Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth
Abstract
1. Introduction
2. Results
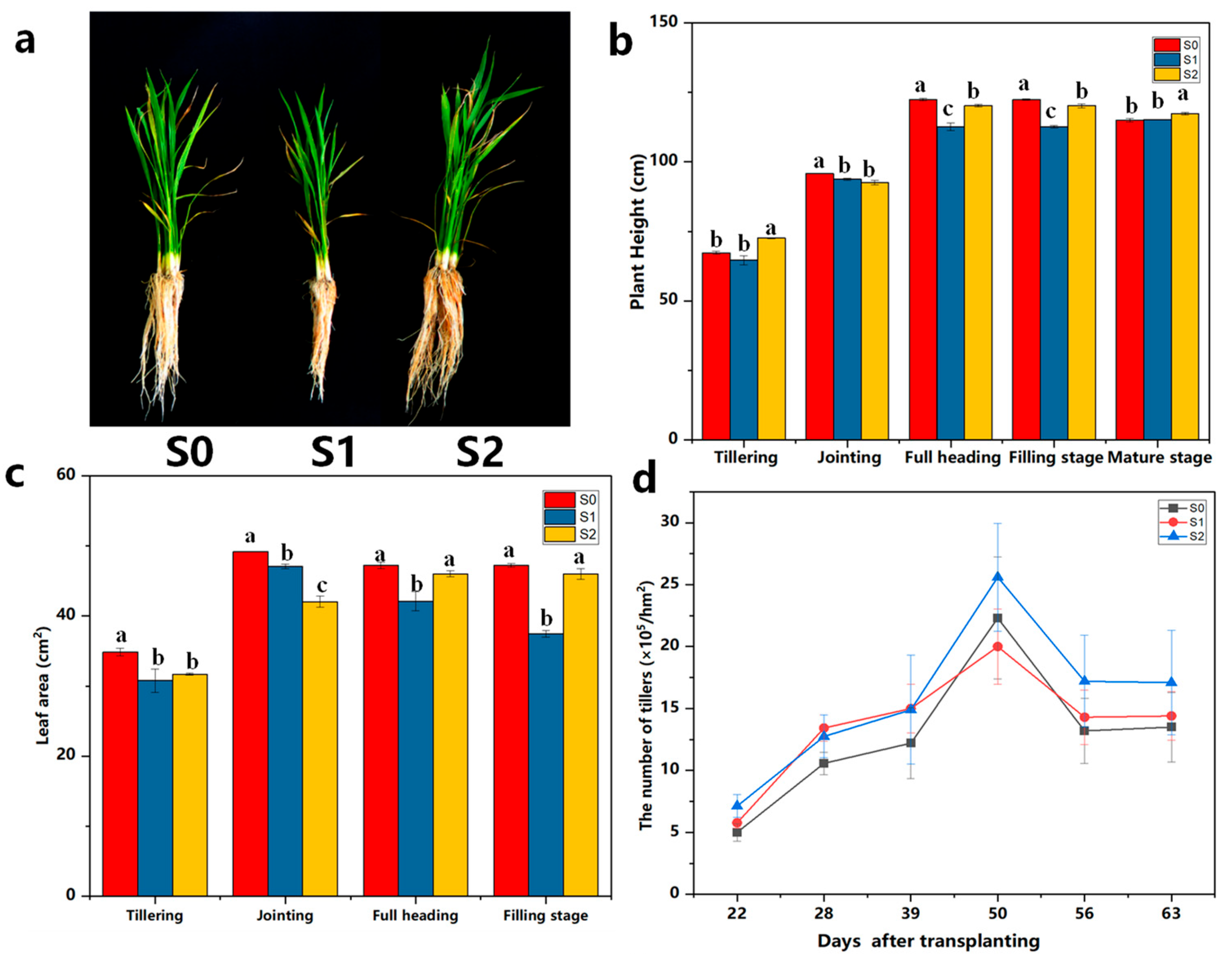
2.1. The Impacts of Exogenous-Degrading Bacteria Treatments on the Morphological and Physiological Characteristics of Rice
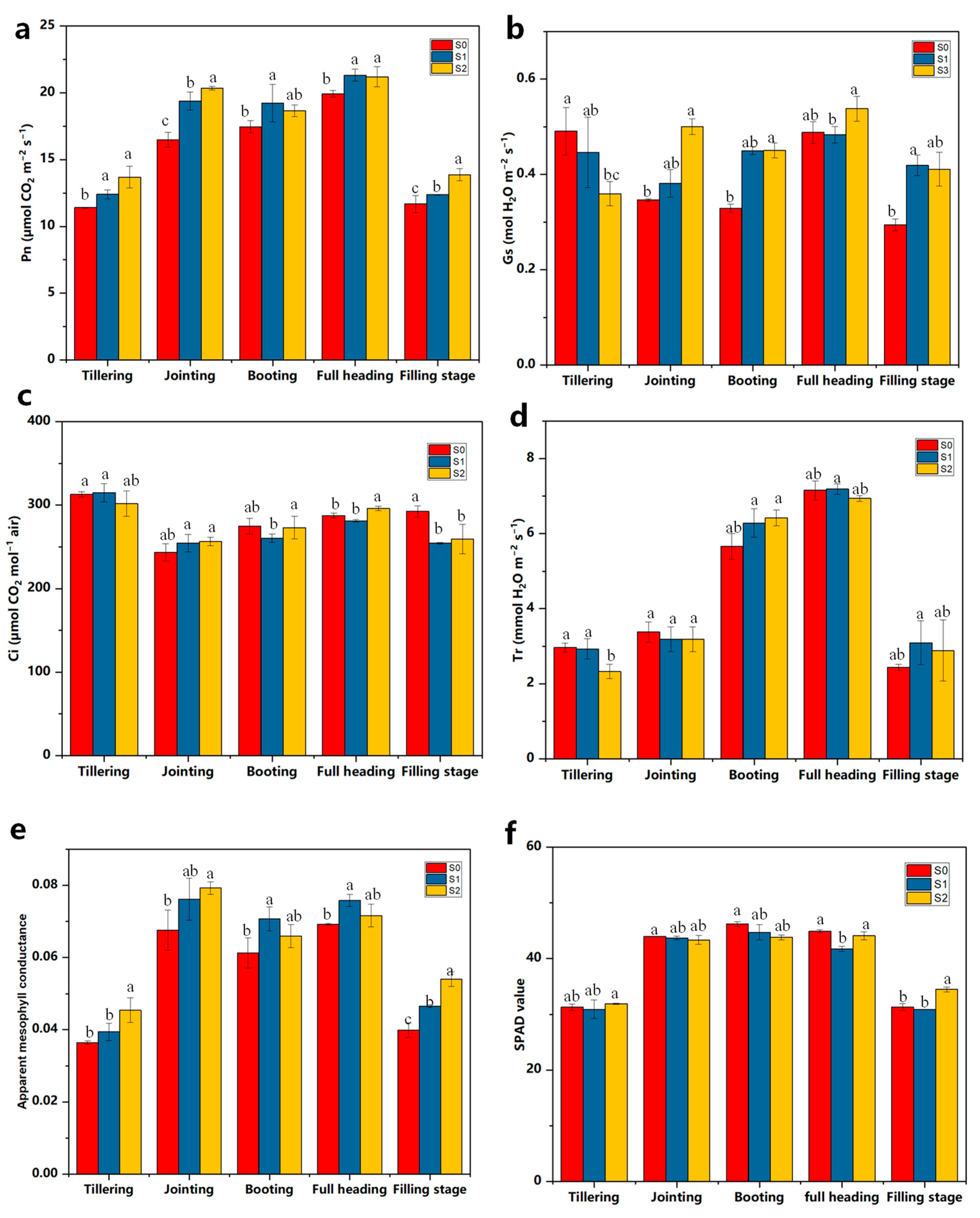
2.2. Effects of the Addition of Straw-Degrading Bacteria on the Photosynthetic Capacity of Rice
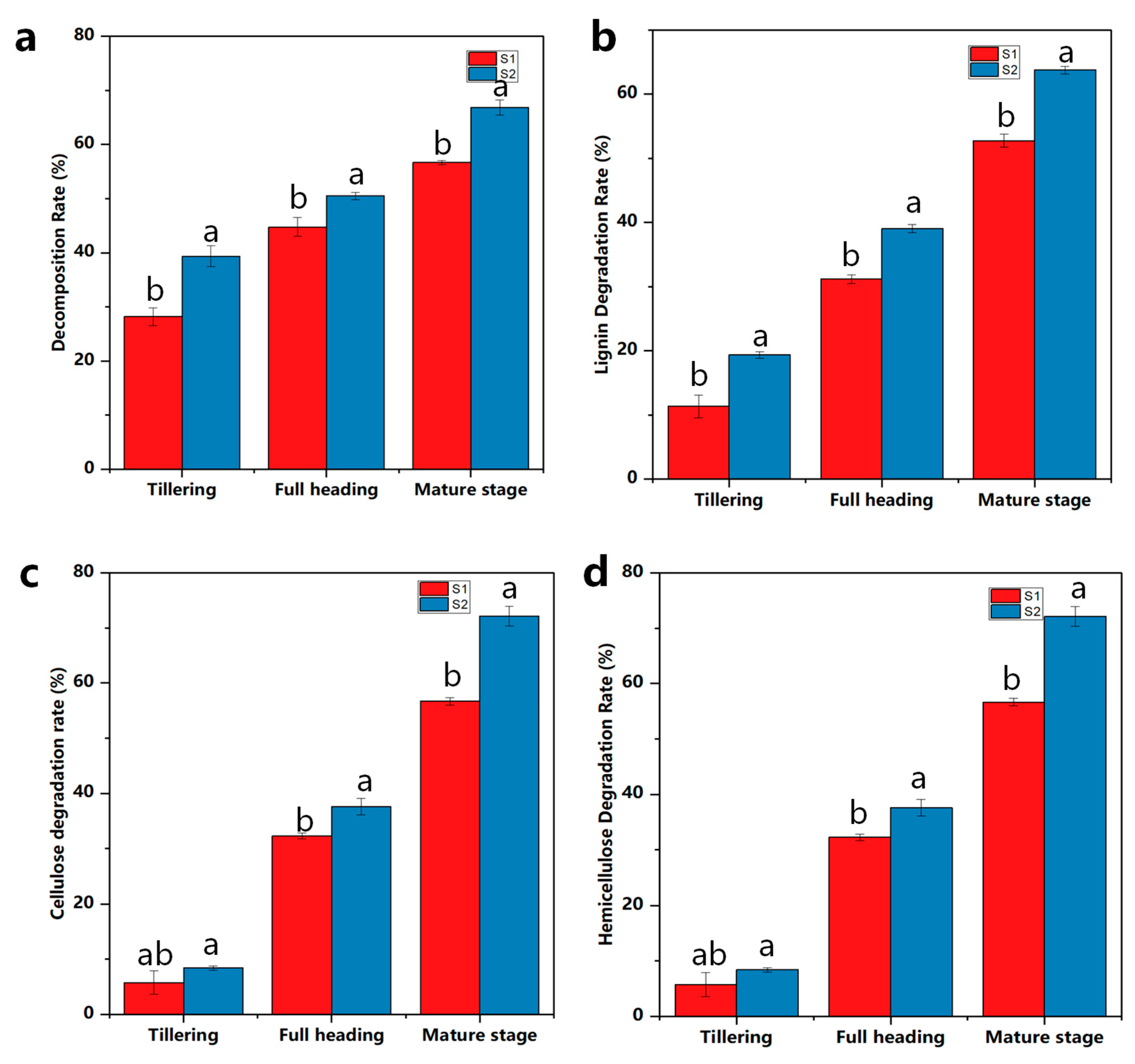
2.3. Impacts on Rice Straw Degradation Rate
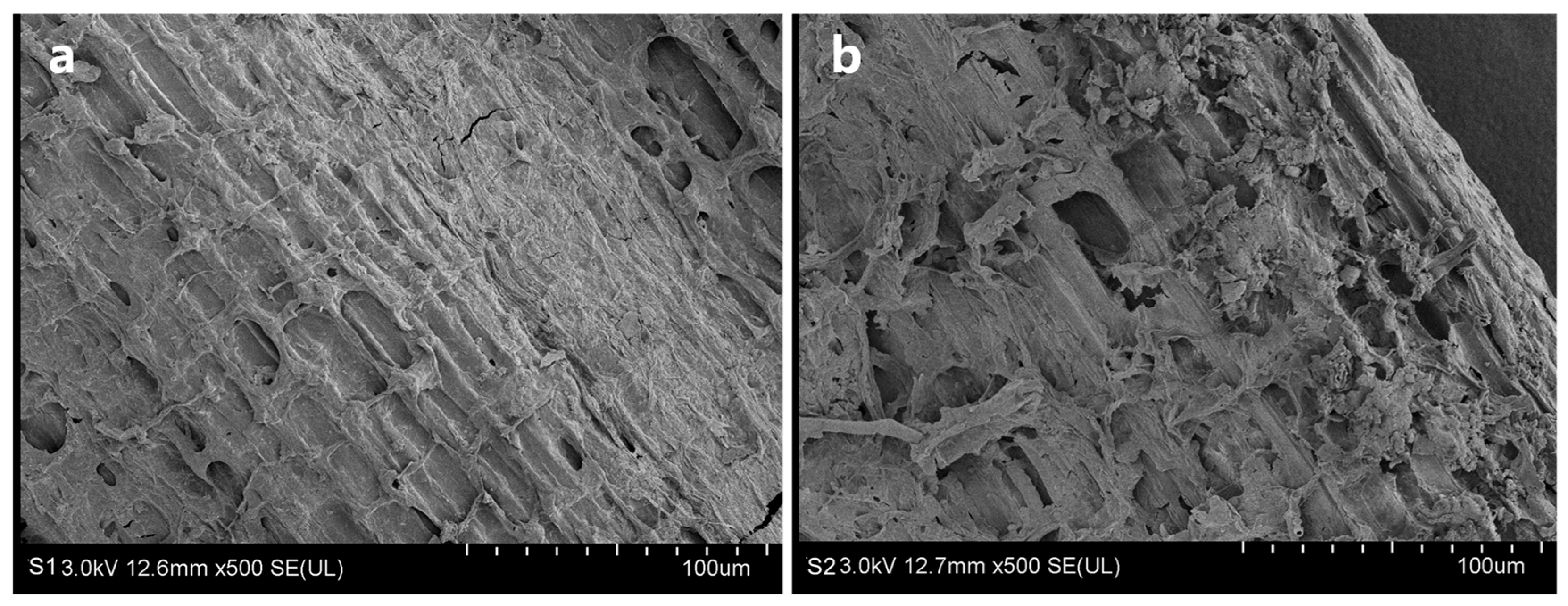
2.4. SEM (Scanning Electron Microscopic) Observation on the Degradation of Straw
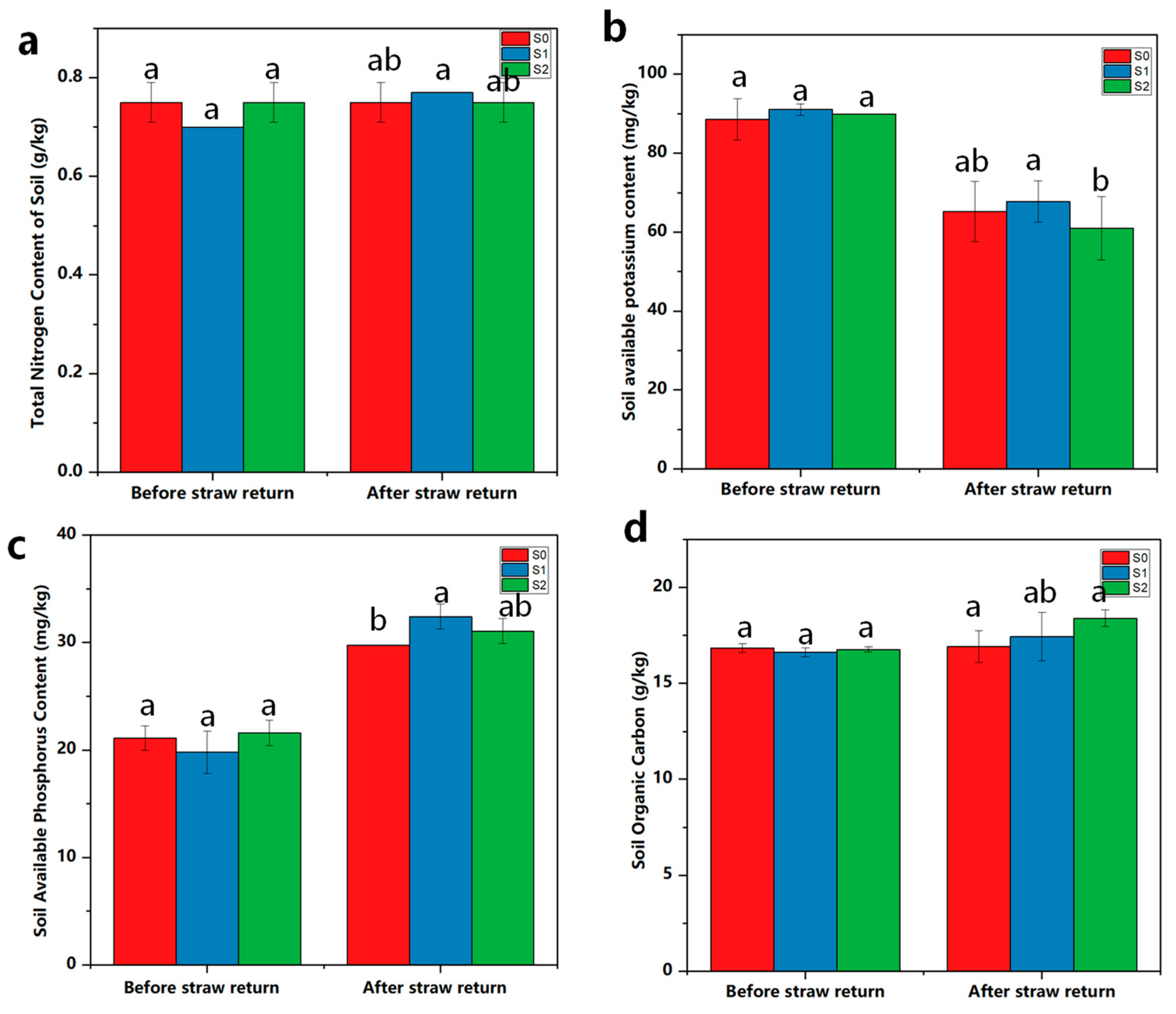
2.5. Soil Physicochemical Characteristics
2.6. Enzymatic Activity
2.7. Effects of Straw Return on the α-Diversity of the Soil Bacterial and Fungal Communities
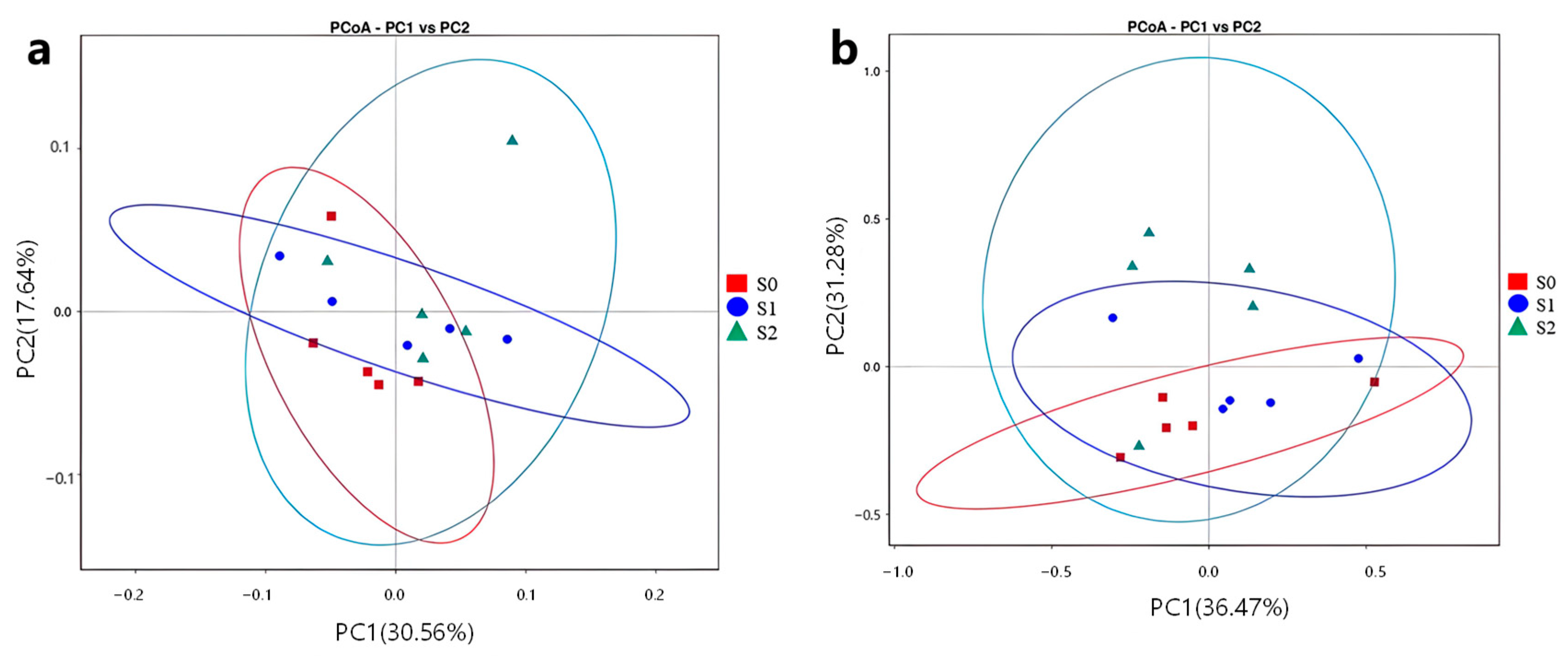
2.8. Effect of Straw-Degrading Bacteria on the Microbial β-Diversity
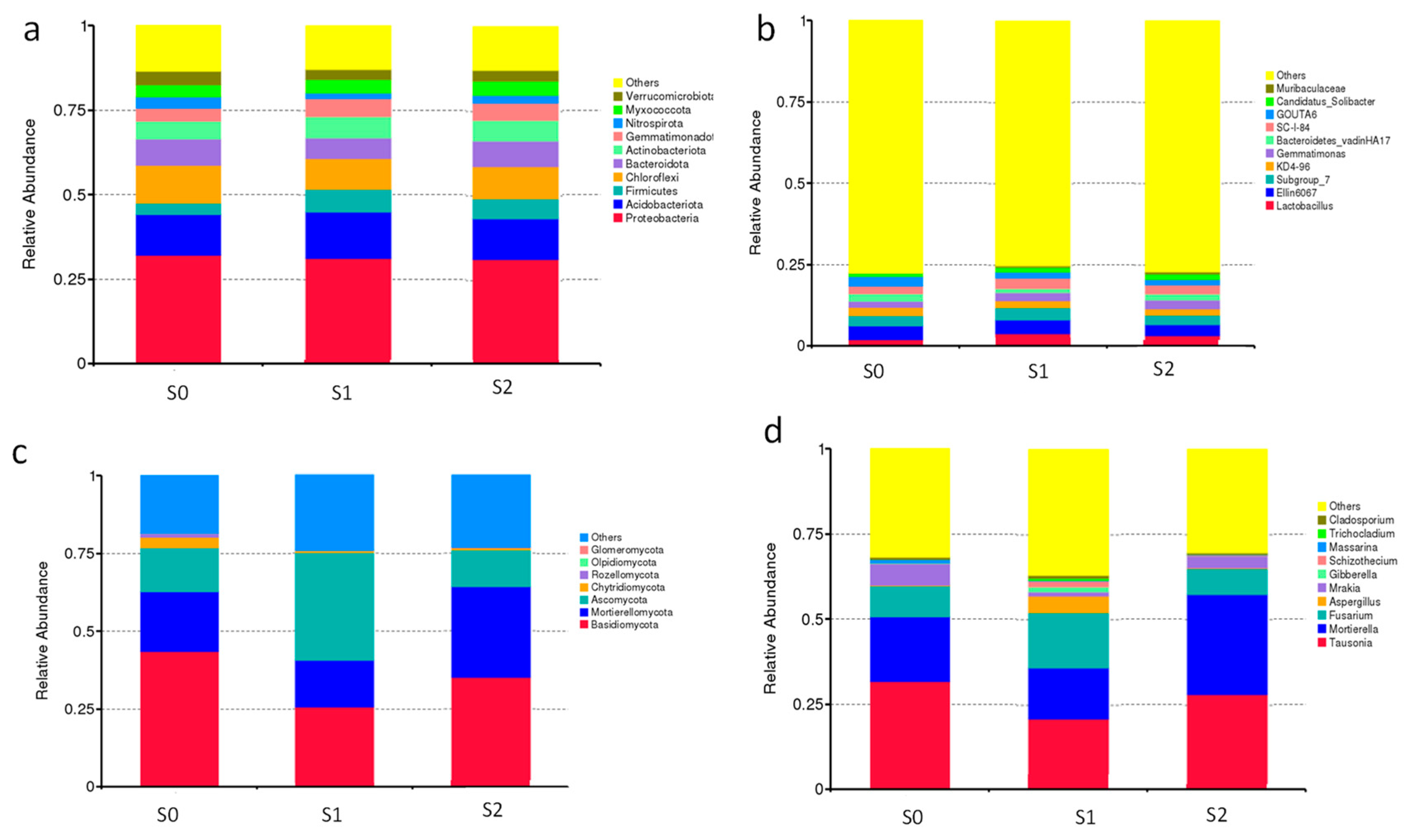
2.9. Microbial Community Structure and Composition
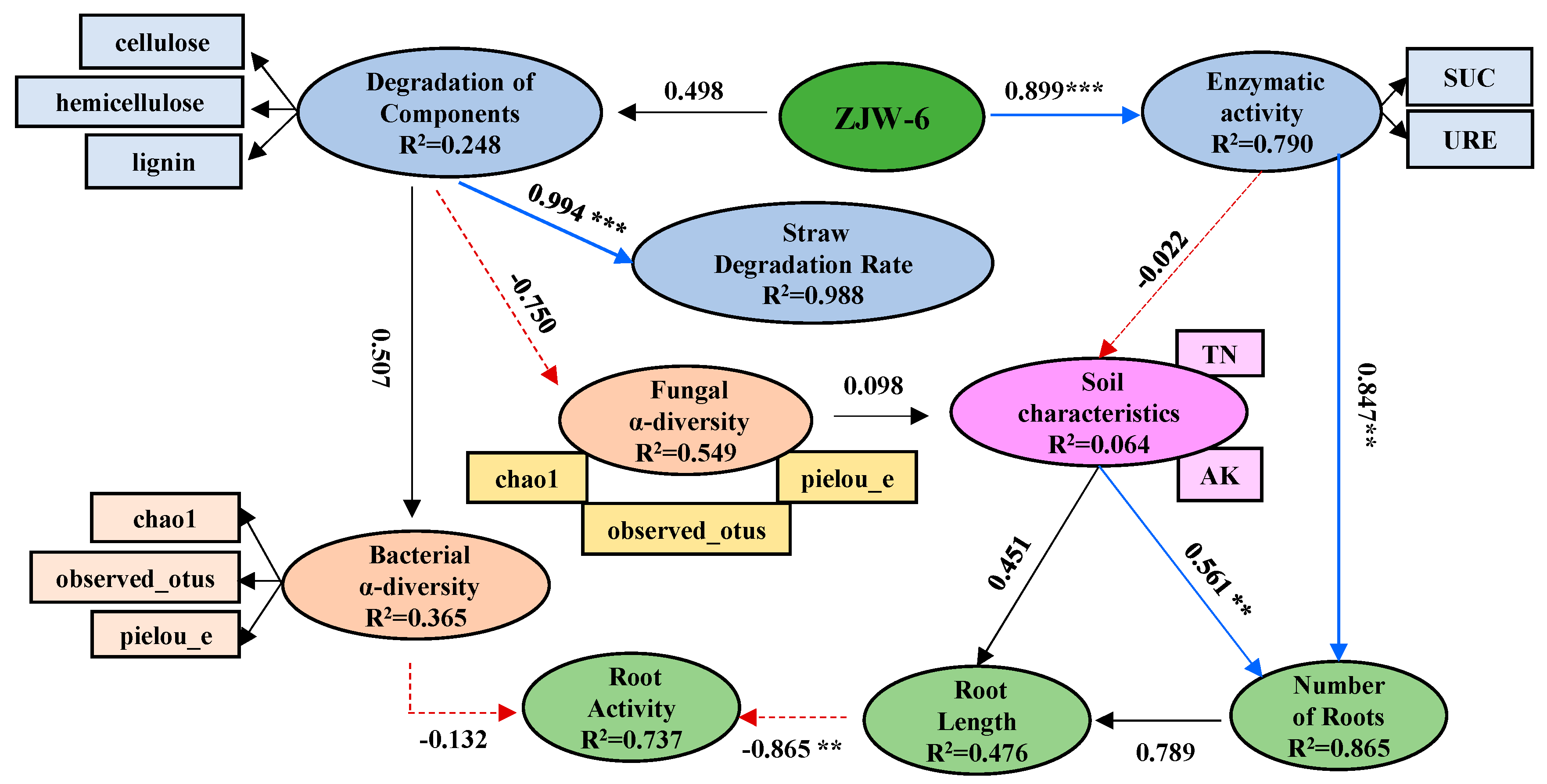
2.10. Regulatory Effects of Straw Incorporation and Degradative Bacteria on the Properties of Soil
3. Discussion
4. Materials and Methods
4.1. Sample Source
4.2. Experimental Design and Management
4.3. Measurement of the Parameters That Rice Growth under Field Conditions
4.4. Measurement of the Tiller Number and Aboveground Biomass
4.5. Determination of the Rate of Degradation
4.6. Electron Microscopy Observations
4.7. Measurements of Soil Enzymes Activities
4.8. Analysis of the Microbial Community Structure
4.9. Bioinformatics Analysis and Statistics of the High-Throughput Sequencing Data
