PTEN Depletion Increases Radiosensitivity in Response to Ataxia Telangiectasia-Related-3 (ATR) Inhibition in Non-Small Cell Lung Cancer (NSCLC)
Abstract
1. Introduction
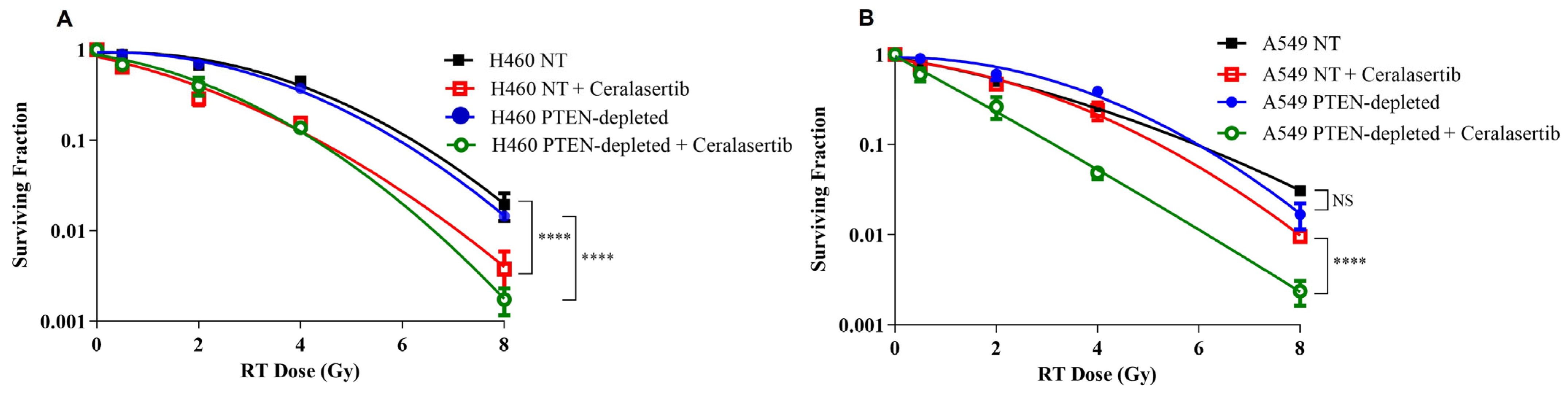
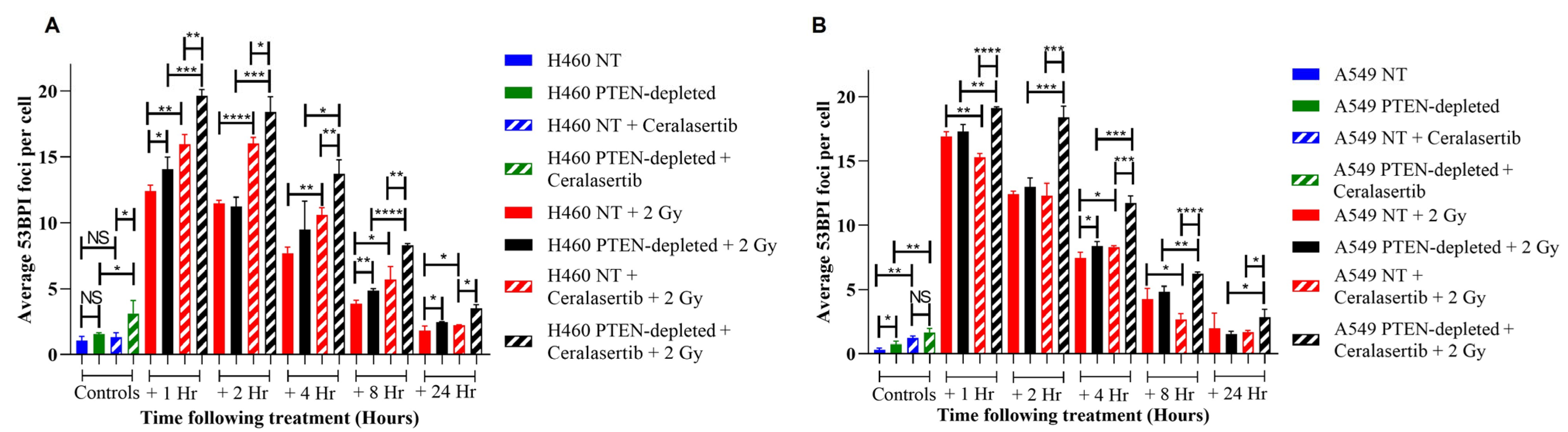
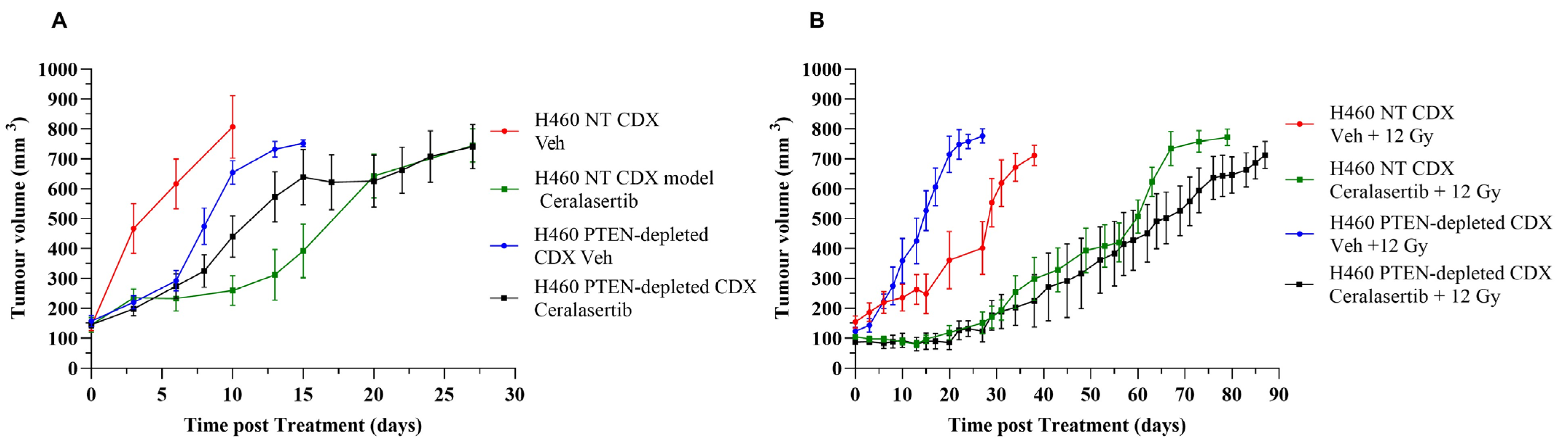
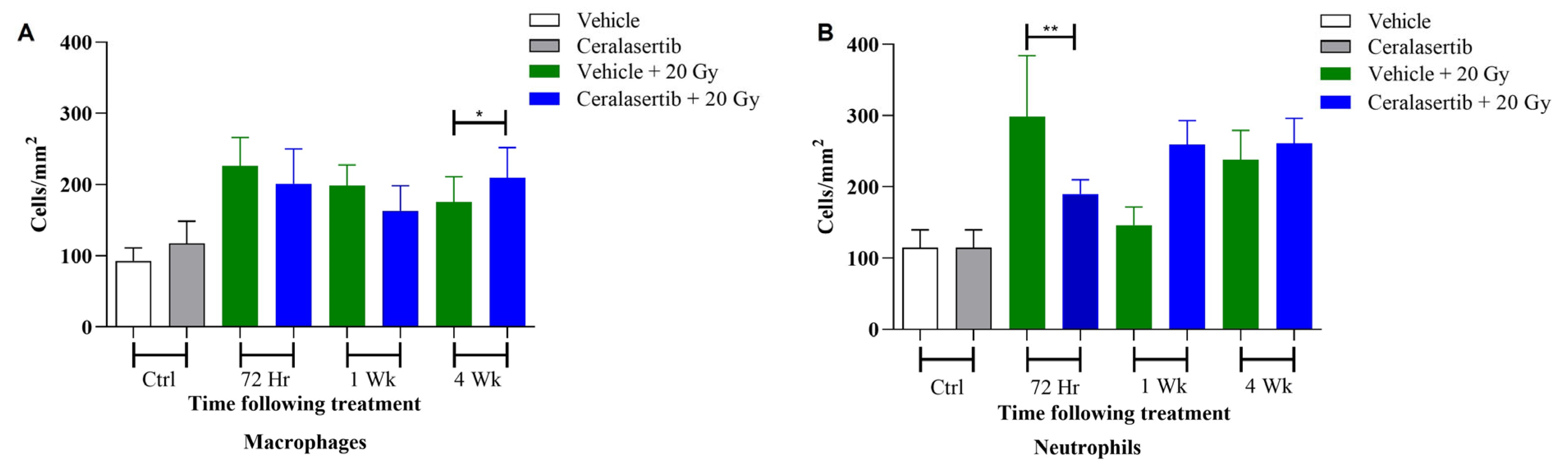
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Drugs, and Reagents
4.2. In Vitro X-Irradiation
4.3. Clonogenic Assay, DNA Damage, Flow Cytometry Analysis, and Western Blotting
4.4. Animals and Maintenance
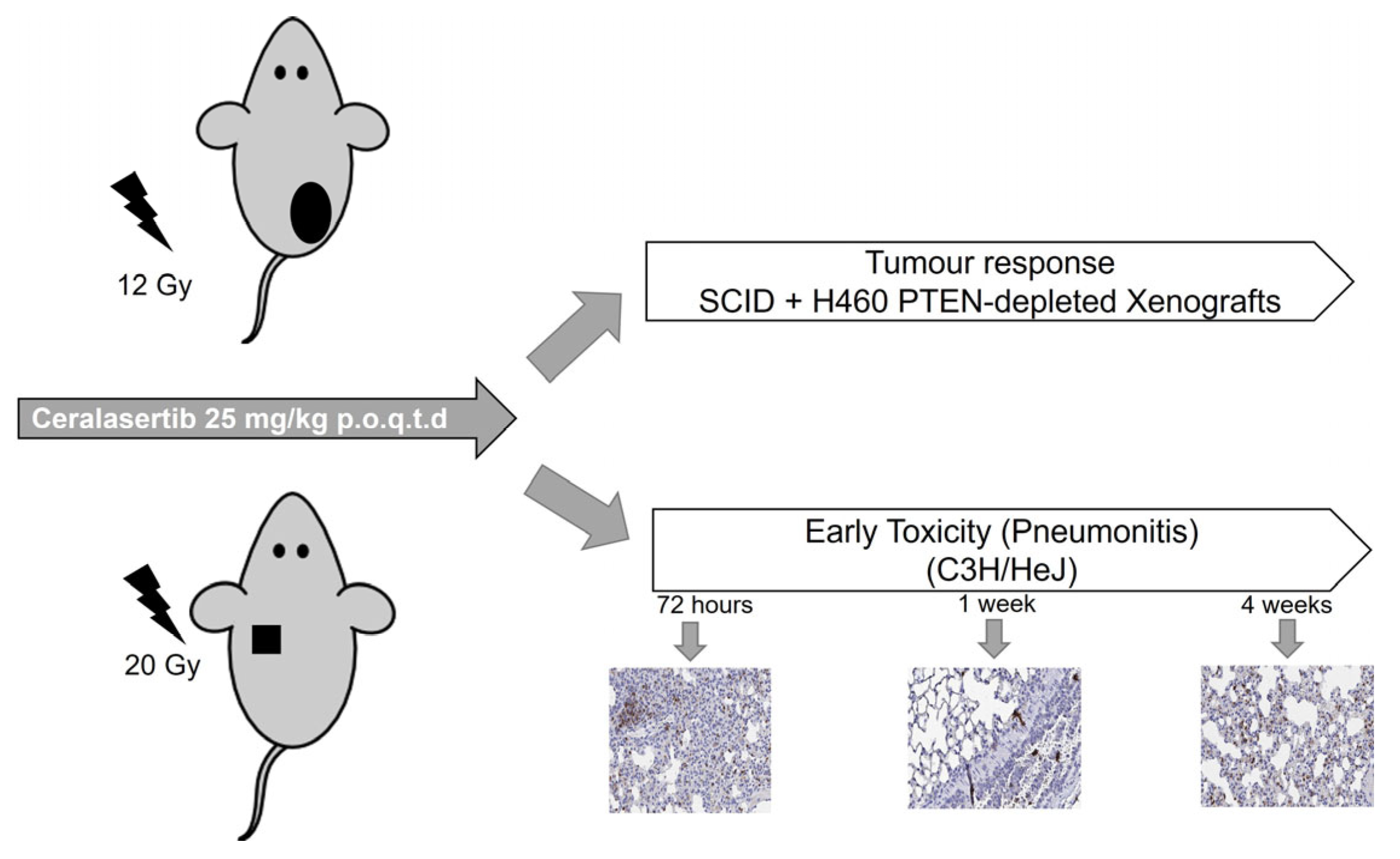
4.5. Pre-Clinical Study Design
4.6. Histological Assessment of Early Inflammatory Response
4.7. Data Fitting and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Rivera, M.P. Targeted Therapy for the Treatment of Advanced Non-small Cell Lung Cancer. Chest 2005, 128, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Guo, X.; Wang, J.; Stancu, A.L.; Joslin, P.M.N.; Zhang, D.; Zhu, S. Targeted therapies for advanced non-small cell lung cancer. Oncotarget 2018, 9, 37589–37607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Karachaliou, N.; Morales-Espinosa, D.; Costa, C.; Molina, M.A.; Sansano, I.; Gasco, A.; Viteri, S.; Massuti, B.; Wei, J.; et al. Adaptive resistance to targeted therapies in cancer. Transl. Lung Cancer Res. 2013, 2, 152–159. [Google Scholar] [PubMed]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized medicine in non-small cell lung cancer: A review from a pharmacogenomics perspective. Acta Pharm. Sin. B 2018, 8, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Kim, M.J.; Jeon, H.S.; Choi, J.E.; Kim, D.S.; Lee, E.B.; Cha, S.I.; Yoon, G.S.; Kim, C.H.; Jung, T.H.; et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer 2010, 69, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, R.J.; Zhang, X.; Patel, P.R.; Shelton, J.W.; Escott, C.E.; Sica, G.L.; Rossi, M.R.; Hill, C.E.; Steuer, C.E.; Pillai, R.N.; et al. Next-generation sequencing and clinical outcomes of patients with lung adenocarcinoma treated with stereotactic body radiotherapy. Cancer 2017, 123, 3681–3690. [Google Scholar] [CrossRef]
- Mehrad, M.; Roy, S.; Bittar, H.T.; Dacic, S. Next-Generation Sequencing Approach to Non–Small Cell Lung Carcinoma Yields More Actionable Alterations. Arch. Pathol. Lab. Med. 2018, 142, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, N.; Leduc, C.; Kohler, D.; Saieg, M.A.; John, T.; Sykes, J.; Yoshimoto, M.; Pintile, M.; Squire, J.; Shepherd, F.A.; et al. Loss of phosphatase and tensin homolog protein expression is an independent poor prognostic marker in lung adenocarcinoma. J. Thorac. Oncol. 2012, 7, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, C.-P.; He, B.-X.; Chen, X.; Lu, X.-X.; Xie, M.-X.; Li, W.; He, S.-Y.; You, S.-J.; Chen, Q. PTEN expression is a prognostic marker for patients with non-small cell lung cancer: A systematic review and meta-analysis of the literature. Oncotarget 2016, 7, 57832–57840. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Diao, X.-Y.; Zhang, X.; Shao, Q.; Feng, Y.-F.; An, X.; Wang, W.-Y. Identification of genetic alterations associated with primary resistance to EGFR-TKIs in advanced non-small-cell lung cancer patients with EGFR sensitive mutations. Cancer Commun. 2019, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; He, Y.