Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications
Abstract
:1. Introduction
2. Polysaccharides Modified by Oxidation for the Introduction of Carbonyl Groups
2.1. Introduction of Carbonyl Groups in Polysaccharide Chains
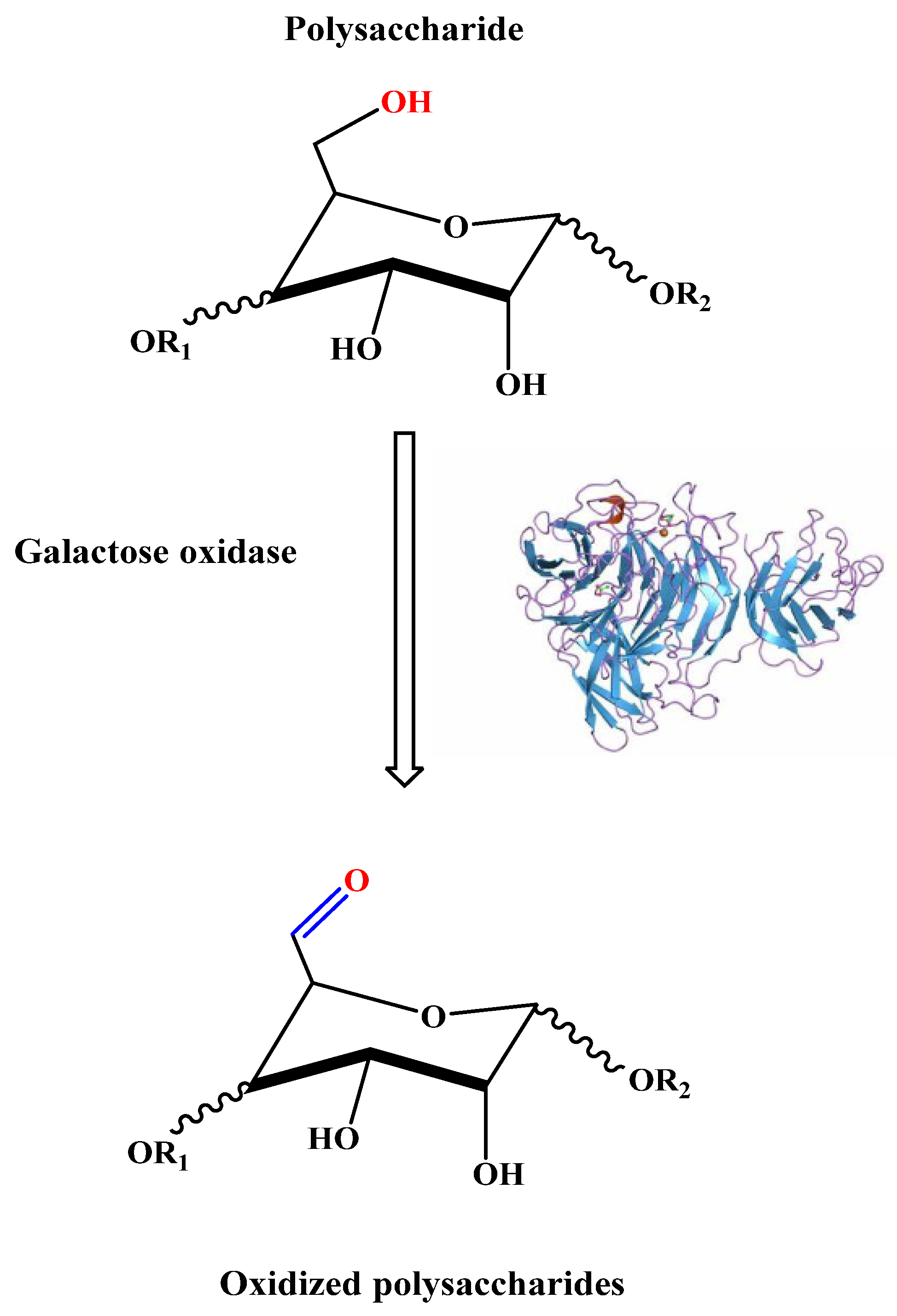
2.1.1. Enzymatic Oxidation
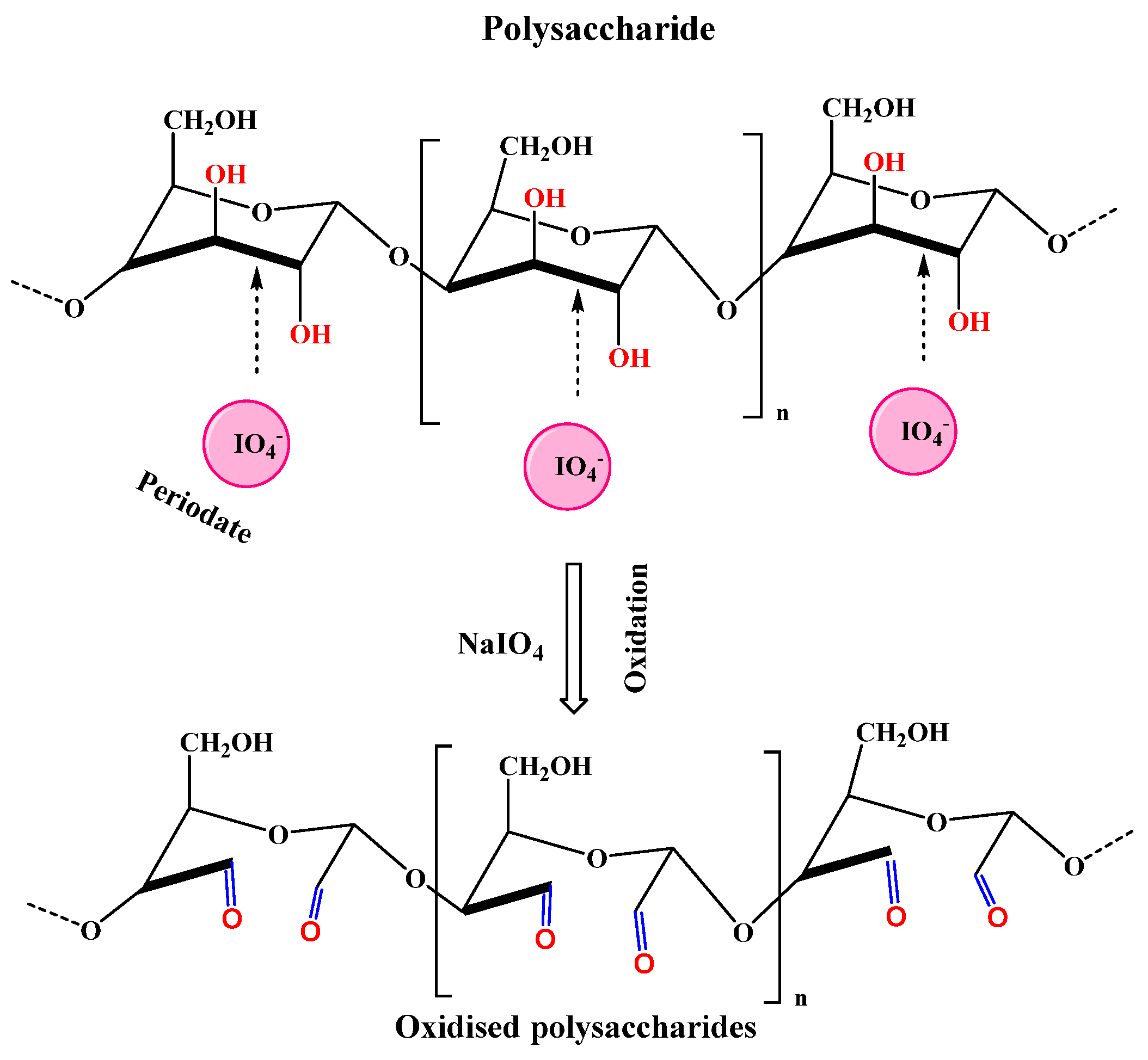
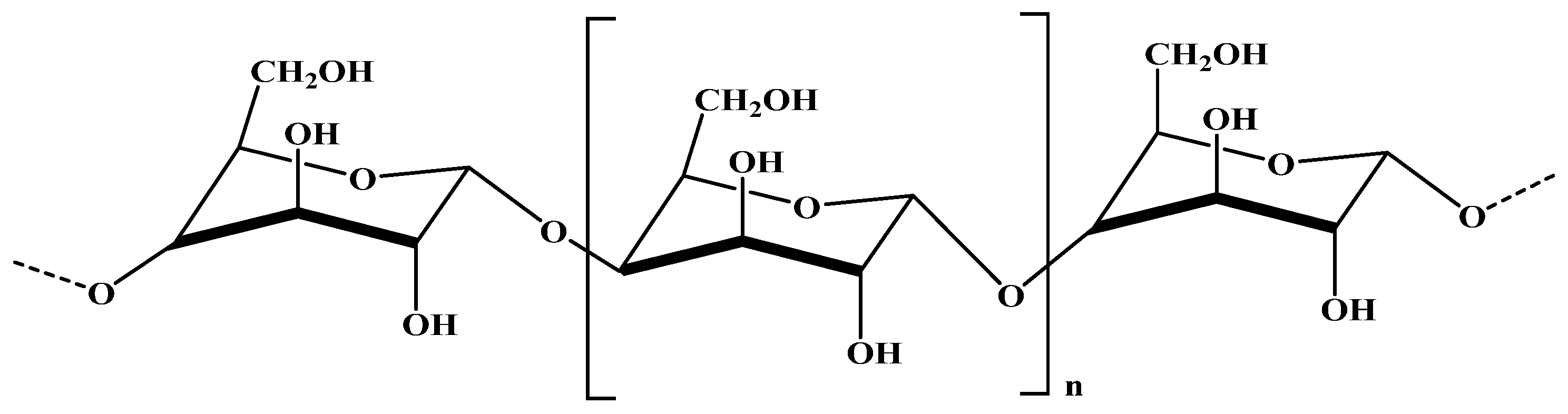
2.1.2. Periodate Oxidation
2.2. Functionalities and Characteristics of Polysaccharides Modified by the Addition of Carbonyl Groups
2.2.1. Functionalities
Chemical Reactivity
- Cross-Linking Ability
- Oxidation–Reduction Reactions
- Bioactivity
2.2.2. Characteristics
Enhanced Hydrophilicity
Mechanical Properties
Biocompatibility and Biodegradability
Controlled Gelation and Swelling
2.3. Example of Polysaccharides Modified by Oxidation
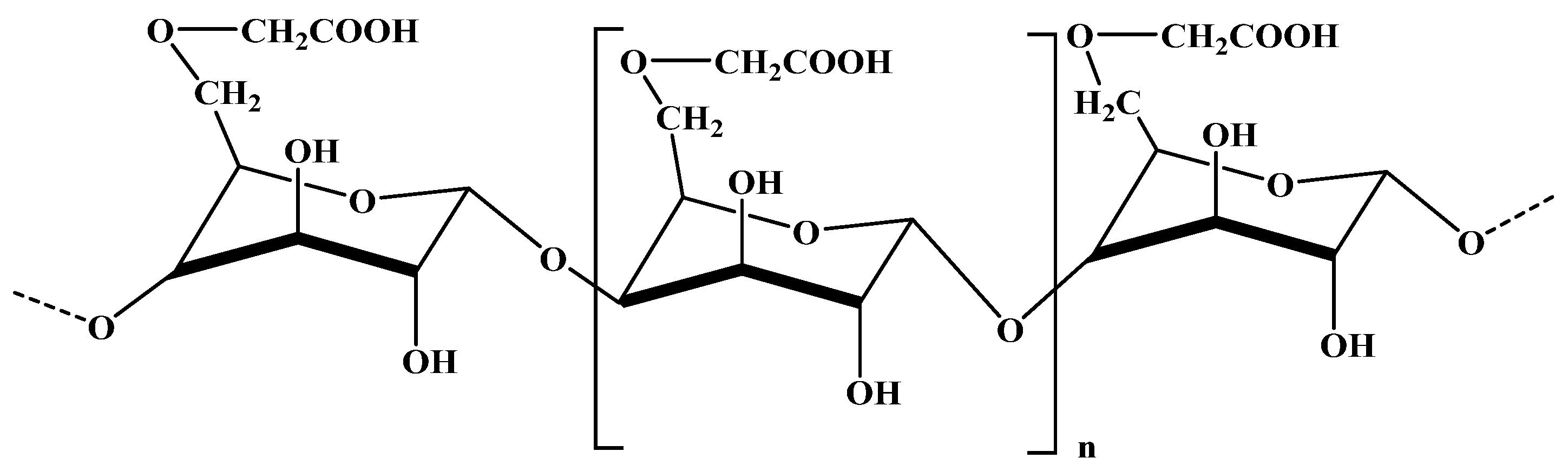
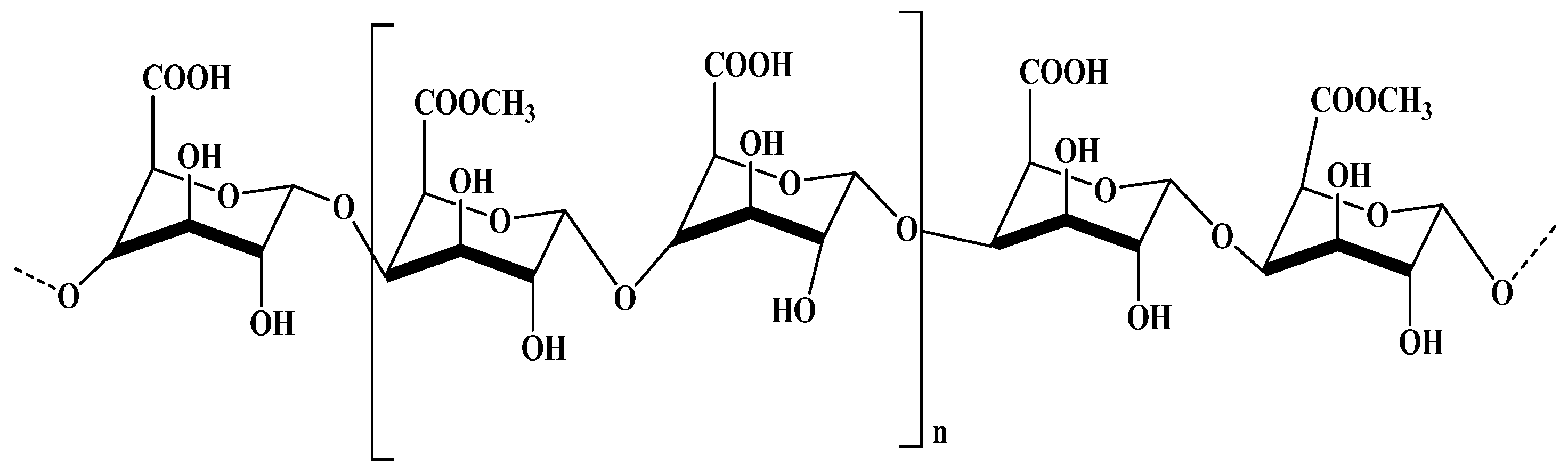
2.3.1. Alginate
2.3.2. Chitosan
2.3.3. Pullulan
2.3.4. Carboxymethylcellulose
2.3.5. Pectin
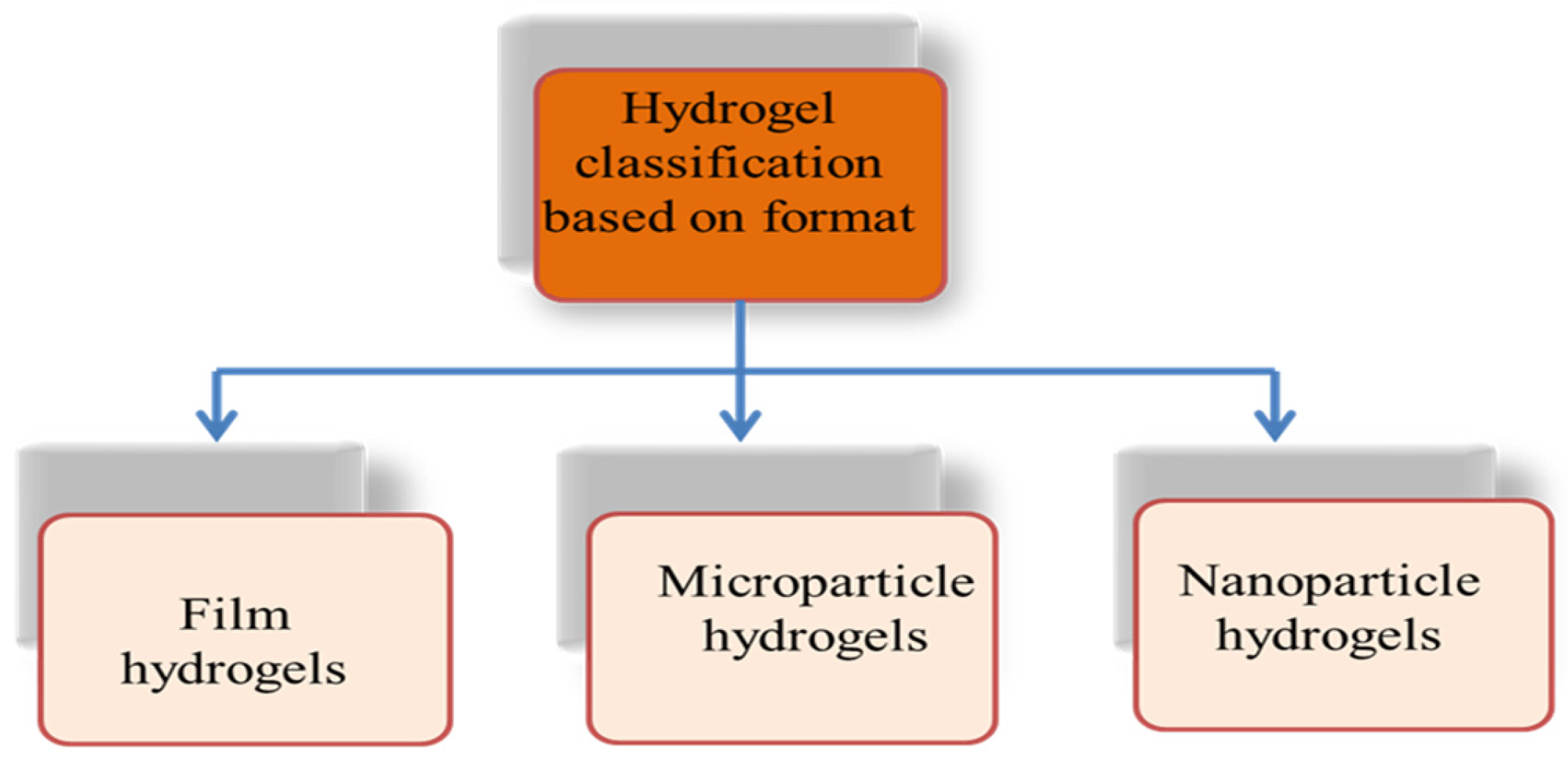
3. Hydrogels
3.1. Synthesis of Hydrogels
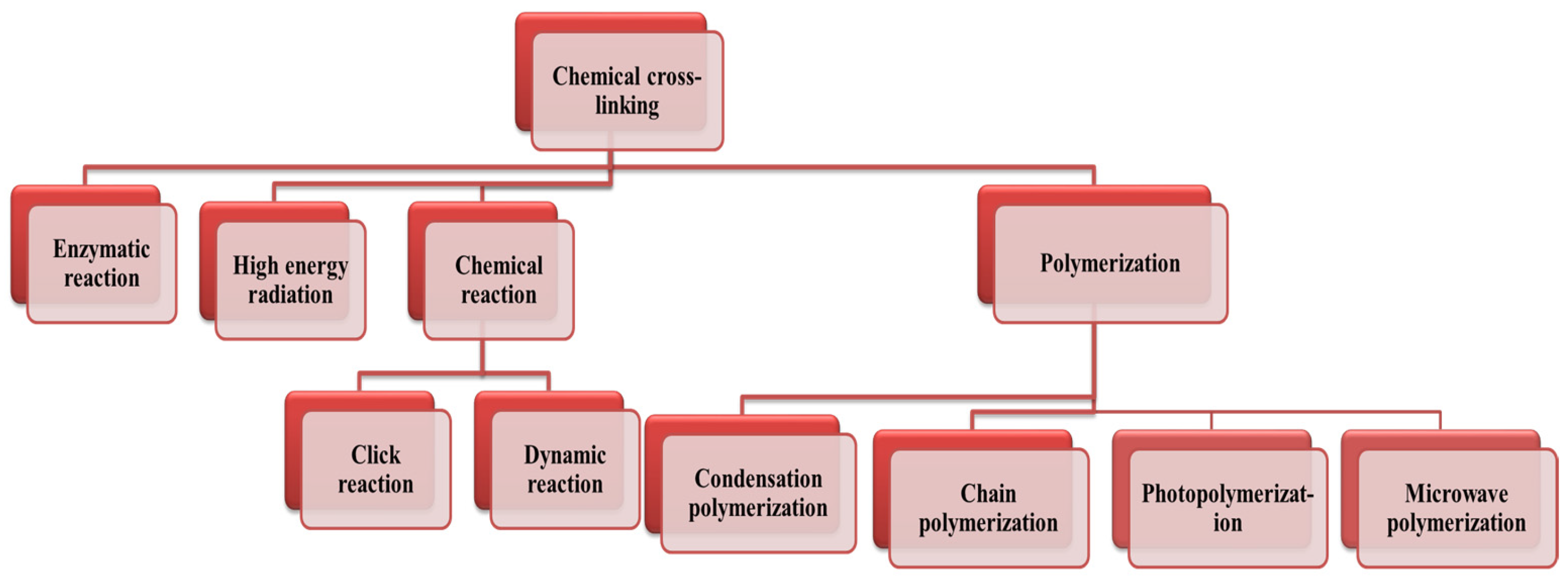
3.1.1. Chemical Cross-Linking
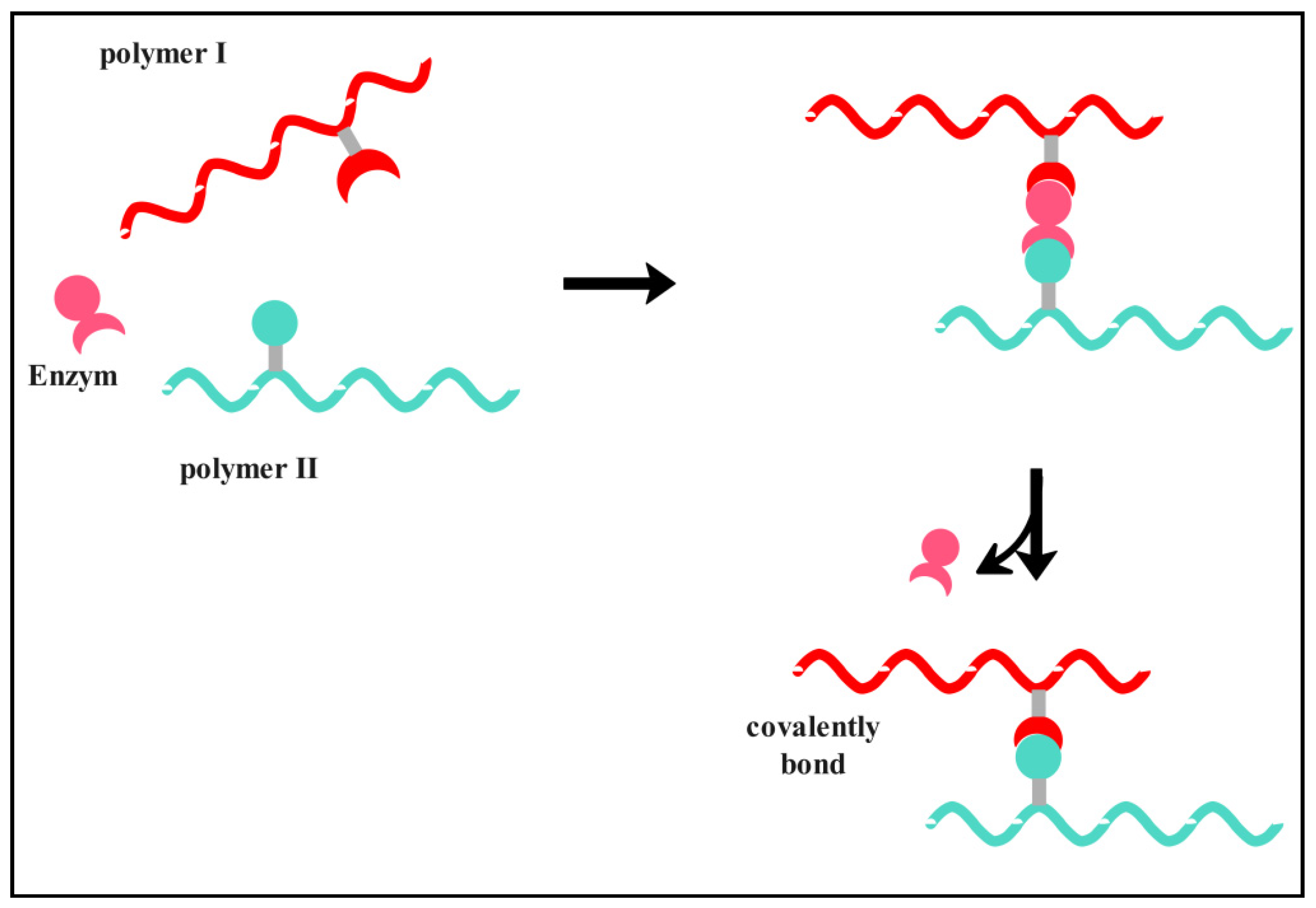
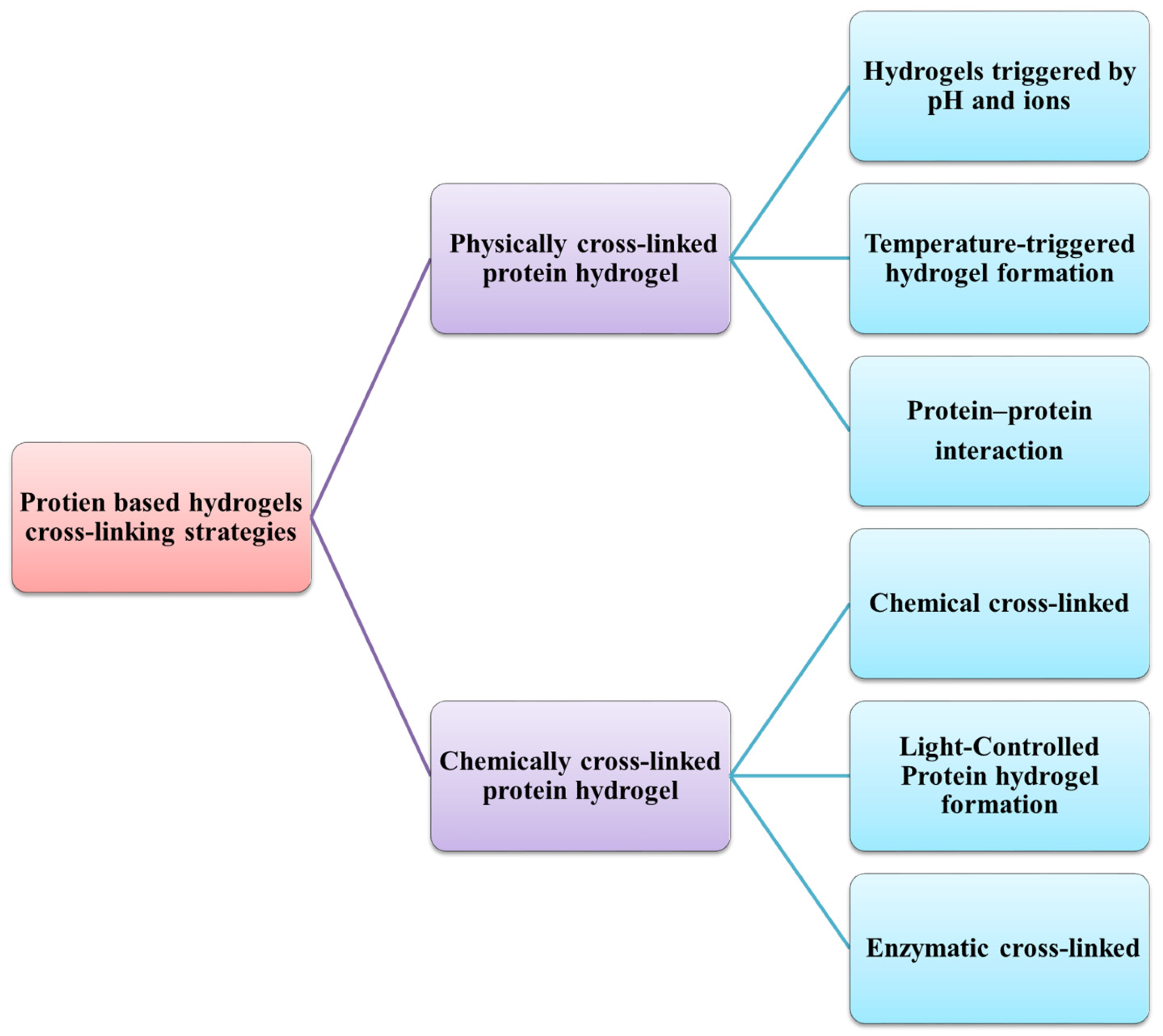
3.1.2. Physical Cross-Linking
4. Protein
4.1. The Shape and Structure of Proteins
4.2. Proteins-Based Hydrogels
4.3. Strategies for Reinforcing the Gel Network
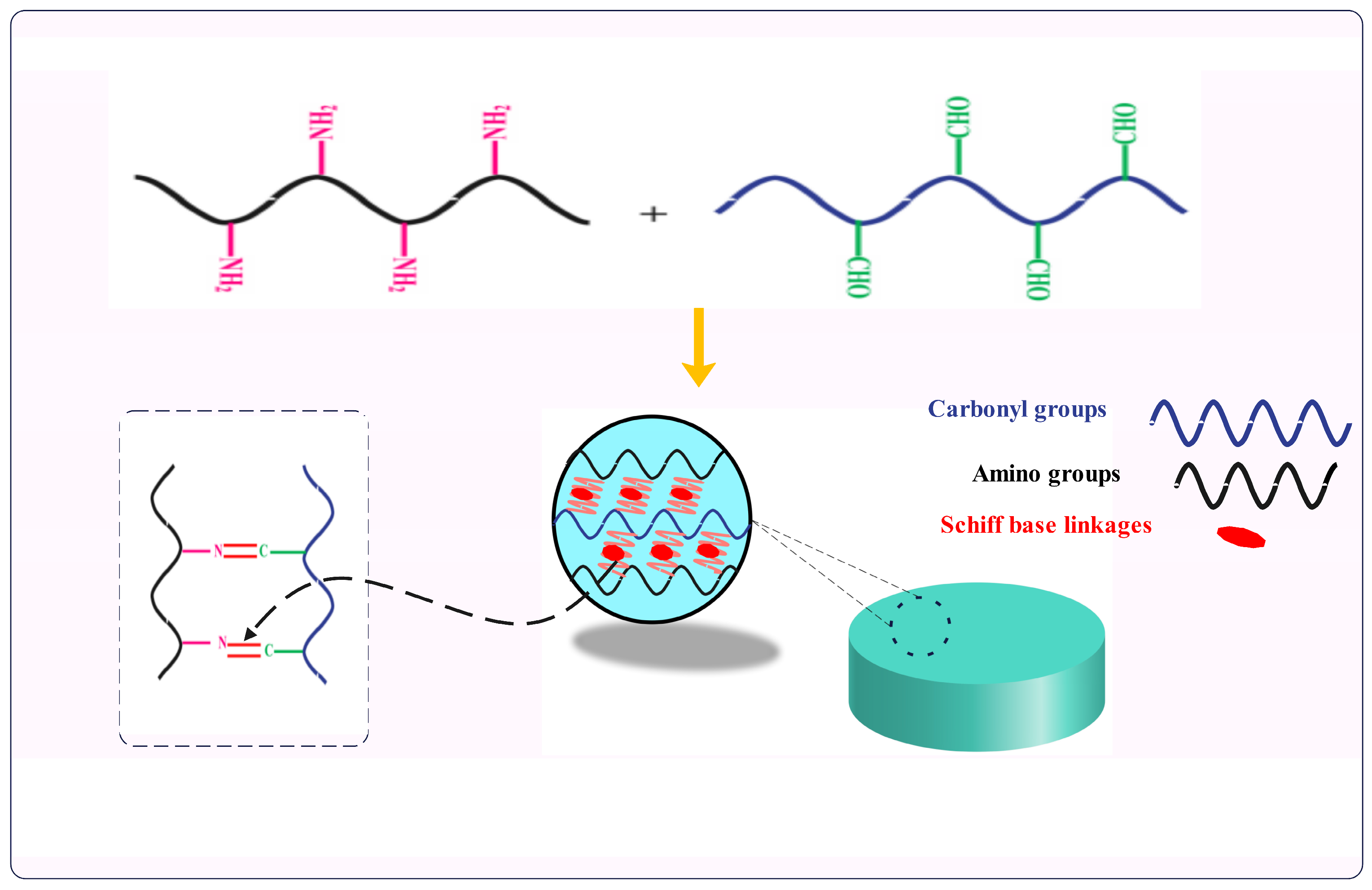
5. Cross-Linking with Carbonyl Derivatives of Polysaccharides—Schiff Base Linkages
5.1. Particular Cases of Schiff Bases
5.1.1. Hydrazone and Acyl Hydrazone Bonds-Based Hydrogels
5.1.2. Oxime Bond-Based Hydrogels
6. Biomedical Applications of Hydrogels Based on Cross-Linked Proteins with Carbonyl Derivatives of Polysaccharides
6.1. Biomedical Applications of Hydrogels Obtained through Schiff Base Chemistry
6.1.1. Biomedical Applications of Schiff Base Hydrogel Films
- Drug delivery
- Tissue engineering
- Wound healing
6.1.2. Biomedical Applications of Hydrogel Microparticles Containing Schiff Bases
- Drug delivery applications
- Cell encapsulation
- Bone regeneration applications
6.1.3. Biomedical Applications of Hydrogel Nanoparticles (Nanogels) Obtained by Schiff Base Reaction
- Drug Delivery Applications
- Anti-tumor and cancer therapy
- Antibacterial Applications
7. Conclusions
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Chaudhary, S.; Jain, V.P.; Jaiswar, G. The Composition of Polysaccharides: Monosaccharides and Binding, Group Decorating, Polysaccharides Chains. In Innovation in Nano-Polysaccharides for Eco-Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–118. [Google Scholar]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer Polysaccharides from Natural Resources: A Review of Recent Research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Li, Q.; He, X.; Wang, X.; Wen, Y.; Zeng, L.; Yu, W.; Hu, P.; Chen, H. A Multifunctional Hydrogel Based on Nature Polysaccharide Fabricated by Schiff Base Reaction. Eur. Polym. J. 2023, 197, 112330. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-Healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 1–18. [Google Scholar] [CrossRef]
- Yang, C.; Gao, L.; Liu, X.; Yang, T.; Yin, G.; Chen, J.; Guo, H.; Yu, B.; Cong, H. Injectable Schiff Base Polysaccharide Hydrogels for Intraocular Drug Loading and Release. J. Biomed. Mater. Res. Part A 2019, 107, 1909–1916. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, M.; Guan, Y.; Zhang, Y. Multiple Responsive Hydrogel Films Based on Dynamic Schiff Base Linkages. Polym. Chem. 2014, 5, 7081–7089. [Google Scholar] [CrossRef]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently Polysaccharide-Based Alginate/Chitosan Hydrogel Embedded Alginate Microspheres for BSA Encapsulation and Soft Tissue Engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- Weng, L.; Rostamzadeh, P.; Nooryshokry, N.; Le, H.C.; Golzarian, J. In Vitro and in Vivo Evaluation of Biodegradable Embolic Microspheres with Tunable Anticancer Drug Release. Acta Biomater. 2013, 9, 6823–6833. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In Situ Hydrogel Constructed by Starch-Based Nanoparticles via a Schiff Base Reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Su, M.; Ruan, L.; Dong, X.; Tian, S.; Lang, W.; Wu, M.; Chen, Y.; Lv, Q.; Lei, L. Current State of Knowledge on Intelligent-Response Biological and Other Macromolecular Hydrogels in Biomedical Engineering: A Review. Int. J. Biol. Macromol. 2023, 227, 472–492. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Tomasik, P. Chemical Modifications of Polysaccharides. Chem. Funct. Prop. Food Sacch. 2003, 2013, 123–130. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Cooperstone, J.L.; Cichon, M.J.; Joachim, H.V.; Monica, G. Colorants Fennema’s Food Chemistry; Damodaran, S., Parkin, L.K., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Abdalla, T.H.; Nasr, A.S.; Bassioni, G.; Harding, D.R.; Kandile, N.G. Fabrication of Sustainable Hydrogels-Based Chitosan Schiff Base and Their Potential Applications. Arab. J. Chem. 2022, 15, 103511. [Google Scholar] [CrossRef]