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.Y.; Liu, W.L.; Li, X.Q.; Zhang, W.Z.; Bu, S.L.; Wang, A.J. A novel fungal agent for straw returning to enhance straw decomposition and nutrients release. Environ. Technol. Innov. 2023, 30, 103064. [Google Scholar] [CrossRef]
- Yin, L.J.; Huang, P.S.; Lin, H.H. Isolation of cellulase-producing bacteria and characterization of the cellulase from the isolated bacterium Cellulomonas sp. YJ5. J. Agric. Food. Chem. 2010, 58, 9833–9837. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Cheng, L.L.; Huang, X.L.; Zhang, Y.; Yin, C.B.; Lebailly, P. Incentive mechanism to promote corn stalk return sustainably in Henan, China. Sci. Total Environ. 2020, 738, 139775. [Google Scholar] [CrossRef]
- Xu, M.G.; Lou, Y.L.; Sun, X.L.; Wang, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fertil. Soils. 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huangm, G.; Xiao, W.; Han, L. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Ma, J.X.; Rong, Z.Y.; Zeng, D.D.; Wang, Y.C.; Hu, S.J.; Ye, W.W.; Zheng, X.B. Wheat Straw Return Influences Nitrogen-Cycling and Pathogen Associated Soil Microbiota in a Wheat–Soybean Rotation System. Front. Microbiol. 2019, 10, 1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Liu, D.B.; Wu, M.Q.; Xia, Y.; Zhang, F.L.; Fan, X.P. Long-term straw returning improve soil K balance and potassium supplying ability under rice and wheat cultivation. Sci. Rep. 2021, 11, 22260. [Google Scholar] [CrossRef]
- Sinovuyo, N.B.; Dou, S.; Zhang, X.W.; Zhang, Y.F.; Ma, R.; Liu, X. Tillage effects on humus composition and humic acid structural characteristics in soil aggregate-size fractions. Soil Tillage Res. 2021, 213, 105090. