, L.; Lu, X.; Yao, F.; Liang Lei Wang, L.; Wang, X.; Chen, H.; Sun, H.; et al. Weighted correlation network analysis of the genes in the eyes of juvenile Plectropomus leopardus provides novel insights into the molecular mechanisms of the adaptation to the background color. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101123. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Schulter, D. Colour plasticity and background matching in a Threespine Stickleback species pair. Biol. J. Linn. Soc. 2011, 102, 902–914. [Google Scholar] [CrossRef]
- Kelley, J.; Merilaita, S. Testing the role of background matching and self-shadow concealment in explaining countershading coloration in wild-caught rainbowfish. Biol. J. Linn. Soc. 2015, 114, 915–928. [Google Scholar] [CrossRef]
- Galloway, J.; Green, S.; Stevens, M.; Kelley, L. Finding a signal hidden among noise: How can predators overcome camouflage strategies? Philos. Trans. R. Soc. B 2020, 375, 20190478. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R. Coloration and chromatophores. In The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 1993; pp. 535–562. [Google Scholar]
- Kobayashi, Y.; Hamamoto, A.; Takahashi Saito, Y. Dimerization of melanocortin receptor 1 (MC1R) and MC5R creates a ligand-dependent signal modulation: Potential participation in physiological color change in the Flounder. Gen. Comp. Endocrinol. 2016, 230, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang ZGuo, Y.; Liu, L.; Yu, J.; Zhang, S.; Liu, S.; Liu, C. Morphological characters and transcriptome profiles associated with black skin and red skin in Crimson Snapper (Lutjanus erythropterus). Int. J. Mol. Sci. 2015, 16, 26991–27004. [Google Scholar] [CrossRef]
- Goda, M.; Fujii, R. Blue chromatophores in two species of callionymid fish. Zool. Sci. 1995, 12, 811–813. [Google Scholar] [CrossRef]
- Pinto, P.; Guerreiro, C.; Costa, R.; Martinez-Blanch, J.; Carballo, C.; Codoñer, F.; Manchado, M.; Power, D. Understanding pseudo-albinism in the sole (Solea senegalensis): A transcriptomics and metagenomics approach. Sci. Rep. 2019, 9, 13604. [Google Scholar] [CrossRef]
- Vissio, P.; Darias, M.; DiYorio, M.; Pérez, D.; Sirkin Delgadin, T. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. Gen. Comp. Endocrinol. 2021, 301, 113662. [Google Scholar] [CrossRef]
- Braasch, I.; Volff, J.; Schartl, M. The evolution of teleost pigmentation and the fish-specific genome duplication. J. Fish Biol. 2008, 73, 1891–1918. [Google Scholar] [CrossRef]
- Aritaki, M.; Tagawa, M. Pseudoalbinism and ambicoloration in hatchery-reared pleuronectids as malformations of asymmetrical formation. Fish Sci. 2012, 78, 327–335. [Google Scholar] [CrossRef]
- Long, Y.; Li, Q.; Zhou, B.; Song, G.; Li, T.; Cui, Z. De Novo assembly of Mud Loach (Misgurnus anguillicaudatus) skin transcriptome to identify putative genes involved in immunity and epidermal mucus. PLoS ONE 2013, 8, e56998. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.; Kanazawa, A. Fatty Acid Composition of Neural Tissues of Normally Pigmented and Unpigmented Juveniles of Japanese Flounder Using Rotifer and Artemia Enriched in n-3 HUFA. Fish. Sci. 1996, 62, 88–93. [Google Scholar]
- Villalta, M.; Estevez, A.; Bransden, M.; Bell, J. Arachidonic acid, arachidonic/eicosapentaenoic acid ratio, stearidonic acid and eicosanoids are involved in dietary-induced albinism in Senegal sole (Solea senegalensis). Aquac. Nutr. 2008, 14, 120–128. [Google Scholar] [CrossRef]
- Boglino, A.; Wishkerman, A.; Darias MDe la Iglesia, P.; Estévez, A.; Andree, K.; Gisbert, E. The effects of dietary arachidonic acid on Senegalese Sole morphogenesis: A synthesis of recent findings. Aquaculture 2014, 432, 443–452. [Google Scholar] [CrossRef]
- Lund, I.; Steenfeldt, S.; Banta, G.; Hansen, B. The influence of dietary concentrations of arachidonic acid and eicosapentaenoic acid at various stages of larval ontogeny on eye migration, pigmentation, and prostaglandin content of common sole larvae (Solea solea L.). Aquaculture 2008, 276, 143–153. [Google Scholar] [CrossRef]