- Buwalda, S. Bio-Based Composite Hydrogels for Biomedical Applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering Multifunctional Dynamic Hydrogel for Biomedical and Tissue Regenerative Applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Ballance, S.; Potthast, A.; Christensen, B.E. An Evaluation of Tritium and Fluorescence Labelling Combined with Multi-Detector SEC for the Detection of Carbonyl Groups in Polysaccharides. Carbohydr. Polym. 2009, 76, 196–205. [Google Scholar] [CrossRef]
- Matsumura, K.; Rajan, R. Oxidized Polysaccharides as Green and Sustainable Biomaterials. Curr. Org. Chem. 2021, 25, 1483–1496. [Google Scholar] [CrossRef]
- Bragd, P.L.; Van Bekkum, H.; Besemer, A.C. TEMPO-Mediated Oxidation of Polysaccharides: Survey of Methods and Applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Jiang, B.; Drouet, E.; Milas, M.; Rinaudo, M. Study on TEMPO-Mediated Selective Oxidation of Hyaluronan and the Effects of Salt on the Reaction Kinetics. Carbohydr. Res. 2000, 327, 455–461. [Google Scholar] [CrossRef]
- Parikka, K.; Leppänen, A.S.; Pitkänen, L.; Reunanen, M.; Willför, S.; Tenkanen, M. Oxidation of Polysaccharides by Galactose Oxidase. J. Agric. Food Chem. 2010, 58, 262–271. [Google Scholar] [CrossRef]
- Jaušovec, D.; Vogrinčič, R.; Kokol, V. Introduction of Aldehyde vs. Carboxylic Groups to Cellulose Nanofibers Using Laccase/TEMPO Mediated Oxidation. Carbohydr. Polym. 2015, 116, 74–85. [Google Scholar] [CrossRef]
- Klein-Koerkamp, C.; Granet, R.; Zerrouki, R.; Villandier, N.; Jérôme, F.; Barrault, J.; Krausz, P. Efficient Oxidative Modification of Polysaccharides in Water Using H2O2 Activated by Iron Sulfophthalocyanine. Carbohydr. Polym. 2009, 78, 938–944. [Google Scholar] [CrossRef]
- Teleman, A.; Kruus, K.; Ämmälahti, E.; Buchert, J.; Nurmi, K. Structure of Dicarboxyl Malto-Oligomers Isolated from Hypochlorite-Oxidised Potato Starch Studied by 1H and 13C NMR Spectroscopy. Carbohydr. Res. 1999, 315, 286–292. [Google Scholar] [CrossRef]
- Ghafar, A.; Gurikov, P.; Subrahmanyam, R.; Parikka, K.; Tenkanen, M.; Smirnova, I.; Mikkonen, K.S. Mesoporous Guar Galactomannan Based Biocomposite Aerogels through Enzymatic Crosslinking. Compos. Part A Appl. Sci. Manuf. 2017, 94, 93–103. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Suuronen, J.P.; Ghafar, A.; Serimaa, R.; Tenkanen, M. Enzymatic Oxidation as a Potential New Route to Produce Polysaccharide Aerogels. RSC Adv. 2014, 4, 11884–11892. [Google Scholar] [CrossRef]
- Parikka, K.; Master, E.; Tenkanen, M. Oxidation with Galactose Oxidase: Multifunctional Enzymatic Catalysis. J. Mol. Catal. B Enzym. 2015, 120, 47–59. [Google Scholar] [CrossRef]
- Cooper, J.A.; Smith, W.; Bacila, M.; Medina, H. Galactose Oxidase from Polyporus Circinatus, Fr. J. Biol. Chem. 1959, 234, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Parikka, K.; Tenkanen, M. Oxidation of Methyl α-d-Galactopyranoside by Galactose Oxidase: Products Formed and Optimization of Reaction Conditions for Production of Aldehyde. Carbohydr. Res. 2009, 344, 14–20. [Google Scholar] [CrossRef]
- Mollerup, F.; Haara, M.; Parikka, K.; Xu, C.; Tenkanen, M.; Master, E. Enzymatic Oxidation of Plant Polysaccharides Adsorbed to Cellulose Surfaces. New Biotechnol. 2014, 31, S7–S8. [Google Scholar] [CrossRef]
- Patel, I.; Ludwig, R.; Haltrich, D.; Rosenau, T.; Potthast, A. Studies of the Chemoenzymatic Modification of Cellulosic Pulps by the Laccase-TEMPO System. Holzforschung 2011, 65, 475–481. [Google Scholar] [CrossRef]
- Aracri, E.; Vidal, T. Enhancing the Effectiveness of a Laccase—TEMPO Treatment Has a Biorefining Effect on Sisal Cellulose Fibres. Cellulose 2012, 19, 867–877. [Google Scholar] [CrossRef]
- Xu, S.; Song, Z.; Qian, X.; Shen, J. Introducing Carboxyl and Aldehyde Groups to Softwood-Derived Cellulosic Fibers by Laccase/TEMPO-Catalyzed Oxidation. Cellulose 2013, 20, 2371–2378. [Google Scholar] [CrossRef]
- Ding, W.; Wang, Y.N.; Zhou, J.; Shi, B. Effect of Structure Features of Polysaccharides on Properties of Dialdehyde Polysaccharide Tanning Agent. Carbohydr. Polym. 2018, 201, 549–556. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate Oxidation of Polysaccharides for Modification of Chemical and Physical Properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Malaprade, L. Oxidation of Some Polyalcohols by Periodic Acid-Applications. Comptes Rendus 1928, 186, 382–384. [Google Scholar]
- Malaprade, L. Action of Polyalcohols on Periodic Acid. Analytical Application. Bull. Soc. Chim. Fr. 1928, 43, 683–696. [Google Scholar]
- Criegee, R. Oxidation with Quadrivalent Lead Salts. II. Oxidative Cleavage of Glycols. Ber. Dtsch. Chem. Ges. B 1931, 64, 260–266. [Google Scholar] [CrossRef]
- Fleury, P.; Lange, J. Sur l’oxydation des acides alcools et des sucres par l’acid periodique. Comptes Rendus Acad. Sci. 1932, 195, 1395–1397. [Google Scholar]