-Y. PTEN in DNA damage repair. Cancer Lett. 2012, 319, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Ho, J.; Srikumar, T.; Dowling, R.J.O.; Gorrini, C.; Miller, S.J.; Mak, T.W.; Neel, B.G.; Raught, B.; Stambolic, V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 2013, 341, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Bayman, N.; McCloskey, P.; Rowbottom, C.; Faivre-Finn, C. New radiotherapy approaches in locally advanced non-small cell lung cancer. Eur. J. Cancer 2014, 50, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef]
- Morgan, M.A.; Lawrence, T.S. Molecular Pathways: Overcoming Radiation Resistance by Targeting DNA Damage Response Pathways. Clin. Cancer Res. 2015, 21, 2898–2904. [Google Scholar] [CrossRef]
- Sharma, R.A.; Plummer, R.; Stock, J.K.; Greenhalgh, T.A.; Ataman, O.; Kelly, S.; Clay, R.; Adams, R.R.; Baird, R.D.; Billingham, L.; et al. Clinical development of new drug–radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016, 13, 627–642. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef]
- Tang, S.; Li, Z.; Yang, L.; Shen, L.; Wang, Y. A potential new role of ATM inhibitor in radiotherapy: Suppressing ionizing Radiation-Activated EGFR. Int. J. Radiat. Biol. 2020, 96, 461–468. [Google Scholar] [CrossRef]
- Dunne, V.; Ghita, M.; Small, D.M.; Coffey, C.B.M.; Weldon, S.; Taggart, C.C.; Osman, S.O.; McGarry, C.M.; Prise, K.M.; Hanna, G.G.; et al. Inhibition of ataxia telangiectasia related-3 (ATR) improves therapeutic index in preclinical models of non-small cell lung cancer (NSCLC) radiotherapy. Radiother. Oncol. 2017, 124, 475–481. [Google Scholar] [CrossRef]
- Takeuchi, M.; Tanikawa, M.; Nagasaka, K.; Oda, K.; Kawata, Y.; Oki, S.; Agapiti, C.; Sone, K.; Miyagawa, Y.; Hiraike, K.; et al. Anti-Tumor Effect of Inhibition of DNA Damage Response Proteins, ATM and ATR, in Endometrial Cancer Cells. Cancers 2019, 11, 1913. [Google Scholar] [CrossRef]