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, Y.; Mei, Y.; Liu, J.; Kalkhajeh, Y.K.; Hu, H.; Huang, J. Effects of continuous straw returning on bacterial community structure and enzyme activities in rape-rice soil aggregates. Sci. Rep. 2023, 13, 2357. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Jin, J.; Fu, M. Effects of different nitrogen applications and straw return depth on straw microbial and carbon and nitrogen cycles in paddy fields in the cool zone. Sci. Rep. 2024, 14, 6424. [Google Scholar] [CrossRef]
- Su, P.D.; Gao, X.Y.; Zhang, J.K.; Djellabi, R.; Yang, B.; Wu, Q.; Wen, Z. Enhancing the adsorption function of biochar by mechanochemical graphitization for organic pollutant removal. Front. Env. Sci. Eng. 2021, 15, 130. [Google Scholar] [CrossRef]
- Gong, X.J.; Zou, H.T.; Qian, C.R.; Yu, Y.; Hao, Y.B.; Li, L.; Wang, Q.J.; Jiang, Y.B.; Ma, J.T. Construction of in situ degradation bacteria of corn straw and analysis of its degradation efficiency. Ann. Microbiol. 2020, 70, 62. [Google Scholar] [CrossRef]
- Zheng, G.X.; Yin, T.; Lu, Z.X.; Boboua, S.Y.B.; Li, J.C.; Zhou, W.L. Degradation of rice straw at low temperature using a novel microbial consortium LTF-27 with efficient ability. Bioresour. Technol. 2020, 304, 123064. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.L.; Lou, Z.X.; An, X.Y.; Li, X.; Jiang, X.B.; Wang, W.D.; Zhao, H.Y.; Fu, M.J.; Cui, Z.J. The effects of adding exogenous lignocellulose degrading bacteria during straw incorporation in cold regions on degradation characteristics and soil indigenous bacteria communities. Front. Microbiol. 2023, 14, 1141545. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, S.S.; Liu, N.; He, H.; Cao, X.Y.; Lv, C.; Zhang, K.; Dai, J.L. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef] [PubMed]