- Perlin, A.S. Glycol-Cleavage Oxidation. Adv. Carbohydr. Chem. Biochem. 2006, 60, 183–250. [Google Scholar] [PubMed]
- Ding, W.; Wu, Y. Sustainable Dialdehyde Polysaccharides as Versatile Building Blocks for Fabricating Functional Materials: An Overview. Carbohydr. Polym. 2020, 248, 116801. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhou, J.; Zeng, Y.; Wang, Y.N.; Shi, B. Preparation of Oxidized Sodium Alginate with Different Molecular Weights and Its Application for Crosslinking Collagen Fiber. Carbohydr. Polym. 2017, 157, 1650–1656. [Google Scholar] [CrossRef]
- Painter, T.; Larsen, B. Formation of hemiacetals between neighboring hexuronic acid residues during the periodate oxidation of alginate. Acta Chem. Scandinava 1970, 24, 813–833. [Google Scholar] [CrossRef]
- Kholiya, F.; Chaudhary, J.P.; Vadodariya, N.; Meena, R. Synthesis of Bio-Based Aldehyde from Seaweed Polysaccharide and Its Interaction with Bovine Serum Albumin. Carbohydr. Polym. 2016, 150, 278–285. [Google Scholar] [CrossRef]
- Wongsagon, R.; Shobsngob, S.; Varavinit, S. Preparation and Physicochemical Properties of Dialdehyde Tapioca Starch. Starch/Staerke 2005, 57, 166–172. [Google Scholar] [CrossRef]
- Bobbitt, J.M. Periodate Oxidation of Carbohydrates. Adv. Carbohydr. Chem. 1956, 11, 1–41. [Google Scholar] [CrossRef]
- Dewar, M.J. Structure of Stipitatic Acid. Nature 1945, 155, 50–51. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent Advances in Polysaccharide-Based Hydrogels for Synthesis and Applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Ye, Y.; Ren, H.; Zhu, S.; Tan, H.; Li, X.; Li, D.; Mu, C. Synthesis of Oxidized β-Cyclodextrin with High Aqueous Solubility and Broad-Spectrum Antimicrobial Activity. Carbohydr. Polym. 2017, 177, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Darwin, K.H.; Stanley, S.A. The Aldehyde Hypothesis: Metabolic Intermediates as Antimicrobial Effectors. Open Biol. 2022, 12, 220010. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Zhu, M.; Li, X.; Xu, Y.; Wei, H.; Li, D.; Mu, C. Effects of Carboxyl and Aldehyde Groups on the Antibacterial Activity of Oxidized Amylose. Carbohydr. Polym. 2018, 192, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Paiva, M.T.P.; Kishima, J.O.F.; Silva, J.B.M.D.; Mantovan, J.; Colodi, F.G.; Mali, S. Crosslinking Methods in Polysaccharide-Based Hydrogels for Drug Delivery Systems. Biomed. Mater. Devices 2024, 2, 288–306. [Google Scholar] [CrossRef]
- Li, X.; Weng, Y.; Kong, X.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Wang, X.; Chen, H. A Covalently Crosslinked Polysaccharide Hydrogel for Potential Applications in Drug Delivery and Tissue Engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2857–2865. [Google Scholar] [CrossRef]
- Jabeen, N.; Iram, F.; Atif, M.; Fatimah, N.E. Functionalization of Arabinoxylan in Chitosan Based Blends by Periodate Oxidation for Drug Release Study. ChemistrySelect 2024, 9, e202303496. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodríguez-fernández, J.; Gallego- Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for in Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Kurita, O.; Miyake, Y.; Yamazaki, E. Chemical Modification of Citrus Pectin to Improve Its Dissolution into Water. Carbohydr. Polym. 2012, 87, 1720–1727. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) Forwound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Chen, S.; Lin, Y.; Yue, Y. A Schiff Base Hydrogel Dressing Loading Extracts from Periplaneta Americana for Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 230, 123256. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.X.; Mo, X.M.; Pan, J.F. In Situ Forming Hydrogel of Natural Polysaccharides through Schiff Base Reaction for Soft Tissue Adhesive and Hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Chaudhary, V.; Sharma, B.; Kumar, A.; Bhagi, A.K.; Singh, K.P. Sustainable Polysaccharide Hydrogels Based on Dynamic Schiff Base Linkages as Versatile Building Blocks for Fabricating Advanced Functional Materials. J. Polym. Environ. 2023, 31, 1257–1278. [Google Scholar] [CrossRef]
- Perera, M.M.; Ayres, N. Dynamic Covalent Bonds in Self-Healing, Shape Memory, and Controllable Stiffness Hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Li, K.; Yao, F.; Oderinde, O.; Fu, G.; Xu, L. Polysaccharide-Templated Preparation of Mechanically-Tough, Conductive and Self-Healing Hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-Cross-Linking Biopolymers as Injectable in Situ Forming Biodegradable Scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Metaxa, A.F.; Kordas, G. Modified Polysaccharides as Drug Delivery. In Polysaccharides Bioactivity and Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; pp. 1805–1835. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Behrooznia, Z.; Nourmohammadi, J. Polysaccharide-Based Materials as an Eco-Friendly Alternative in Biomedical, Environmental, and Food Packaging. Giant 2024, 19, 100301. [Google Scholar] [CrossRef]