- Walls, G.M.; Oughton, J.B.; Chalmers, A.J.; Brown, S.; Collinson, F.; Forster, M.D.; Franks, K.N.; Gilbert, A.; Hanna, G.G.; Hannaway, N.; et al. CONCORDE: A phase I platform study of novel agents in combination with conventional radiotherapy in non-small-cell lung cancer. Clin. Transl. Radiat. Oncol. 2020, 25, 61–66. [Google Scholar] [CrossRef]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Al-Subhi, N.; Ali, R.; Abdel-Fatah, T.; Moseley, P.M.; Chan, S.Y.T.; Green, A.R.; Ellis, I.O.; Rakha, E.A.; Madhusudan, S. Targeting ataxia telangiectasia-mutated- and Rad3-related kinase (ATR) in PTEN-deficient breast cancers for personalized therapy. Breast Cancer Res. Treat. 2018, 169, 277–286. [Google Scholar] [CrossRef]
- Li, K.; Yan, H.; Guo, W.; Tang, M.; Zhao, X.; Tong, A.; Peng, Y.; Li, Q.; Yuan, Z. ATM inhibition induces synthetic lethality and enhances sensitivity of PTEN-deficient breast cancer cells to cisplatin. Exp. Cell Res. 2018, 366, 24–33. [Google Scholar] [CrossRef]
- McCabe, N.; Hanna, C.; Walker, S.M.; Gonda, D.; Li, J.; Wikstrom, K.; Savage, K.I.; Butterworth, K.T.; Chen, C.; Harkin, D.P.; et al. Mechanistic Rationale to Target PTEN-Deficient Tumor Cells with Inhibitors of the DNA Damage Response Kinase ATM. Cancer Res. 2015, 1, 2159–2165. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014, 5, e1437. [Google Scholar] [CrossRef]
- Hanna, C.; Dunne, V.L.; Walker, S.M.; Butterworth, K.T.; McCabe, N.; Waugh, D.J.J.; Kennedy, R.; Prise, K.M. ATM Kinase Inhibition Preferentially Sensitises PTEN-Deficient Prostate Tumour Cells to Ionising Radiation. Cancers 2020, 13, 79. [Google Scholar] [CrossRef]
- Mansour, W.Y.; Tennstedt, P.; Volquardsen, J.; Oing, C.; Kluth, M.; Hube-Magg, C.; Borgmann, K.; Simon, R.; Petersen, C.; Dikomey, E.; et al. Loss of PTEN-assisted G2/M checkpoint impedes homologous recombination repair and enhances radio-curability and PARP inhibitor treatment response in prostate cancer. Sci. Rep. 2018, 8, 3947. [Google Scholar] [CrossRef]
