Assessment of Pyrogenic Response of Medical Devices and Biomaterials by the Monocyte Activation Test (MAT): A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Research Question and Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection
2.6. Study Risk of Bias Assessment
2.7. Synthesis of Results
3. Results and Discussion
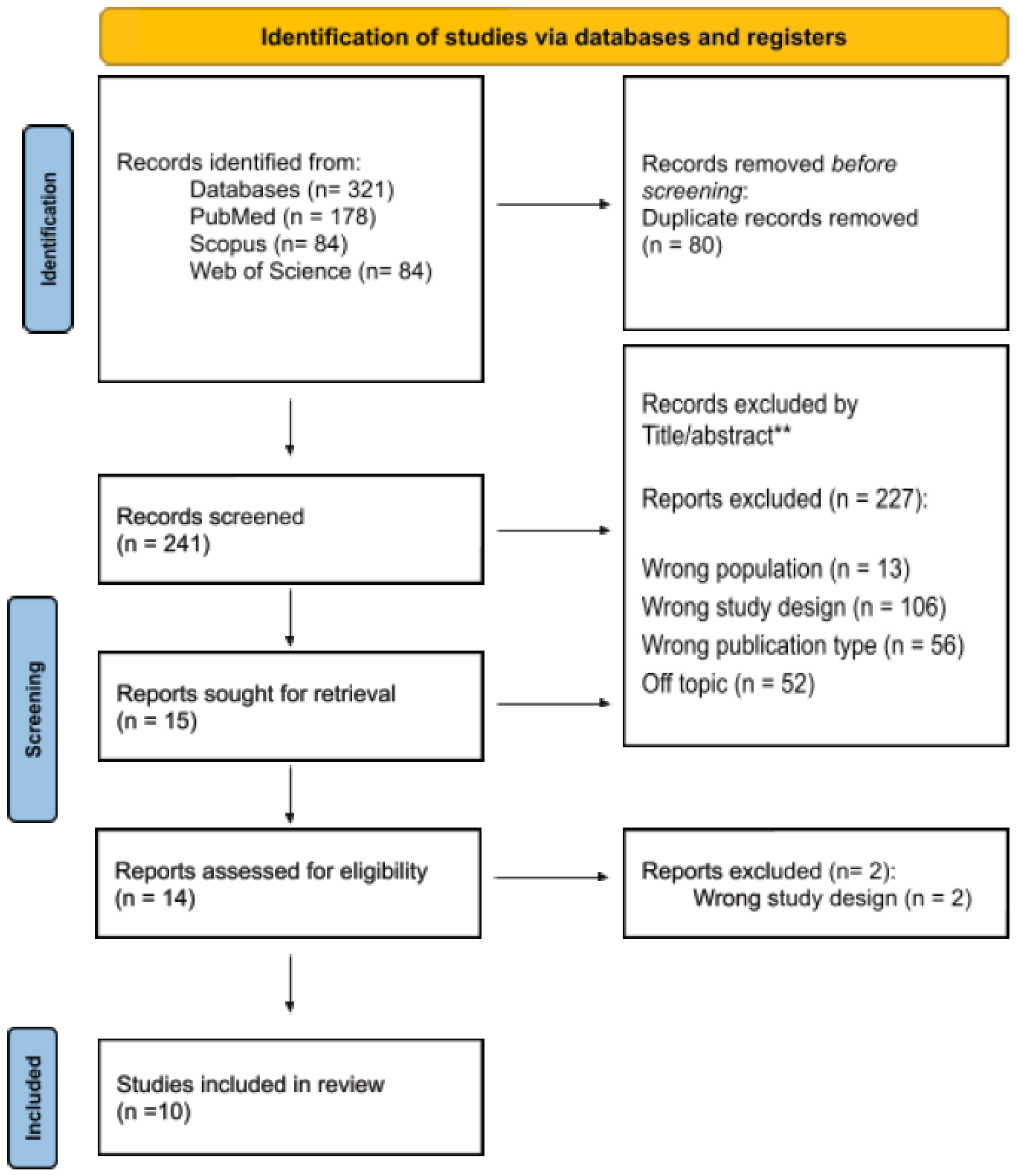
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Synthesis of Results
3.5. Discussion
3.5.1. Device Categories
3.5.2. Exposure Protocols
3.5.3. Dependance on the Biological Matrix
3.5.4. Pyrogenicity Detection Parameters
3.5.5. Endotoxin Stimuli and Interference Test
3.5.6. Correlations between the Rabbit Pyrogen Test (RPT), Limulus Amebocyte Lysate (LAL), and Monocyte Activation Test (MAT)
3.5.7. Summary of Evidence
3.5.8. Review Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fennrich, S.; Wendel, A.; Hartung, T. New Applications of the Human Whole Blood Pyrogen Assay (PyroCheck). ALTEX Altern. Anim. Exp. 1999, 16, 146–149. [Google Scholar]
- Pammolli, F.; Riccaboni, M.; Oglialoro, L.; Magazzini, G.; Baio, N.; Salerno, C. Munich Personal RePEc Archive Medical Devices Competitiveness and Impact on Public Health Expenditure. Entreprise Directorate-General, European Commission 2005. Available online: https://mpra.ub.uni-muenchen.de/16021/ (accessed on 15 May 2023).