- Aleksanyan, K.V. Polysaccharides for Biodegradable Packaging Materials: Past, Present, and Future (Brief Review). Polymers 2023, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, M.; Campbell, S.; Kinio, D.; Hoare, T. Tuning Gelation Time and Morphology of Injectable Hydrogels Using Ketone-Hydrazide Cross-Linking. Biomacromolecules 2014, 15, 781–790. [Google Scholar] [CrossRef] [PubMed]
- FitzSimons, T.M.; Anslyn, E.V.; Rosales, A.M. Effect of PH on the Properties of Hydrogels Cross-Linked via Dynamic Thia-Michael Addition Bonds. ACS Polym. Au 2022, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable Polymeric Hydrogels for the Delivery of Therapeutic Agents: A Review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Payne, M.J. An Evaluation of the Local Reaction and Biodegradation of Calcium Sodium Alginate (Kaltostat) Following Subcutaneous Implantation in the Rat. J. R. Coll. Surg. Edinb. 1994, 39, 284–288. [Google Scholar]
- Balakrishnan, B.; Lesieur, S.; Labarre, D.; Jayakrishnan, A. Periodate Oxidation of Sodium Alginate in Water and in Ethanol-Water Mixture: A Comparative Study. Carbohydr. Res. 2005, 340, 1425–1429. [Google Scholar] [CrossRef]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of Sodium Alginate and Characterization of the Oxidized Derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling Alginate Oxidation Conditions for Making Alginate-Gelatin Hydrogels. Carbohydr. Polym. 2018, 198, 509–517, Erratum in Carbohydr. Polym. 2019, 208, 200. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xie, Y.J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate Composites for Bone Tissue Engineering: A Review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.K. Role of Alginate in Bone Tissue Engineering. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 73, pp. 45–57. [Google Scholar]
- Lai, H.L.; Abu’Khalil, A.; Craig, D.Q.M. The Preparation and Characterisation of Drug-Loaded Alginate and Chitosan Sponges. Int. J. Pharm. 2003, 251, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ramesh Babu, V.; Sairam, M.; Hosamani, K.M.; Aminabhavi, T.M. Preparation of Sodium Alginate-Methylcellulose Blend Microspheres for Controlled Release of Nifedipine. Carbohydr. Polym. 2007, 69, 241–250. [Google Scholar] [CrossRef]
- Orive, G.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of Microcapsules for Cell Immobilization Elaborated with Different Type of Alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.X.; Hwang, D.S. A Biomimetic Chitosan Composite with Improved Mechanical Properties in Wet Conditions. Biotechnol. Prog. 2013, 29, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Kim, Y.K.; Arote, R.; Nah, J.W.; Cho, M.H.; Choi, Y.J.; Akaike, T.; Cho, C.S. Chitosan-Graft-Polyethylenimine as a Gene Carrier. J. Control. Release 2007, 117, 273–280. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Li, F. A Novel Sensing Platform Based on Periodate-Oxidized Chitosan. Anal. Methods 2010, 2, 2011–2016. [Google Scholar] [CrossRef]
- Janjic, S.; Kostic, M.; Vucinic, V.; Dimitrijevic, S.; Popovic, K.; Ristic, M.; Skundric, P. Biologically Active Fibers Based on Chitosan-Coated Lyocell Fibers. Carbohydr. Polym. 2009, 78, 240–246. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S.; Ramadan, A.M.; Al-Sehemi, A.G.; Irfan, A.; Bondock, S. An Unexpected Reactivity during Periodate Oxidation of Chitosan and the Affinity of Its 2, 3-Di-Aldehyde toward Sulfa Drugs. Carbohydr. Polym. 2017, 175, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Bennamara, A.; Abdelmjid, A.; Berrada, M.; Charhouf, I.; Bennamara, A.; Abourriche, A.; Chenite, A.; Zhu, J.; Berrada, M. Characterization of a Dialdehyde Chitosan Generated by Periodate Oxidation. Artic. Int. J. Sci. Basic Appl. Res. 2014, 4531, 336–348. [Google Scholar]
- Christensen, B.E.; Vold, I.M.N.; Vårum, K.M. Chain Stiffness and Extension of Chitosans and Periodate Oxidised Chitosans Studied by Size-Exclusion Chromatography Combined with Light Scattering and Viscosity Detectors. Carbohydr. Polym. 2008, 74, 559–565. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Christensen, B.E. Periodate Oxidation of Chitosans with Different Chemical Compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef]
- Bruneel, D.; Schacht, E. Chemical Modification of Pullulan: 1. Periodate Oxidation. Polymer 1993, 34, 2628–2632. [Google Scholar] [CrossRef]
- Mishra, B.; Suneetha, V.; Rath, K. The Role of Microbial Pullulan, a Biopolymer in Pharmaceutical Approaches: A Review. J. Appl. Pharm. Sci. 2011, 1, 45–50. [Google Scholar]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning Structure and Properties of Gelatin Edible Films through Pullulan Dialdehyde Crosslinking. Lwt 2021, 138, 110607. [Google Scholar] [CrossRef]
- Li, S.; Yi, J.; Yu, X.; Wang, Z.; Wang, L. Preparation and Characterization of Pullulan Derivative/Chitosan Composite Film for Potential Antimicrobial Applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef]