- Fraser, M.; Harding, S.M.; Zhao, H.; Coackley, C.; Durocher, D.; Bristow, R.G. MRE11 promotes AKT phosphorylation in direct response to DNA double-strand breaks. Cell Cycle 2011, 10, 2218–2232. [Google Scholar] [CrossRef]
- Kao, G.D.; Jiang, Z.; Fernandes, A.M.; Gupta, A.K.; Maity, A. Inhibition of Phosphatidylinositol-3-OH Kinase/Akt Signaling Impairs DNA Repair in Glioblastoma Cells following Ionizing Radiation. J. Biol. Chem. 2007, 282, 21206–21212. [Google Scholar] [CrossRef]
- Pappas, G.; Zumstein, L.A.; Munshi, A.; Hobbs, M.; Meyn, R.E. Adenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cells by suppressing DNA repair capacity. Cancer Gene Ther. 2007, 14, 543–549. [Google Scholar] [CrossRef]
- Brown, K.H.; Ghita-Pettigrew, M.; Kerr, B.N.; Mohamed-Smith, L.; Walls, G.M.; McGarry, C.K.; Butterworth, K.T. Characterisation of quantitative imaging biomarkers for inflammatory and fibrotic radiation-induced lung injuries using preclinical radiomics. Radiother. Oncol. 2024, 192, 110106. [Google Scholar] [CrossRef]
- Zhang, H.; Han, G.; Liu, H.; Chen, J.; Ji, X.; Zhou, F.; Xie, C. The development of classically and alternatively activated macrophages has different effects on the varied stages of radiation-induced pulmonary injury in mice. J. Radiat. Res. 2011, 52, 717–726. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chou, C.H.; Shun, C.T.; Wei, M.F.; Kuo, S.H. The Expression of CXCL16 During Lung Irradiation May Lead to Radiation Pneumonitis and Fibrosis Through Inducing Neutrophil and Macrophage Infiltration in Lung Tissue. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, S65–S66. [Google Scholar] [CrossRef]