- Vizcaíno-Ochoa, V.; Lazo, J.; Barón-Sevilla, B.; Drawbridge, M. The effect of dietary docosahexaenoic acid (DHA) on growth, survival, and pigmentation of California Halibut Paralichthys californicus larvae (Ayres, 1810). Aquaculture 2010, 302, 228–234. [Google Scholar] [CrossRef]
- Bohorez-Cruz, M.; Rodriguez, S.; Sonnenholzer, S.; Arguello-Guevara, W. Early development and juvenile culture technique of Speckled Flounder Paralichthys woolmani (Jordan& William, 1897) under ambient seawater. J. Appl. Ichthyol. 2018, 34, 610–616. [Google Scholar]
- Kanazawa, A. Nutritional mechanisms involved in the occurrence of abnormal pigmentation in hatchery-reared flatfish. J. World Aquac. Soc. 1993, 24, 162–166. [Google Scholar] [CrossRef]
- Bolker, J.; Hill, C. Pigmentation development in hatchery-reared flatfishes. J. Fish Biol. 2005, 56, 1029–1052. [Google Scholar]
- Fisher, M. Stock assessment of Southern Flounder (Paralichthys lethostigma) in Texas waters. In The Flounder Fishery of the Gulf of Mexico, United States: A Regional Management Plan; VanderKooy, S.J., Ed.; Gulf States Marine Fisheries Commission: Ocean Springs, MS, USA, 2000. [Google Scholar]
- Froeschke, B.F.; Sterba-Boatwright, B.; Stunz, G.W. Assessing Southern Flounder Paralichthys lethostigma long-term population trends in the Northern Gulf of Mexico using time series analyses. Fish. Res. 2011, 108, 291–298. [Google Scholar] [CrossRef]
- Martinez-Andrade, F. Trends in Relative Abundance and Size of Selected Finfishes and Shellfishes along the Texas Coast: November 1975–December 2016; Management Data Series No. 293; Texas Parks and Wildlife Coastal Fisheries Division: Austin, TX, USA, 2018; 175p. [Google Scholar]
- Texas Parks and Wildlife Department. State Wildlife Acton Plan for Texas. 2023 Comprehensive Revision. Editor, Kelly Conrad Simon, State Wildlife Action Plan Coordinator. Austin, Texas. Online SWAP: Texas. 2023. Available online: https://tpwd.texas.gov/wildlife/wildlife-diversity/swap/ (accessed on 7 July 2024).
- Araki, H.; Berejikian, B.A.; Ford, M.J.; Blouin, M.S. The fitness of hatchery-reared salmonids in the wild. Evol. Appl. 2008, 2, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, K.; Che, J.; Zhao, N.; Jia, L.; Zhao, D.; Huang, Y.; Liao, Y.; He, X.; Gong, X.; et al. Single-nucleotide polymorphisms responsible for pseudo-albinism and hypermelanosis in Japanese flounder (Paralichthys olivaceus) and reveal two genes related to malpigmentation. Fish Physiol. Biochem. 2021, 47, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yoo, J.; Takeuchi, T.; Tagawa, M.; Seikai, T. Effect of Thyroid Hormones on the Stage-specific Pigmentation of the Japanese Flounder Paralichthys olivaceus. Zool. Sci. 2000, 17, 1101–1106. [Google Scholar]
- Mizusawa, K.; Kobayashi, Y.; Yamanome, Y.; Saito, Y.; Takahashi, A. Interrelation between the melanocyte-stimulating hormone and melanin-concentrating hormone in physiological body color change: Roles emerging from barfin flounder Verasper moseri. Gen. Comp. Endocrinol. 2013, 181, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Denson, M.; Smith, T. Diet and light intensity affect survival, growth, and pigmentation of Southern Flounder Paralichthys lethostigma. J. World Aquac. Soc. 1997, 28, 366–373. [Google Scholar] [CrossRef]
- Luo, M.; Lu, G.; Yin, H.; Wang, L.; Atuganile, M.; Dong, Z. Fish pigmentation and coloration: Molecular mechanisms and aquaculture perspectives. Rev. Aquac. 2021, 13, 2395–2412. [Google Scholar] [CrossRef]
- Kittilsen, J.; Schjolden, I.; Beitnes-Johansen, J.; Shaw Pottinger, T.; Sørensen, T.; Braastad, B.; Bakken, M.; Øverli, O. Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm. Behav. 2009, 56, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Zhu, W.B.; Yang, J.; Miao, L.H.; Dong, J.J.; Song, F.B.; Dong, Z.J. Effects of dietary cystine and tyrosine on melanogenesis pathways involved in skin color differentiation of Malaysian red tilapia. Aquaculture 2018, 490, 149–155. [Google Scholar] [CrossRef]