- Selvakumar, G.; Lonchin, S. Fabrication and Characterization of Collagen-Oxidized Pullulan Scaffold for Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 1592–1599. [Google Scholar] [CrossRef]
- Scomparin, A.; Salmaso, S.; Bersani, S.; Satchi-Fainaro, R.; Caliceti, P. Novel Folated and Non-Folated Pullulan Bioconjugates for Anticancer Drug Delivery. Eur. J. Pharm. Sci. 2011, 42, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial Sources, Production and Applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Saini, N.; Pandit, V.; Ali, S. An Insight To Pullulan: A Biopolymer in Pharmaceutical Approaches. Int. J. Basic Appl. Sci. 2012, 1, 202–219. [Google Scholar] [CrossRef]
- Zhang, L.R.; Zhang, A.L.; Zhang, H.Z.; Liu, J.; Tang, K.Y. Preparation and Application of Dialdehyde Pullulan for the Construction of Gelatin Hydrogels. IOP Conf. Ser. Mater. Sci. Eng. 2019, 504, 012029. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant Degradation in Periodate Oxidation of Carboxymethyl Cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, Characterization and Feasibility Study of Dialdehyde Carboxymethyl Cellulose as a Novel Crosslinking Reagent. Carbohydr. Polym. 2016, 137, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, A.; Wasiak, I.; Ciach, T. Carboxymethyl Cellulose Oxidation to Form Aldehyde Groups. Chall. Mod. Technol. 2013, 4, 11–18. [Google Scholar]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-Linking of Dialdehyde Carboxymethyl Cellulose with Silk Sericin to Reinforce Sericin Film for Potential Biomedical Application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef]
- Ding, W.; Yi, Y.; Wang, Y.N.; Zhou, J.; Shi, B. Peroxide-Periodate Co-Modification of Carboxymethylcellulose to Prepare Polysaccharide-Based Tanning Agent with High Solid Content. Carbohydr. Polym. 2019, 224, 115169. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A.; Ji, H.F. Synthesis and Application of Photo-Active Carboxymethyl Cellulose Derivatives. React. Funct. Polym. 2016, 102, 137–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Shao, S.; Chang, X.; Li, M. Periodate Oxidation of Carboxymethyl Cellulose under Controlled Conditions. ChemistrySelect 2020, 5, 6765–6773. [Google Scholar] [CrossRef]
- Dellali, M.; Iurciuc, C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules 2021, 26, 2185. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Mincke, S.; Folens, K.; Vanden Bussche, F.; Lapeire, L.; Verbeken, K.; Van Der Voort, P.; Tessema, D.A.; Du Laing, G.; Stevens, C.V. Dialdehyde Carboxymethyl Cellulose Cross-Linked Chitosan for the Recovery of Palladium and Platinum from Aqueous Solution. React. Funct. Polym. 2019, 141, 145–154. [Google Scholar] [CrossRef]
- Lü, S.; Liu, M.; Ni, B. An Injectable Oxidized Carboxymethylcellulose/N-Succinyl-Chitosan Hydrogel System for Protein Delivery. Chem. Eng. J. 2010, 160, 779–787. [Google Scholar] [CrossRef]
- Applications, F. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Functionalization of Pectin by Periodate Oxidation. Carbohydr. Polym. 2013, 98, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Preparation and Characterization of In-Situ Crosslinked Pectin-Gelatin Hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, Q.; Shi, X.; Xie, Z.; Hu, J.; Liu, Y. Effects of Preparation Parameters on the Properties of the Crosslinked Pectin Nanofiber Mats. Carbohydr. Polym. 2021, 269, 118314. [Google Scholar] [CrossRef]
- Shi, X.; Cui, S.; Song, X.; Rickel, A.P.; Sanyour, H.J.; Zheng, J.; Hu, J.; Hong, Z.; Zhou, Y.; Liu, Y. Gelatin-Crosslinked Pectin Nanofiber Mats Allowing Cell Infiltration. Mater. Sci. Eng. C 2020, 112, 110941. [Google Scholar] [CrossRef]
- Fan, L.; Sun, Y.; Xie, W.; Zheng, H.; Liu, S. Oxidized Pectin Cross-Linked Carboxymethyl Chitosan: A New Class of Hydrogels. J. Biomater. Sci. Polym. Ed. 2012, 23, 2119–2132. [Google Scholar] [CrossRef]
- Ahadi, F.; Khorshidi, S.; Karkhaneh, A. A Hydrogel/Fiber Scaffold Based on Silk Fibroin/Oxidized Pectin with Sustainable Release of Vancomycin Hydrochloride. Eur. Polym. J. 2019, 118, 265–274. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Tanzi, M.C.; Barbosa, M.A.; Granja, P.L. Biofunctional Chemically Modified Pectin for Cell Delivery. Soft Matter 2012, 8, 4731–4739. [Google Scholar] [CrossRef]
- Bharskar, G. A Review on Hydrogel. World J. Pharm. Pharm. Sci. 2020, 9, 1288–1298. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Su, J. Thiol-Mediated Chemoselective Strategies for in Situ Formation of Hydrogels. Gels 2018, 4, 72. [Google Scholar] [CrossRef]
- Trengove, A.; Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Di Bella, C.; O’Connor, A.J. Microbial Transglutaminase Improves Ex Vivo Adhesion of Gelatin Methacryloyl Hydrogels to Human Cartilage. Front. Med. Technol. 2021, 3, 773673. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled Release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Buchert, J.; Cura, D.E.; Ma, H.; Gasparetti, C.; Monogioudi, E.; Faccio, G.; Mattinen, M.; Boer, H.; Partanen, R.; Selinheimo, E.; et al. Crosslinking Food Proteins for Improved Functionality. Annu. Rev. Food Sci. Technol. 2010, 1, 113–138. [Google Scholar] [CrossRef] [PubMed]