- Abernathy, L.M.; Fountain, M.D.; Rothstein, S.E.; David, J.M.; Yunker, C.K.; Rakowski, J.; Lonardo, F.; Joiner, M.C.; Hillman, G.G. Soy Isoflavones Promote Radioprotection of Normal Lung Tissue by Inhibition of Radiation-Induced Activation of Macrophages and Neutrophils. J. Thorac. Oncol. 2015, 10, 1703–1712. [Google Scholar] [CrossRef]
- Schoppy, D.W.; Ragland, R.L.; Gilad, O.; Shastri, N.; Peters, A.A.; Murga, M.; Fermamdez-Capetillo, O.; Diehl, J.A.; Brown, E.J. Oncogenic stress sensitizes murine cancers to hypomorphic suppression of ATR. J. Clin. Investig. 2012, 122, 241–252. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Brown, S.; Ryan, A.; Greystoke, A. The UK at the Forefront of Innovative Drug–Radiotherapy Combination Clinical Trials: Introducing the CONCORDE Platform. Clin. Oncol. 2020, 32, 358–362. [Google Scholar] [CrossRef]
- Maxwell, P.J.; Coulter, J.; Walker, S.M.; McKechnie, M.; Neisen, J.; McCabe, N.; Kennedy, R.D.; Salto-Tellez, M.; Albanese, C.; Waugh, D.J.J. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur. Urol. 2013, 64, 177–188. [Google Scholar] [CrossRef]
- Puck, T.T.; Marcus, P.I. Action of x-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; Moreira, H.; Redmond, K.M.; O’Sullivan, J.M.; Alshehri, A.H.D.; Wright, T.C.; Dunne, V.L.; Campfield, C.; Biggart, S.; McMahon, S.J.; et al. Differential responses to 223Ra and Alpha-particles exposure in prostate cancer driven by mitotic catastrophe. Front. Oncol. 2022, 12, 877302. [Google Scholar] [CrossRef]

| Treatment Group | Cell Model | p Value (NT- vs. PTEN-Depleted) | |
|---|---|---|---|
| H460 NT Tumour Quadrupling Time (Days) | H460 PTEN-Depleted Tumour Quadrupling Time (Days) | ||
| Vehicle | 3 ± 2.53 | 8 ± 1.6 | 0.001765 |