- Cal, L.; Suarez-Bregua, P.; Cerdá-Reverter, L.; Braasch, I.; Rotllant, J. Fish pigmentation and the melanocortin system. Comp. Biochem. Physiol. 2017, 211, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhu, Y.; Yan, J.; Zhang, L.; Zhu, S.; An, L.; Meng q Zhang, Z.; Wang, X. Full-length transcriptome sequencing analysis reveals differential skin color regulation in snakehead fish Channa argus. Aquac. Fish. 2022, 9, 590–596. [Google Scholar] [CrossRef]
- Malachowicz, M.; Wenne, R.; Burzynski, A. De novo assembly of the sea trout (Salmo trutta) skin transcriptome to identify putative genes involved in the immune response and epidermal mucus secretion. PLoS ONE 2017, 12, e0172282. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Bao, B.; Xie, Z.; Chen, X.; Li, B.; Jia, X.; Yao, Q.; Orti, G.; Li, W.; Li, X.; et al. The genome and transcriptome of Japanese Flounder provide insights into flatfish asymmetry. Nat. Genet. 2017, 49, 119–124. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing genomic data quality and beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef] [PubMed]
- Chao, B.; Li, R.; Wen, Z.; Ge, W.; Shi, Q. Phylogenetic Analysis of Core Melanin Synthesis Genes Provides Novel Insights into the Molecular Basis of Albinism in Fish. Front. Genet. 2021, 12, 707228. [Google Scholar]
- Li, W.; Sancar, A. Methodologies for detecting environmentally induced DNA damage and repair. Environ. Mol. Mutagen. 2020, 7, 664–679. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Xiao, Y.; Gui, B.; Zhu, B.; Xu, G.; Sun, M.; Xiao, J.; Mahboob, S.; Al-Ghanim, K.; et al. Gene Expression Variations of Red—White Skin Coloration in Common Carp (Cyprinus carpio). Int. J. Mol. Sci. 2015, 16, 21310–21329. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodeling in tumor progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Giatagana, E.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ham, S.; Harrison, C.; de Kretser, D.; Wallace, E.M.; Southwick, G.; Temple-Smith, P. Potential treatment of keloid pathogenesis with follistatin 288 by blocking the activin molecular pathway. Exp. Dermatol. 2021, 30, 402–408. [Google Scholar] [CrossRef]
- Haq, F.; Nabeel, A.; Quasim, M. Comparative genomic analysis of collagen gene diversity. Biotech 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review of recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.; Philpott, M.; Kloepper, E.; Paus, R. Human hair follicle organ culture: Theory, application, and perspectives. Exp. Dermatol. 2015, 24, 903–987. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.; Costa, R.; Sadoul, B.; Bégout, M.; Cousin, X.; Canario, A.; Power, D. Thermal imprinting during embryogenesis modifies skin repair in juvenile European sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 2023, 134, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Nyström, L.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- Won, J.; Lee, M.; Kim, M.; Min, K.; Ahn, G.; Han, J.; Shim, J. A potential dermal substitute using decellularized dermis extracellular matrix-derived bio-ink. Artif. Cells Nanomed. Biotechnol. 2019, 47, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Golebiowska, A.; Intravaia, J.; Sathe, V.; Kumbar, S.; Nukavarapu, S. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges, and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.; Baribault, H.; Holmes, D.; Graham, H.; Kadler, K.; Iozzo, R. Targeted disruption of Decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Nikolovska, K.; Renke, J.; Jungmann, O.; Grobe, K.; Iozzo, R.; Zamfir, A.; Seidler, D. A Decorin-deficient matrix affects skin chondroitin/dermatan sulfate levels and keratinocyte function. Matrix Biol. 2014, 35, 91–102. [Google Scholar] [CrossRef]