- Nicol, E. Photopolymerized Porous Hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Zhang, J.; Bethel, K.; Liu, Y.; Davis, E.M.; Zeng, H.; Kong, Z.; Johnson, B.N. Closed-Loop Controlled Photopolymerization of Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 40365–40378. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Xu, M.; Zhang, L.; Zhang, J.; Dong, W. Mechanical Testing and Reinforcing Mechanisms of a Magnetic Field-Sensitive Hydrogel Prepared by Microwave-Assisted Polymerization. Polym. Test. 2018, 71, 344–351. [Google Scholar] [CrossRef]
- Sosnik, A.; Gotelli, G.; Abraham, G.A. Microwave-Assisted Polymer Synthesis (MAPS) as a Tool in Biomaterials Science: How New and How Powerful. Prog. Polym. Sci. 2011, 36, 1050–1078. [Google Scholar] [CrossRef]
- Tanan, W.; Saengsuwan, S. Microwave Assisted Synthesis of Poly (Acrylamide-Co-2-Hydroxyethyl Methacrylate)/Poly(Vinyl Alcohol) Semi-IPN Hydrogel. Energy Procedia 2014, 56, 386–393. [Google Scholar] [CrossRef]
- Weerasinghe, M.A.S.N.; Dodo, O.J.; Rajawasam, C.W.H.; Raji, I.O.; Wanasinghe, S.V.; Konkolewicz, D.; De Alwis Watuthanthrige, N. Educational Series: Turning Monomers into Crosslinked Polymer Networks. Polym. Chem. 2023, 14, 4503–4514. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in Polysaccharide and Protein Films and Coatings for Food Contact—A Review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Jus, S.; Stachel, I.; Fairhead, M.; Meyer, M.; Thöny-Meyer, L.; Guebitz, G.M. Enzymatic Cross-Linking of Gelatine with Laccase and Tyrosinase. Biocatal. Biotransformation 2012, 30, 86–95. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of Transglutaminase-Crosslinked Whey Protein/Pectin Films as Water Barrier Coatings in Fried and Baked Foods. Food Bioprocess Technol. 2014, 7, 447–455. [Google Scholar] [CrossRef]
- Battisti, R.; Fronza, N.; Vargas Júnior, Á.; da Silveira, S.M.; Damas, M.S.P.; Quadri, M.G.N. Gelatin-Coated Paper with Antimicrobial and Antioxidant Effect for Beef Packaging. Food Packag. Shelf Life 2017, 11, 115–124. [Google Scholar] [CrossRef]
- Benbetta, N. Tuning the Functional Properties of Polysaccharide—Protein Bio-Based Edible Films by Chemical, Enzymatic, and Physical Cross-Linking. Compr. Rev. Food Sci. Food Saf. 2016, 15, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Pruett, S.B.; Fan, R.; Zheng, Q.; Schwab, C. Patterns of Immunotoxicity Associated with Chronic as Compared with Acute Exposure to Chemical or Physical Stressors and Their Relevance with Regard to the Role of Stress and with Regard to Immunotoxicity Testing. Toxicol. Sci. 2009, 109, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Z.; Jeon, J.; Jiang, B.; Subramani, S.V.; Li, J.; Zhang, F. Protein-Based Hydrogels and Their Biomedical Applications. Molecules 2023, 28, 4988. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M. Development of Hydrogels and Study the Effect of Their Mechanical Properties on Podocyte Behaviors. Ph.D. Thesis, Université Montpellier, Montpellier, France, 2020; pp. 23–27. [Google Scholar]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.K.; Zhong, M.; Zhang, L.Q.; Liu, X.Y.; Xie, X.M. Robust and Self-Healable Nanocomposite Physical Hydrogel Facilitated by the Synergy of Ternary Crosslinking Points in a Single Network. J. Mater. Chem. B 2016, 4, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zuo, H.; Gao, W.; Ning, N.; Tian, M.; Zhang, L. A Robust, Self-Healable, and Shape Memory Supramolecular Hydrogel by Multiple Hydrogen Bonding Interactions. Macromol. Rapid Commun. 2018, 39, 1800138. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Liu, Z.; Gao, X.; Long, S.; Zhang, G. Integrated Functional High-Strength Hydrogels with Metal-Coordination Complexes and H-Bonding Dual Physically Cross-Linked Networks. Macromol. Rapid Commun. 2018, 39, 1800400. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597, Erratum in Macromol. Biosci. 2007, 7, 1299. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Hyon, S.H.; Ikada, Y. Stereocomplex Formation between Enantiomeric Poly(Lactic Acid)s. 2. Stereocomplex Formation in Concentrated Solutions. Macromolecules 1991, 24, 2719–2724. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte Complexes: Mechanisms, Critical Experimental Aspects, and Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef]
- Rembaum, A. Polyelectrolyte Complexes. J. Macromol. Sci. Part A Chem. 1969, 3, 87–99. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, L.; Yuan, J.; Chen, Y.; Leng, Y. Physical Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128164211. [Google Scholar]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive Sol-Gel Reversible Hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Taniguchi, N. Amino Acids and Proteins. Available online: https://www.academia.edu/117395586/Amino_Acids_and_Proteins (accessed on 9 July 2024).