- Abebe, N.; Bayu, D.; Milkyas, A.; Melkamu, M.; Gebermedihin, A.; Goytom, B.; Armaye, B.; Abebayheu, A. Effect of microbial inoculation on nutrient turnover and lignocellulose degradation during composting: A meta-analysis. Waste Manag. 2021, 125, 220–234. [Google Scholar] [CrossRef]
- Wimon, P.; Wanwipa, K.; Frank, R. Inoculation of cellulolytic and ligninolytic microorganisms accelerates decomposition of high C/N and cellulose rich sugarcane straw in tropical sandy soils. Appl. Soil Ecol. 2022, 172, 104355. [Google Scholar] [CrossRef]
- Chu, X.; Awasthi, M.K.; Liu, Y.; Cheng, Q.; Qu, J.; Sun, Y. Studies on the degradation of corn straw by combined bacterial cultures. Bioresour. Technol. 2020, 320, 124174. [Google Scholar] [CrossRef]
- Eliana, P.P.; Joana, F.S. Construction of Effective Minimal Active Microbial Consortia for Lignocellulose Degradation. Microb. Ecol. 2018, 76, 419–429. [Google Scholar] [CrossRef]
- Qin, S.; Jiao, K.; Lyu, D.; Shi, L.; Liu, L. Effects of maize residue and cellulose-decomposing bacteria inocula on soil microbial community, functional diversity, organic fractions, and growth of Malus hupehensis Rehd. Arch. Agron. Soil Sci. 2015, 61, 173–184. [Google Scholar] [CrossRef]
- Zhang, M.; Dang, P.; Haegeman, B.; Han, X.; Wang, X.; Pu, X.; Qin, X.; Siddique, K.H. The effects of straw return on soil bacterial diversity and functional profiles: A meta-analysis. Soil Biol. Biochem. 2024, 195, 109484. [Google Scholar] [CrossRef]