- Gubbiotti, M.; Vallet, S.; Ricard-Blum, S.; Iozzo, R. Decorin interacting network: A comprehensive analysis of Decorin-binding partners and their versatile functions. Matrix Biol. 2016, 55, 7–21. [Google Scholar] [CrossRef]
- Hirayoshi, K.; Kudo, H.; Takechi, H.; Nakai, A.; Iwamatsu, A.; Yamada, K.; Nagata, K. HSP47: A tissue-specific, transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol. Cell. Biol. 1991, 8, 4036–4044. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, G.; Rieger, S.; Wang, F.; Smolen, F.; Gonzalez, R.; Buchanan, J.; Sagasti, A. Coordinate development of skin cells and cutaneous sensory axons in zebrafish. J. Comp. Neurol. 2011, 520, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Wu, A.; Chen, X.; Tian, Y.; Lin, X. Enolase I, a moonlighting protein, as a potential target for cancer treatment. Int. J. Biol. Sci. 2021, 14, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Satani, N.; Hammoudi, N.; Pisaneschi, F.; Leonard, P.; Maxwell, D. Pomhex: A cell-permeable high potency enolase inhibitor with utility for collateral lethality treatment of cancer. Mol. Cancer Ther. 2017, 16, A39. [Google Scholar] [CrossRef]
- Zhu, X.; Yu Li, B.; Quan, J.; Zeng, Z.; Li, G. Targeting a LncRNA P5848-ENO1 axis inhibits tumor growth in hepatocellular carcinoma. Biosci. Rep. 2019, 39, BSR20180896. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Oehlers, L.; Heater, S.; Booth, R.; Walter, R.; David, W. Proteomic analyses of the Xiphophorus Gordon–Kosswig melanoma model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 55, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Bhide, K.; Bhide, M.; Iversen, M.; Brinchmann, M. Proteomic and structural differences in lumpfish skin among the dorsal, caudal, and ventral regions. Sci. Rep. 2019, 9, 6990. [Google Scholar] [CrossRef] [PubMed]
- Higdon, C.; Mitra, R.; Johnson, S. Gene expression analysis of zebrafish melanocytes, iridophores, and retinal pigmented epithelium reveals indicators of biological function and developmental origin. PLoS ONE 2013, 7, e67801. [Google Scholar] [CrossRef]
- García-Arroyo, R.; Domènech, E.B.; Herrera-Úbeda, C.; Asensi, M.A.; Núñez de Arenas, C.; Cuezva, J.M.; Garcia-Fernàndez, J.; Pallardó, F.V.; Mirra, S.; Marfany, G. Exacerbated Response to Oxidative Stress in the Retinitis Pigmentosa CerklKD/KO Mouse Model Triggers Retinal Degeneration Pathways upon Acute Light Stress. Redox Biol. 2023, 66, 102862. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Anderson, P. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 2002, 30, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Neuffer, S.; Cooper, C. Zebrafish Syndromic Albinism Models as Tools for Understanding and Treating Pigment Cell Disease in Humans. Cancers 2022, 14, 1752. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Hu, J.; Han, Q.; Ge, Z. LSM2 is associated with a poor prognosis and promotes cell proliferation, migration, and invasion in skin cutaneous melanoma. BMC Med. Genom. 2023, 16, 129. [Google Scholar] [CrossRef]
- Sloan, D.; Broz, A.; Sharbrough, J.; Wu, Z. Detecting Rare Mutations and DNA Damage with Sequencing-Based Methods. Trends Biotechnol. 2018, 7, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Arcand-Hoy, L.; Benson, W. Fish Reproduction: An ecologically relevant indicator of endocrine disruption. Environ. Toxicol. Chem. 1998, 17, 7228–7230. [Google Scholar] [CrossRef]
- Golshan, M.; Mohammad, S.; Alavi, H. Androgen signaling in male fishes: Examples of anti-androgenic chemicals that cause reproductive disorders. Theriogenology 2019, 139, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Nakamura, I.; Kondo, S. Pigment cell distributions in different zebrafish tissues, with special reference to the striped pigment pattern. Dev. Dyn. 2005, 234, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Nüsslein-Volhard, C.; Singh, A. How fish color their skin: A paradigm for development and evolution of adult patterns. Bioassays 2017, 39, 1600231. [Google Scholar] [CrossRef]