- Fortune Business Insights. Medical devices: Global Market Analysis, insights and Forecast, 2024–2032. Fortune Bus. Insights 2023. Available online: https://www.fortunebusinessinsights.com/industry-reports/medical-devices-market-100085 (accessed on 15 May 2023).
- Stang, K.; Fennrich, S.; Krajewski, S.; Stoppelkamp, S.; Burgener, I.A.; Wendel, H.P.; Post, M. Highly sensitive pyrogen detection on medical devices by the monocyte activation test. J. Mater. Sci. Mater. Med. 2014, 25, 1065–1075. [Google Scholar] [CrossRef]
- Fennrich, S.; Hennig, U.; Toliashvili, L.; Schlensak, C.; Wendel, H.P.; Stoppelkamp, S. More than 70 years of Pyrogen detection: Current state and future perspectives. Altern. Lab. Anim. 2016, 44, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.C.; De Oliveira, C.B.N.; Carneiro, P.D.S.; Marengo, E.B.; De Mattos, K.A.; De Almeida, R.S.C.; Janaína, S.; Gomes, A.G.; França, P.O.A.; Fernandes, D.I. Métodos alternativos para a detecção de pirogênios em produtos e ambientes sujeitos a Vigilância Sanitária: Avanços e perspectivas no Brasil a partir do reconhecimento internacional do Teste de Ativação de Monócitos. Vigil. Sanit. Debate 2018, 6, 137. [Google Scholar] [CrossRef]
- Melandri, V.C.R.; Faria, G.C.; Caldeira, C.; Presgrave, O.A. Utilização de métodos alternativos na determinação da contaminação pirogênica no controle de produtos injetáveis sujeitos à vigilância sanitária. Univ. Ciênc. Saúde 2011, 8, 2. [Google Scholar] [CrossRef]
- Hannon, G.; Prina-Mello, A. Endotoxin contamination of engineered nanomaterials: Overcoming the hurdles associated with endotoxin testing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1738. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, R.; Fluri, A. Analysis of endotoxin residues on cleaned implant materials. J. ASTM Int. 2008, 5, 1–7. [Google Scholar] [CrossRef]
- Wilson, M.E.; Boggild, A.K. Fever and Systemic Symptoms. In Tropical Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2011; pp. 925–938. [Google Scholar] [CrossRef]
- Probey, T.F.; Pittman, M. The pyrogenicity of bacterial contaminants found in biologic products. J. Bacteriol. 1945, 50, 397–411. [Google Scholar] [CrossRef]
- Atkins, E.; Huang, W.C. Studies on the pathogenesis of fever with influenzal viruses. I. The appearance of an endogenous pyrogen in the blood following intravenous injection of virus. J. Exp. Med. 1958, 107, 383–401. [Google Scholar] [CrossRef]
- Monn, C.; Becker, S. Cytotoxicity and Induction of Proinflammatory Cytokines from Human Monocytes Exposed to Fine (PM 2.5) and Coarse Particles (PM 10–2.5) in Outdoor and Indoor Air. Toxicol. Appl Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Borton, L.K.; Coleman, K.P. Material-mediated pyrogens in medical devices: Applicability of the in vitro Monocyte Activation Test. ALTEX Altern. Anim. Exp. 2018, 35, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Clippinger, A.J.; Briglia, C.F.; Casey, W.; Coleman, K.; Fritsch, A.; Hartung, T.; Maouyo, D.; Muller, T.; Reich, J.; et al. Using the Monocyte Activation Test as a Stand-Alone Release Test for Medical Devices. ALTEX Altern. Anim. Exp. 2021, 38, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.-J.; Tan, D.-J. Comparison of temperature rise interpretations in the rabbit pyrogen test among Chinese, Japanese, European, and United States pharmacopeias and 2-2-2 theoretical models proposed by S. Hoffmann. Innate Immun. 2011, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Garcia-Recio, V.; Jiménez, P.; Garrosa, M.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Endotoxins from a Pharmacopoeial Point of View. Toxins 2018, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, C.; Freitas, J.C.; Gimenes, I.; Silva, S.A.; Cabello, P.; Presgrave, O.A. Normal temperature variation in New Zealand white rabbits during restraint for preliminary pyrogen test. Pharmeuropa Bio. Sci. Notes 2014, 2014, 118–123. [Google Scholar]