- Koner, S.; Chen, J.S.; Hsu, B.M.; Rathod, J.; Huang, S.W.; Chen, H.Y.; Hussain, B.; Chan, M.W.Y. Depth-resolved microbial diversity and functional profiles of trichloroethylene-contaminated soils for Biolog EcoPlate-based biostimulation strategy. J. Hazard. Mater. 2022, 424, 127266. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wei, Z.; Xiao, Y.S.; Li, P.F.; Gu, S.S.; Wu, S.L.; Zhai, Z.G.; Feng, K.; Deng, Y.; Hu, Q.L. Fungi-Bacteria Associations in Wilt Diseased Rhizosphere and Endosphere by Interdomain Ecological Network Analysis. Front. Microbiol. 2021, 12, 722626. [Google Scholar] [CrossRef]
- Reshma, S.; Kumar, S.D.; Prabhakar, M.; Kumar, N.S.; Nandkishore, T.; Kumar, S.A. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Yang, Q.Z.; Wang, Y.X.; Cheng, Z.a.; Cao, N.a.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Bowker, M.A.; Grace, J.B.; Powell, J.R. From patterns to causal understanding: Structural equation modeling (SEM) in soil ecology. Pedobiol. J. Soil Ecol. 2015, 58, 65–72. [Google Scholar] [CrossRef]
- Huang, S.; Zeng, Y.J.; Wu, J.F.; Shi, Q.H.; Pan, X.H. Effect of crop residue retention on rice yield in China: A meta-analysis. Field Crop. Res. 2013, 154, 188–194. [Google Scholar] [CrossRef]
- Bijay-Singh; Shan, Y.H.; Johnson-Beebout, S.E.; Yadvinder-Singh; Buresh, R.J. Chapter 3 Crop Residue Management for Lowland Rice-Based Cropping Systems in Asia. Adv. Agron. 2008, 98, 117–199. [Google Scholar] [CrossRef]
- Chang, L.; Meng, L.; Jun, C.; Bo, L.; Changming, F. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar]
- Shan, Y.H.; Cai, Z.C.; Han, Y.; Johnson, S.E.; Buresh, R.J. Organic acid accumulation under flooded soil conditions in relation to the incorporation of wheat and rice straws with different C:N ratios. Soil Sci. Plant Nutr. 2008, 54, 46–56. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Zhang, H.; Li, L.; Lin, Q. Effects of coupling of precise straw return and nitrogen fertilizer on photosynthetic characteristics after anthesis and yield of winter wheat. Acta Agric. Boreal.-Sin. 2012, 6, 21–26. [Google Scholar]
- Yin, W.; Chai, Q.; Guo, Y.; Feng, F.; Zhao, C.; Yu, A.; Hu, F. Analysis of leaf area index dynamic and grain yield components of intercropped wheat and maize under straw mulch combined with reduced tillage in arid environments. J. Agric. Sci. 2016, 8, 26–42. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Crop Residue Removal Impacts on Soil Productivity and Environmental Quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Yan, F.J.; Sun, Y.J.; Hui, X.; Jiang, M.J.; Xiang, K.H.; Wu, Y.X.; Zhang, Q.; Tang, Y.; Yang, Z.Y.; Sun, Y.Y. The effect of straw mulch on nitrogen, phosphorus and potassium uptake and use in hybrid rice. Paddy Water Environ. 2019, 17, 23–33. [Google Scholar] [CrossRef]
- Itziar, A.; Ana, A.; Patrick, R.; Isabel, A.; Ibone, A.; Carlos, G. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Parkinson, D. Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol. Biochem. 2000, 32, 59–66. [Google Scholar] [CrossRef]
- Liang, W.; Wu, Z.B.; Cheng, S.P.; Zhou, Q.H.; Hu, H.Y. Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol. Eng. 2003, 21, 191–195. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.H.; Zhu, A.N.; Zhang, J.B.; Chen, L.J. Application of 31 P NMR spectroscopy in determining phosphatase activities and P composition in soil aggregates influenced by tillage and residue management practices. Soil Tillage Res. 2014, 138, 35–43. [Google Scholar] [CrossRef]