- Adhikary, S.; Eilers, M. Transcriptional regulation, and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 2005, 6, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Uechi, T.; Nakajima, Y.; Nakao, A.; Torihara, H.; Chakraborty, A.; Inoue, K.; Kenmochi, N. Ribosomal Protein Gene Knockdown Causes Developmental Defects in Zebrafish. PLoS ONE 2006, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Zarbalis, K. The emerging roles of ribosome biogenesis in craniofacial development. Front. Physiol. 2014, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Rami, M.; Khattala, K.; Elmadi, A.; Afifi, M.; Youssef, B. Shah-Waardenburg syndrome. Pan Afr. Med. J. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.C.; Amiel, J.; Lyonnet, S. Molecular bases of human neurocristopathies. Adv. Exp. Med. Biol. 2006, 589, 213–234. [Google Scholar] [CrossRef]
- Knight, R.; Schilling, T. Cranial neural crest and development of the head skeleton. Adv. Exp. Med. Biol. 2006, 589, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Horton, L.; Bushell, M.; Barth-Baus, D.; Tilleray, V.; Clemens, M.; Henshold, J. P53 activation results in rapid dephosphorylation of the elF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase, and inhibition of translation initiation. Oncogene 2002, 21, 5325–5334. [Google Scholar] [CrossRef]
- Johansson, J.; Marie, K.; Lu, Y.; Brombin, A.; Santoriello, C.; Zeng, Z.; Zich, J.; Gautier, P.; von Kriegsheim, A.; Brunsdon, H.; et al. PRL3-DDX21 Transcriptional Control of Endolysosomal Genes Restricts Melanocyte Stem Cell Differentiation. Dev. Cell 2020, 54, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Tong XLiang, F.; Zhang, Y.; Kuok, C.; Zhang, Y.; Liu, X.; Zhu, Z.; Lin, S.; Zhang, B. Eif3ba regulates cranial neural crest development by modulating p53 in zebrafish. Dev. Biol. 2013, 381, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Souto-Neto, J.A.; David, D.D.; Zanetti, G.; Sua-Cespedes, C.; Freret-Meurer, N.V.; Moraes, M.N.; de Assis, L.V.M.; de Lauro Castrucci, A.M. Light-specific wavelengths differentially affect the exploration rate, opercular beat, skin color change, opsin transcripts, and the oxi-redox system of the longsnout seahorse Hippocampus reidi. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 288, 111551. [Google Scholar] [CrossRef]
- Chou, S.; Yen, Y.; Yuan, F.; Zhang, S.; Chong, C. Neuronal Senescence in the Aged Brain. Aging Dis. 2023, 14, 1618–1632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larson, T.A.; Gordon, T.N.; Lau, H.E.; Parichy, D.M. Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation. Dev. Biol. 2010, 346, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, G.; Han, D.; Kangyu, D.; Gu, Z.; Yang, M.; Xu, W.; Zhang, W.; Mai, K. Comparatively study on the insulin-regulated glucose homeostasis through brain-gut peptides in Japanese flounder Paralichthys olivaceus after intraperitoneal and oral administration of glucose. Gen. Comp. Endocrinol. 2018, 266, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, X.; Louro, B.; Li, X.; Canário, A.; Lu, W. Transcriptomics reveals that the caudal neurosecretory system in the olive flounder (Paralichthys olivaceus) is more responsive in bold individuals and to chronic temperature change. Aquaculture 2021, 544, 73703. [Google Scholar] [CrossRef]
- Wallace, R. High metabolic demand in neural tissues: Information and control theory perspectives on the synergism between rate and stability. J. Theor. Biol. 2016, 409, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Circadian vision in zebrafish: From molecule to cell and from neural network to behavior. J. Biol. Rhythm. 2019, 5, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, S.; Huang, W.; Zhang, J. Physiological and pathological functions of βB-Crystallin in multiple organs: A systematic review. Aging 2021, 11, 15674–15687. [Google Scholar] [CrossRef] [PubMed]