- de Barros, G.P.; Seugling, J.; Bricarello, P.A. Effect of Homeopathic Medicines and a Nosode on Larvae of Cochliomyia hominivorax (Diptera: Calliphoridae). Homeopathy 2019, 108, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, I.; Caldeira, C.; Presgrave, O.A.F.; Moura, W.C.D.; Villas Boas, M.H.S. Assessment of pyrogenic response of lipoteichoic acid by the monocyte activation test and the rabbit pyrogen test. Regul. Toxicol. Pharmacol. 2015, 73, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. Comparison and validation of novel pyrogen tests based on the human fever reaction. Altern. Lab. Anim. 2002, 30 (Suppl. S2), 49–51. [Google Scholar] [CrossRef]
- Mazzotti, F.; Beuttler, J.; Zeller, R.; Fink, U.; Schindler, S.; Wendel, A.; Hartung, T.; Von Aulock, S. In vitro pyrogen test—A new test method for solid medical devices. J. Biomed. Mater. Res. Part A 2007, 80, 276–282. [Google Scholar] [CrossRef]
- ISO 10993-11:2017; Biological Evaluation of Medical Devices-Part 11: Tests for Systemic Toxicity. ISO/TC 194. ISO: Geneva, Switzerland, 2017.
- Spoladore, J.; Gimenes, I.; Bachinski, R.; Negherbon, J.P.; Hartung, T.; Granjeiro, J.M.; Alves, G.G. Standardized pyrogen testing of medical products with the bacterial endotoxin test (BET) as a substitute for rabbit Pyrogen testing (RPT): A scoping review. Toxicol. Vitr. 2021, 74, 105160. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.; Fennrich, S.; Fischer, M.; Montag-Lessing, T.; Wendel, A. Development and evaluation of a pyrogen test based on human whole blood. ALTEX Altern. Anim. Exp. 1998, 15, 9–10. [Google Scholar]
- Schindler, S.; Spreitzer, I.; Löschner, B.; Hoffmann, S.; Hennes, K.; Halder, M.; Brügger, P.; Frey, E.; Hartung, T.; Montag, T. International validation of pyrogen tests based on cryopreserved human primary blood cells. J. Immunol. Methods 2006, 316, 42–51. [Google Scholar] [CrossRef] [PubMed]
- ECA. PharmEuropa—Revision of Chapter 2.6.30 Monocyte Activation Test—ECA Academy. 2022. Available online: https://www.gmp-compliance.org/gmp-news/pharmeuropa-revision-of-chapter-2-6-30-monocyte-activation-test (accessed on 23 July 2022).
- Caldeira, C.; Gimenes, I.; Freitas, J.C.B.; Rolim, D.E.; Presgrave, O.A.F. The use of Mono Mac 6 cells as indicators of endotoxin contamination in the quality control of injectable products. Abstracts of the 5th World Congress on Alternatives & Animal Use in the Life Sciences. ALTEX Altern. Anim. Exp. 2005, 22, 213. [Google Scholar]
- Hoffmann, S.; Peterbauer, A.; Schindler, S.; Fennrich, S.; Poole, S.; Mistry, Y.; Montag-Lessing, T.; Spreitzer, I.; Löschner, B.; van Aalderen, M.; et al. International validation of novel pyrogen tests based on human monocytoid cells. J. Immunol. Methods 2005, 298, 161–173. [Google Scholar] [CrossRef]
- Spreitzer, I. Evolution and Characteristics of the Monocyte Activation Test (MAT). In Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems; Springer: Cham, Switzerland, 2019; pp. 523–535. Available online: https://link.springer.com/chapter/10.1007/978-3-030-17148-3_14 (accessed on 23 July 2022).