| Ceralasertib | 19 ± 2.53 | 10 ± 3.41 | 0.000406 |
| Vehicle + 12 Gy | 25 ± 5.01 | 13 ± 2.34 | 0.000340 |
| Ceralasterib + 12 Gy | 52 ± 6.07 | 70 ± 2.24 | 0.000047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunne, V.L.; Ghita-Pettigrew, M.; Redmond, K.M.; Small, D.M.; Weldon, S.; Taggart, C.C.; Prise, K.M.; Hanna, G.G.; Butterworth, K.T. PTEN Depletion Increases Radiosensitivity in Response to Ataxia Telangiectasia-Related-3 (ATR) Inhibition in Non-Small Cell Lung Cancer (NSCLC). Int. J. Mol. Sci. 2024, 25, 7817. https://doi.org/10.3390/ijms25147817
Dunne VL, Ghita-Pettigrew M, Redmond KM, Small DM, Weldon S, Taggart CC, Prise KM, Hanna GG, Butterworth KT. PTEN Depletion Increases Radiosensitivity in Response to Ataxia Telangiectasia-Related-3 (ATR) Inhibition in Non-Small Cell Lung Cancer (NSCLC). International Journal of Molecular Sciences. 2024; 25(14):7817. https://doi.org/10.3390/ijms25147817
Chicago/Turabian StyleDunne, Victoria L., Mihaela Ghita-Pettigrew, Kelly M. Redmond, Donna M. Small, Sinéad Weldon, Clifford C. Taggart, Kevin M. Prise, Gerard G. Hanna, and Karl T. Butterworth. 2024. "PTEN Depletion Increases Radiosensitivity in Response to Ataxia Telangiectasia-Related-3 (ATR) Inhibition in Non-Small Cell Lung Cancer (NSCLC)" International Journal of Molecular Sciences 25, no. 14: 7817. https://doi.org/10.3390/ijms25147817
APA StyleDunne, V. L., Ghita-Pettigrew, M., Redmond, K. M., Small, D. M., Weldon, S., Taggart, C. C., Prise, K. M., Hanna, G. G., & Butterworth, K. T. (2024). PTEN Depletion Increases Radiosensitivity in Response to Ataxia Telangiectasia-Related-3 (ATR) Inhibition in Non-Small Cell Lung Cancer (NSCLC). International Journal of Molecular Sciences, 25(14), 7817. https://doi.org/10.3390/ijms25147817