- Yu, P.J.; Liu, S.W.; Han, K.X.; Guan, S.C.; Zhou, D.W. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.Q.; Gong, Y.S.; Yang, H.F.; Fan, M.S.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Bhat, A.K. Preserving Microbial diversity of Soil ecosystem: A key to sustainable productivity. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 85–101. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2470361 (accessed on 3 October 2022).
- Zhao, J.; Zhang, R.F.; Xue, C.; Xun, W.B.; Sun, L.; Xu, Y.C.; Shen, Q.R. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 2014, 67, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xun, W.B.; Huang, T.; Zhang, G.S.; Gao, J.S.; Ran, W.; Li, D.C.; Shen, Q.R.; Zhang, R.F. Alteration of the soil bacterial community during parent material maturation driven by different fertilization treatments. Soil Biol. Biochem. 2016, 96, 207–215. [Google Scholar] [CrossRef]
- Ramirez Kelly, S.; Lauber Christian, L.; Knight, R.; Bradford Mark, A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Xing, Z.P.; Hu, Y.J.; Zhang, H.C.; Dai, Q.G.; Huo, Z.Y.; Xu, K.; Wei, H.Y.; Gao, H.; Guo, B.W. Difference of Root Morphological and Several Physiological Characteristics between Indica and Japonica Super Rice Varieties. Acta Agron. Sin. 2014, 40, 1066–1080. [Google Scholar] [CrossRef]
- Wu, L.L.; Yu, Y.J.; Tian, C.; Zhang, L.; Huang, J.; Zhu, L.F.; Zhu, C.Q.; Kong, Y.L.; Zhang, J.H.; Cao, X.C.; et al. Effects of Different Nitrogen Application Regimes on Translocation of Rice Photosynthetic Products and Nitrogen Under Alternate Wetting and Drying Irrigation. Chin. J. Rice Sci. 2022, 36, 295–307. [Google Scholar] [CrossRef]