- Whitford, D. Proteins; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; ISBN 0471498939. [Google Scholar]
- Sun, P.D.; Foster, C.E.; Boyington, J.C. Overview of Protein Structural and Functional Folds. Curr. Protoc. Protein Sci. 2004, 35, 1711–171189. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 5th ed.; W.W. Norton & Company: New York, NY, USA, 2007; p. 1392. [Google Scholar] [CrossRef]
- Jayachandran, B.; Parvin, T.N.; Alam, M.M.; Chanda, K.; MM, B. Insights on Chemical Crosslinking Strategies for Proteins. Molecules 2022, 27, 8124. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen i Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Controlled Degradation of Hydrogels Using Multi-Functional Cross-Linking Molecules. Biomaterials 2004, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.F.; Tronci, G.; Rößle, M.; Neffe, A.T.; Jung, F.; Lendlein, A. Photocrosslinked Co-Networks from Glycidylmethacrylated Gelatin and Poly(Ethylene Glycol) Methacrylates. Macromol. Biosci. 2012, 12, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical Cross-Linking Methods for Cell Encapsulation in Hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current Understanding of the Applications of Photocrosslinked Hydrogels in Biomedical Engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-Graphene Oxide Hydrogels with Embedded Magnetic Iron Oxide Nanoparticles for Dye Removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Mevik, B.-H.; Wehrens, R. PDF Hosted at the Radboud Repository of the Radboud University Nijmegen Article Information. J. Stat. Softw. 2007, 18, 3–6. [Google Scholar]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/Poly(Vinyl Alcohol) Hydrogels Prepared by Freeze/Thaw Method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Schiff, H. Mittheilungen Aus Dem Universitätslaboratorium in Pisa: Eine Neue Reihe Organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Mohammad, N.; Uddin, E.; Uddin, S.; Babar, I.H.; Hossain, S.; Bitu, N.A.; Khan, M.N.; Asraf, A.; Hossen, F.; Kudrat-E-Zahan, M. Exploring Schiff Base Chemistry—An Overview. Int. J. Chem. and Pharm. Sci. 2021, 9, 18–31. [Google Scholar]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, C.; Chen, X. Hydrogels Based on PH-Responsive Reversible Carbon-Nitrogen Double-Bond Linkages for Biomedical Applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Abid Ali, S.; Khan, K.M. Schiff Bases in Medicinal Chemistry: A Patent Review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Raines, R.T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chemie Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, Z. Benzoic-Imine-Based Physiological-PH-Responsive Materials for Biomedical Applications. Chem. Asian J. 2016, 11, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.D.; Joiner, C.S.; Stoddart, J.F. Template-Directed Synthesis Employing Reversible Imine Bond Formation. Chem. Soc. Rev. 2007, 36, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pham, C.Y.; Yu, R.; Petit, E.; Li, S.; Barboiu, M. Dynamic Hydrogels Based on Double Imine Connections and Application for Delivery of Fluorouracil. Front. Chem. 2020, 8, 739. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic Imine Chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Yu, R. Nouveaux Hydrogels à Liaison Imine Double Préparés à Partir d’O-Carboxyméthyl Chitosane et de Jeffamine Par Chimie Covalente Dynamique Pour Applications Biomédicales. Ph.D. Thesis, Université Montpellier, Montpellier, France, 2021. [Google Scholar]
- Lutz, E. Dynamic Covalent Surfactants for the Controlled Release of Bioactive Volatiles. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 2014; pp. 1–254. [Google Scholar]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with PH-Regulated Recovery of Self-Healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar] [CrossRef]
- Ma, L.; Su, W.; Ran, Y.; Ma, X.; Yi, Z.; Chen, G.; Chen, X.; Deng, Z.; Tong, Q.; Wang, X.; et al. Synthesis and Characterization of Injectable Self-Healing Hydrogels Based on Oxidized Alginate-Hybrid-Hydroxyapatite Nanoparticles and Carboxymethyl Chitosan. Int. J. Biol. Macromol. 2020, 165, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Daemi, H.; Ghanian, M.-H.; Foudazi, R. Modified Hydroxyapatite Nanoparticles Reinforced Nanocomposite Hydrogels Based on Gelatin/Oxidized Alginate via Schiff Base Reaction. Carbohydr. Polym. Technol. Appl. 2021, 2, 100056. [Google Scholar] [CrossRef]
- Toh-Boyo, G.M.; Njong, R.N.; Babette, E.M.; Nfor, E.N. Synthesis, Spectroscopic, Molecular Modeling and Anti-Fungal Studies of Some Divalent Metal Complexes of 4-Hydroxyacetophenone Isonicotinoyl Hydrazone. Open J. Inorg. Chem. 2021, 11, 95–109. [Google Scholar] [CrossRef]
- Lehn, J.M. Dynamers: Dynamic Molecular and Supramolecular Polymers. Prog. Polym. Sci. 2005, 30, 814–831. [Google Scholar] [CrossRef]
- Patenaude, M.; Smeets, N.M.B.; Hoare, T. Designing Injectable, Covalently Cross-Linked Hydrogels for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Apostolides, D.E.; Patrickios, C.S. Dynamic Covalent Polymer Hydrogels and Organogels Crosslinked through Acylhydrazone Bonds: Synthesis, Characterization and Applications. Polym. Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Wang, L.L.; Highley, C.B.; Yeh, C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single-and double-network hydrogels containing dynamic covalent crosslinks. J. Biomed. Mater. Res. A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Collins, J.; Nadgorny, M.; Xiao, Z.; Connal, L.A. Doubly Dynamic Self-Healing Materials Based on Oxime Click Chemistry and Boronic Acids. Macromol. Rapid Commun. 2017, 38, 1600760. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yu, J.; Zhang, X.; Chen, G. Recent Advances on Catalytic Asymmetric Hydrogenation of Oximes and Oxime Ethers. Tetrahedron Lett. 2024, 136, 154914. [Google Scholar] [CrossRef]
- Lin, F.; Yu, J.; Tang, W.; Zheng, J.; Defante, A.; Guo, K.; Wesdemiotis, C.; Becker, M.L. Peptide-Functionalized Oxime Hydrogels with Tunable Mechanical Properties and Gelation Behavior. Biomacromolecules 2013, 14, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hill, M.R.; Sumerlin, B.S. Self-Healing Hydrogels Containing Reversible Oxime Crosslinks. Soft Matter 2015, 11, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Farahani, P.E.; Adelmund, S.M.; Shadish, J.A.; DeForest, C.A. Photomediated Oxime Ligation as a Bioorthogonal Tool for Spatiotemporally-Controlled Hydrogel Formation and Modification. J. Mater. Chem. B 2017, 5, 4435–4442. [Google Scholar] [CrossRef]
- Deforest, C.A.; Tirrell, D.A. A Photoreversible Protein-Patterning Approach for Guiding Stem Cell Fate in Three-Dimensional Gels. Nat. Mater. 2015, 14, 523–531. [Google Scholar] [CrossRef]
- Labowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Mondal, S.S.; Jaiswal, N.; Bera, P.S.; Tiwari, R.K.; Behera, J.N.; Chanda, N.; Ghosal, S.; Saha, T.K. Cu (II) and Co (II/III) Complexes of N,O-Chelated Schiff Base Ligands: DNA Interaction, Protein Binding, Cytotoxicity, Cell Death Mechanism and Reactive Oxygen Species Generation Studies. Appl. Organomet. Chem. 2021, 35, e6026. [Google Scholar] [CrossRef]
- Wani, M.Y.; Malik, M.A.; Islamia, O.J.M. Gold and Its Complexes in Anticancer Chemotherapy; Springer: Singapore, 2021; ISBN 9789813363144. [Google Scholar]
- Uddin, N.; Rashid, F.; Ali, S.; Tirmizi, S.A.; Ahmad, I.; Zaib, S.; Zubair, M.; Diaconescu, P.L.; Tahir, M.N.; Iqbal, J.; et al. Synthesis, Characterization, and Anticancer Activity of Schiff Bases. J. Biomol. Struct. Dyn. 2020, 38, 3246–3259. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Reversible Covalent Immobilization of Cinnamaldehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioprocess Technol. 2015, 8, 526–538. [Google Scholar] [CrossRef]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A Dynamic and Self-Crosslinked Polysaccharide Hydrogel with Autonomous Self-Healing Ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Jia, Q.; Shan, S. Synthesis and Characterization of Schiff Base Contained Dextran Microgels in Water-in-Oil Inverse Microemulsion. Carbohydr. Polym. 2016, 152, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P. Synthetic Biopolymers. In Synthetic Biology; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319227085. [Google Scholar]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable Macroporous Hydrogel Formed by Enzymatic Cross-Linking of Gelatin Microgels. ACS Appl. Bio Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, X.; Dong, H.; He, H.; Cao, X. High-Throughput Generation of Hyaluronic Acid Microgels via Microfluidics-Assisted Enzymatic Crosslinking and/or Diels–Alder Click Chemistry for Cell Encapsulation and Delivery. Appl. Mater. Today 2017, 9, 49–59. [Google Scholar] [CrossRef]
- Jia, X.; Yeo, Y.; Clifton, R.J.; Jiao, T.; Kohane, D.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Hyaluronic Acid-Based Microgels and Microgel Networks for vocal fold regeneration. Biomacromolecules 2006, 7, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Kesselman, L.R.B.; Shinwary, S.; Selvaganapathy, P.R.; Hoare, T. Synthesis of Monodisperse, Covalently Cross-Linked, Degradable “Smart” Microgels Using Microfluidics. Small 2012, 8, 1092–1098. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Lü, S.; Gao, C.; Chen, J. In Vitro Degradation and Drug-Release Properties of Water-Soluble Chitosan Cross-Linked Oxidized Sodium Alginate Core-Shell Microgels. J. Biomater. Sci. Polym. Ed. 2012, 23, 2007–2024. [Google Scholar] [CrossRef]
- Hachet, E.; Sereni, N.; Pignot-Paintrand, I.; Ravaine, V.; Szarpak-Jankowska, A.; Auzély-Velty, R. Thiol-Ene Clickable Hyaluronans: From Macro-to Nanogels. J. Colloid Interface Sci. 2014, 419, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, G.; Shojaosadati, S.A.; Abdollahi, M. Synthesis and Characterization of Schiff Base Containing Bovine Serum Albumin-Gum Arabic Aldehyde Hybrid Nanogels via Inverse Miniemulsion for Delivery of Anticancer Drug. Int. J. Biol. Macromol. 2021, 170, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Asmatulu, R. Hydrogels in Various Biomedical Applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center: Badajoz, Spain, 2015; Volume 1845, pp. 248–257. [Google Scholar]
- Dalei, G.; Das, S.; Ranjan Jena, S.; Jena, D.; Nayak, J.; Samanta, L. In Situ Crosslinked Dialdehyde Guar Gum-Chitosan Schiff-Base Hydrogels for Dual Drug Release in Colorectal Cancer Therapy. Chem. Eng. Sci. 2023, 269, 118482. [Google Scholar] [CrossRef]