- Whal, S.; Engelhardt, M.; Shaupp, P.; Lappe, C.; Ivanov, I. The inner clock blue light sets the human rhythm. J. Biophoton. 2019, 12, e201900102. [Google Scholar] [CrossRef] [PubMed]
- Omura, Y.; Shiozawa, S.; Tabata, K. Proliferation of rod cells in the mature retina of the Japanese Flounder Paralichthys olivaceus. Fish. Sci. 2004, 70, 80–86. [Google Scholar] [CrossRef]
- Abalo, X.; Lagman, D.; Heras, G.; Del Pozo, A.; Eggert, J.; Larhammar, D. Circadian regulation of the phosphodiesterase 6 genes in zebrafish differs between cones and rods: Implications for photopic and scotopic vision. Vis. Res. 2020, 166, 43–51. [Google Scholar] [CrossRef]
- Grabherr, M.; Haas, B.; Yassour, M.; Levin, J.; Thompson, D.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huerta-Cepas, J.; Szlarczyk, D.; Heller, D.; Hernandez-Plaza, A.; Forslund, S.; Cook, H.; Mende, D.; Letunic, I.; Rattei, T.; Jensen, L.; et al. eggNOG 5.0: A hierarchical, functionally, and phylogenetically annotated orthology resource based on 5 on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.; Pimentel, H.; Melsted, P. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Nat. Preced. 2010, 11, R106. [Google Scholar]
- Schurch, N.J.; Schofield, P.; Gierliński, M.; Cole, C.; Sherstnev, A.; Singh, V.; Wrobel, N.; Gharbi, K.; Simpson, G.G.; Owen-Hughes, T.; et al. How Many Biological Replicates Are Needed in an RNA-Seq Experiment and Which Differential Expression Tool Should You Use? RNA 2016, 22, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Subramanian, A.; Tamayo Pablo Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; Mesirov, J.P. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Throvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Vaisin, V.; Kucera, M.; Tannus-Lopez, C.; Rostamaufar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway Enrichment Analysis and visualization of omics using g: Profiler, GSEA, Cytoscape, and Enrichment Map. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Chicco, D.; Agapito, G. Nine quick tips for pathway enrichment analysis. PLoS Comput. Biol. 2022, 18, e1010348. [Google Scholar] [CrossRef]



| Category | Count |
|---|---|
| Total Number of Transcripts | 1,589,613 |
| Gene representation | 952,825 |
| Assembled genes | 162,000 |
| Gene names | 106,916 |
| Unique gene names | 13,844 |
| PFAMs | 47,363 |
| Reads mapped to the assembly | 99.43% |
| N50 | 1778 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blandon, I.R.; DiBona, E.; Battenhouse, A.; Vargas, S.; Mace, C.; Seemann, F. Analysis of the Skin and Brain Transcriptome of Normally Pigmented and Pseudo-Albino Southern Flounder (Paralichthys lethostigma) Juveniles to Study the Molecular Mechanisms of Hypopigmentation and Its Implications for Species Survival in the Natural Environment. Int. J. Mol. Sci. 2024, 25, 7775. https://doi.org/10.3390/ijms25147775
Blandon IR, DiBona E, Battenhouse A, Vargas S, Mace C, Seemann F. Analysis of the Skin and Brain Transcriptome of Normally Pigmented and Pseudo-Albino Southern Flounder (Paralichthys lethostigma) Juveniles to Study the Molecular Mechanisms of Hypopigmentation and Its Implications for Species Survival in the Natural Environment. International Journal of Molecular Sciences. 2024; 25(14):7775. https://doi.org/10.3390/ijms25147775
Chicago/Turabian StyleBlandon, Ivonne R., Elizabeth DiBona, Anna Battenhouse, Sean Vargas, Christopher Mace, and Frauke Seemann. 2024. "Analysis of the Skin and Brain Transcriptome of Normally Pigmented and Pseudo-Albino Southern Flounder (Paralichthys lethostigma) Juveniles to Study the Molecular Mechanisms of Hypopigmentation and Its Implications for Species Survival in the Natural Environment" International Journal of Molecular Sciences 25, no. 14: 7775. https://doi.org/10.3390/ijms25147775