- Serrano-Gamboa, J.G.; Rojas-Herrera, R.A.; González-Burgos, A.; Folch-Mallol, J.L.; Jiménez, D.J.; Sánchez-González, M.N. Degradation profile of nixtamalized maize pericarp by the action of the microbial consortium PM-06. AMB Express 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Lu, R.K. Analytical Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; p. 135. [Google Scholar]
- Tanja, M.; Steven, L.S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Nicholas, A.B.; Sathish, S.; Jeremiah, J.F.; Dirk, G.; Jeffrey, I.G.; Rob, K.; David, A.M.; Gregory, C.J. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, W.; Song, Z.; Wang, S.; Geng, S.; Jiang, H.; Wang, Z.; Tian, P.; Wu, Z.; Yang, M. Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth. Int. J. Mol. Sci. 2024, 25, 7835. https://doi.org/10.3390/ijms25147835
Wei X, Li W, Song Z, Wang S, Geng S, Jiang H, Wang Z, Tian P, Wu Z, Yang M. Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth. International Journal of Molecular Sciences. 2024; 25(14):7835. https://doi.org/10.3390/ijms25147835
Chicago/Turabian StyleWei, Xiaoshuang, Wanchun Li, Ze Song, Shiwen Wang, Shujuan Geng, Hao Jiang, Zhenhui Wang, Ping Tian, Zhihai Wu, and Meiying Yang. 2024. "Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth" International Journal of Molecular Sciences 25, no. 14: 7835. https://doi.org/10.3390/ijms25147835
APA StyleWei, X., Li, W., Song, Z., Wang, S., Geng, S., Jiang, H., Wang, Z., Tian, P., Wu, Z., & Yang, M. (2024). Straw Incorporation with Exogenous Degrading Bacteria (ZJW-6): An Integrated Greener Approach to Enhance Straw Degradation and Improve Rice Growth. International Journal of Molecular Sciences, 25(14), 7835. https://doi.org/10.3390/ijms25147835