- Locarno, S.; Arosio, P.; Curtoni, F.; Piazzoni, M.; Pignoli, E.; Gallo, S. Microscopic and Macroscopic Characterization of Hydrogels Based on Poly(Vinyl-Alcohol)–Glutaraldehyde Mixtures for Fricke Gel Dosimetry. Gels 2024, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Nabid, M.R. Synthesis of Chemically Cross-Linked Hydrogel Films Based on Basil Seed (Ocimum basilicum L.) Mucilage for Wound Dressing Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 163, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Locarno, S.; Brambilla, E.; Lenardi, C.; Pignoli, E.; Veronese, I. Dosimetric Characterization of Double Network Fricke Hydrogel Based on PVA-GTA and Phenylalanine Peptide Derivative. J. Phys. D Appl. Phys. 2024, 7, 075303. [Google Scholar] [CrossRef]
- Hunziker, E.; Spector, M.; Libera, J.; Gertzman, A.; Woo, S.L.Y.; Ratcliffe, A.; Lysaght, M.; Coury, A.; Kaplan, D.; Vunjak-Novakovic, G. Translation from Research to Applications. Tissue Eng. 2006, 12, 3341–3364. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.G.; Vijayakumar, V. Hydrogels: Classification, Synthesis, Characterization, and Applications. Encycl. Biomed. Polym. Polym. Biomater. 2015, 11, 3879–3892. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Rajalekshmi, R.; Kaladevi Shaji, A.; Joseph, R.; Bhatt, A. Scaffold for Liver Tissue Engineering: Exploring the Potential of Fibrin Incorporated Alginate Dialdehyde–Gelatin Hydrogel. Int. J. Biol. Macromol. 2021, 166, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, J.; Liu, S.; Zhao, P.; Lu, G.; Chen, J. The Preparation, Characterization and Evaluation of Regenerated Cellulose/Collagen Composite Hydrogel Films. Carbohydr. Polym. 2014, 107, 57–64. [Google Scholar] [CrossRef]
- Liu, Y.; Chan-Park, M.B. Hydrogel Based on Interpenetrating Polymer Networks of Dextran and Gelatin for Vascular Tissue Engineering. Biomaterials 2009, 30, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Giordani, P.; Zuccari, G. Synthesis and Physicochemical Characterization of Gelatine-Based Biodegradable Aerogel-like Composites as Possible Scaffolds for Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiang, L.; Gong, L.; Hu, W.; Huang, W.; Chen, Y.; Asha, A.B.; Srinivas, S.; Chen, L.; Narain, R.; et al. Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties. Chem. Mater. 2019, 31, 2366–2376. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yuan, J. Schiff’s Base as a Stimuli-Responsive Linker in Polymer Chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A Review on Applications of Chitosan-Based Schiff Bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhashemi, Z.; Zohuriaan-Mehr, M.J.; Jahanmardi, R. Antibacterial Activity Induction into Superabsorbent Hydrogel via Schiff-Base-Metal Coordination Modification. Polym. Bull. 2023, 80, 8045–8065. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-Chitosan Oligosaccharide-ZnO Composite Hydrogel for Accelerating Wound Healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Oh, G.W.; Kim, S.C.; Kim, T.H.; Jung, W.K. Characterization of an Oxidized Alginate-Gelatin Hydrogel Incorporating a COS-Salicylic Acid Conjugate for Wound Healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, F.; Han, H. Biomedical Technology Fabricated Technology of Biomedical Micro-Nano Hydrogel. Biomed. Technol. 2023, 2, 31–48, Erratum in Biomed. Technol. 2024, 6, 91–92. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Chen, X.; Chen, X.; Li, S.; Yuan, G.; Zhou, T.; Li, J.; Jia, Y.; Xiong, D.; Tan, H. Covalent Chitosan-Cellulose Hydrogels via Schiff-Base Reaction Containing Macromolecular Microgels for PH-Sensitive Drug Delivery and Wound Dressing. Macromol. Chem. Phys. 2019, 220, 1900399. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, Q.; Miao, S.; Ren, P.; Wu, Y.; Shen, Y. A Hydrogel Microparticle with Sustained Release Properties for Pulmonary Drug Delivery. React. Funct. Polym. 2023, 183, 105489. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Shi, Z.; Xu, Y.; Lian, Q.; Zhong, Q.; Liu, Q.; Chen, Y.; Pan, X.; Chen, R.; et al. Long-Term Antibacterial and Biofilm Dispersion Activity of an Injectable in Situ Crosslinked Co-Delivery Hydrogel/Microgel for Treatment of Implant Infection. Chem. Eng. J. 2022, 433, 134451. [Google Scholar] [CrossRef]
- Hunt, N.C.; Grover, L.M. Cell Encapsulation Using Biopolymer Gels for Regenerative Medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Jang, Y.; Cha, C.; Jung, J.; Oh, J. Interfacial Compression-Dependent Merging of Two Miscible Microdroplets in an Asymmetric Cross-Junction for In Situ Microgel Formation. Macromol. Res. 2018, 26, 1143–1149. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Hu, H.; Gao, C. Supramolecular Microgels/Microgel Scaffolds for Tissue Repair and Regeneration. Supramol. Mater. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Zhou, X.; Xi, K.; Bian, J.; Li, Z.; Wu, L.; Tang, J.; Xiong, C.; Yu, Z.; Zhang, J.; Gu, Y.; et al. Injectable Engineered Micro/Nano-Complexes Trigger the Reprogramming of Bone Immune Epigenetics. Chem. Eng. J. 2023, 462, 142158. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.C.; Li, X.Y.; Zhang, L.L.; Jiang, F. A 3D Porous Microsphere with Multistage Structure and Component Based on Bacterial Cellulose and Collagen for Bone Tissue Engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef]
- Duan, Q.-Y.; Zhu, Y.-X.; Jia, H.-R.; Wang, S.-H.; Wu, F.-G. Nanogels: Synthesis, Properties, and Recent Biomedical Applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Su, H.; Zhang, W.; Wu, Y.; Han, X.; Liu, G.; Jia, Q.; Shan, S. Schiff Base-Containing Dextran Nanogel as PH-Sensitive Drug Delivery System of Doxorubicin: Synthesis and Characterization. J. Biomater. Appl. 2018, 33, 170–181. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R.; Anil Kumar, P.R.; Raj, D.K. Preparation, Characterization and Biological Evaluation of Curcumin Loaded Alginate Aldehyde–Gelatin Nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wu, J. PH-Sensitive Nanogels for Drug Delivery in Cancer Therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Recent Advances in the Biomedical Applications of Functionalized Nanogels. Pharmaceutics 2022, 14, 2832. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Yang, X.; He, L.; Zheng, R.; Min, J.; Su, H.; Shan, S.; Jia, Q. Facile Preparation of PH/Reduction Dual-Stimuli Responsive Dextran Nanogel as Environment-Sensitive Carrier of Doxorubicin. Polymer 2020, 200, 122585. [Google Scholar] [CrossRef]
- Ziaei, A.A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In Situ Forming Alginate/Gelatin Hybrid Hydrogels Containing Doxorubicin Loaded Chitosan/AuNPs Nanogels for the Local Therapy of Breast Cancer. Int. J. Biol. Macromol. 2023, 246, 125640. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Chung, F.Y.; Huang, C.R.; Chen, C.S.; Chen, Y.F. Natural Nanogels Crosslinked with S-Benzyl-L-Cysteine Exhibit Potent Antibacterial Activity. Biomater. Adv. 2023, 153, 213551. [Google Scholar] [CrossRef]
- Gao, F.; Mi, Y.; Wu, X.; Yao, J.; Qi, Q.; Chen, W.; Cao, Z. Preparation of Quaternized Chitosan/Ag Composite Nanogels in Inverse Miniemulsions for Durable and Antimicrobial Cotton Fabrics. Carbohydr. Polym. 2022, 278, 118935. [Google Scholar] [CrossRef] [PubMed]



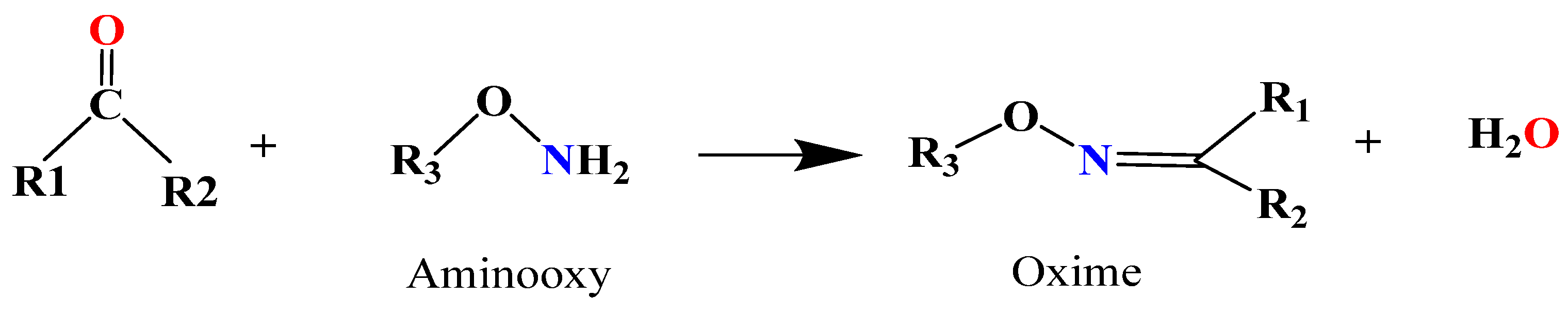


| Polysaccharide | Oxidizing Agent | Oxidized Polysaccharides Properties | References |
|---|---|---|---|
| Sodium Alginate | Sodium periodate |
| [50] |
| Sodium periodate |
| [82] | |
| Sodium periodate |
| [83] | |
| Chitosan | Periodate |
| [98] |
| Periodate |
| [99] | |
| Polullan | Sodium periodate |
| [108] |
| Sodium periodate |
| [102] | |
| Sodium periodate |
| [104] | |
| Carboxymethyl cellulose | Sodium periodate |
| [109] |
| Sodium periodate |
| [115] | |
| Sodium periodate (first method) Hydrogen peroxide in the presence of iron tetrasulfophthalocyanine (second method) |
| [111] | |
| Pectin | Periodic acid |
| [121] |
| Sodium periodate |
| [126] |
| Hydrogel Type | Cross-Linking Types | Materials | Biomedical Application | References |
|---|---|---|---|---|
| Film | Imine bond | Polymers of acrylamide-modified chitin (AMC) containing amino groups and oxidized alginate | Various biomedical fields | [216] |
| Microgel | Enzymatic cross-linking | Gelatin–gelatin (using transglutaminase (mTG)) | Wound healing and tissue engineering | [220] |
| Microgels | Enzymatic cross-linking and Diels–Alder reaction | Furyl amine (furan) and tyramine (TA) grafted hyaluronic acid (HA) molecules (HA–furan/TA) | Cell encapsulation and delivery | [221] |
| Microgel | Hydrazone cross-linking | Hyaluronic acid hydrazide–hyaluronic acid aldehyde Hyaluronic acid hydrazide–poly(ethylene glycol) dialdehyde | Treating vocal fold scarring, not just as biocompatible filler materials; repairs focal defects, smooths the vocal fold margin, and potentially softens and dissolves scar tissue | [222] |
| Microgels | Hydrazone cross-linking | Hydrazide functionalized carboxymethyl cellulose–aldehyde functionalized dextran (DEX-B) | Cell encapsulation or drug delivery | [223] |
| Microgel | Imine cross-linking | Oxidized sodium alginate–chitosan | Polymeric system for the colon-specific delivery of anti-inflammatory drugs, including 5-ASA’ enhances the therapeutic effect of ulcerative colitis | [224] |
| Nanogel | Thiol–ene coupling | Hyaluronic acid–poly(ethylene glycol)–bis(thiol) | Drug delivery | [225] |
| Nanogel | Imine cross-linking | Hybrid bovine serum albumin–arabic gum aldehyde (BSA-GAA) | Drug delivery and other biomedical applications | [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoudi, C.; Tahraoui Douma, N.; Mahmoudi, H.; Iurciuc, C.E.; Popa, M. Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 7839. https://doi.org/10.3390/ijms25147839
Mahmoudi C, Tahraoui Douma N, Mahmoudi H, Iurciuc CE, Popa M. Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications. International Journal of Molecular Sciences. 2024; 25(14):7839. https://doi.org/10.3390/ijms25147839
Chicago/Turabian StyleMahmoudi, Chahrazed, Naïma Tahraoui Douma, Hacene Mahmoudi, Camelia Elena Iurciuc (Tincu), and Marcel Popa. 2024. "Hydrogels Based on Proteins Cross-Linked with Carbonyl Derivatives of Polysaccharides, with Biomedical Applications" International Journal of Molecular Sciences 25, no. 14: 7839. https://doi.org/10.3390/ijms25147839