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Tetz, M.; Mentak, K.; Aldred, M.; Zwisler, W. Detection of pyrogens adsorbed to intraocular lenses. Evaluation of limulus amoebocyte lysate and in vitro pyrogen tests. J. Cataract Refract. Surg. 2009, 35, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Haishima, Y.; Murai, T.; Nakagawa, Y.; Hirata, M.; Yagami, T.; Nakamura, A. Chemical and biological evaluation of endotoxin contamination on natural rubber latex products. J. Biomed. Mater. Res. 2001, 55, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Murai, T.; Hasegawa, C.; Hirata, M.; Tsuchiya, T.; Yagami, T.; Haishima, Y. Endotoxin Contamination in Wound Dressings Made of Natural Biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 347–355. [Google Scholar] [CrossRef]
- Hasiwa, M.; Kullmann, K.; von Aulock, S.; Klein, C.; Hartung, T. An in vitro pyrogen safety test for immune-stimulating components on surfaces. Biomaterials 2007, 28, 1367–1375. [Google Scholar] [CrossRef]
- Mohanan, P.V.; Banerjee, S.; Geetha, C.S. Detection of pyrogenicity on medical grade polymer materials using rabbit pyrogen, LAL and ELISA method. J. Pharm. Biomed. Anal. 2011, 55, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mohanan, P.V. Inflammatory response to pyrogens determined by a novel ELISA method using human whole blood. J. Immunol. Methods 2011, 369, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Harder, S.; Quabius, E.S.; Ossenkop, L.; Mehl, C.; Kern, M. Surface contamination of dental implants assessed by gene expression analysis in a whole-blood in vitro assay. A preliminary study. J. Clin. Periodontol. 2012, 39, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Trunk, S.; Müllerbader, P.; Hennig, U.; Abel, M.; Koggel, A.; Stang, K.; Altreuter, Y.; Steger, V.; Schlensak, C.; Wendel, H.P.; et al. Inflammatory potential of cotton-based surgically invasive devices: Implications for cardiac surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Interagency Coordinating Committee on the Validation of Alternative Methods. ICCVAM Background Review Document: Validation Status of Five In Vitro Test Methods Proposed for Assessing Potential Pyrogenicity of Pharmaceuticals and Other Products Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). National Toxicology Program, 2008, No. 08-6392. Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/docs/pyrogen/tmer/pyrotmer2008.pdf (accessed on 25 May 2023).
- ECA. European Pharmacopoeia 10.0. 2.6.30. monocyte-activation test. Eur. Pharmacopoeia 100 2017, 34, 233–239. [Google Scholar]
- FDA. 0027-Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers. Required 2012, 1, 1–10. [Google Scholar]
- U.S. Food & Drug Administration. Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing within a Risk Management Process” Guidance for Industry and Food and Drug Administration staff. US Dep. Heal Hum. Serv. Food Drug Adm. 2020, 1–68. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 1 June 2023).
- ISO 10993-1:2018; Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing within a Risk Management Process. ISO/TC 194. ISO: Geneva, Switzerland, 2018.
- Brasil Ministério da Saúde. Secretaria-Executiva. Departamento de Economia da Saúde, Investimento e Desempenho. In Padrão Descritivo para Dispositivos Médicos Implantáveis no Catmat; Ministério da Saúde: Brasília, Brazil, 2022; p. 217. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/padrao_dispositivos_medicos_implantaveis_CATMAT.pdf (accessed on 25 May 2023).
- Hartung, T. Pyrogen Testing Revisited on Occasion of the 25th Anniversary of the Whole Blood Monocyte Activation Test. ALTEX Altern. Anim. Exp. 2021, 38, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Taktak, Y.S.; Selkirk, S.; Bristow, A.F.; Carpenter, A.; Ball, C.; Rafferty, B.; Poole, S. Assay of pyrogens by interleukin-6 release from monocytic cell lines. J. Pharm. Pharmacol. 1991, 43, 578–582. [Google Scholar] [CrossRef]
- Hoffmann, S.; Luderitz-Puchel, U.; Montag, T.; Hartung, T. Optimisation of pyrogen testing in parenterals according to different pharmacopoeias by probabilistic modelling. J. Endotoxin Res. 2005, 11, 25–31. [Google Scholar] [CrossRef]
- Petersen, E.J.; Nguyen, A.; Brown, J.; Elliott, J.T.; Clippinger, A.; Gordon, J.; Kleinstreuer, N.; Roesslein, M. Characteristics to consider when selecting a positive control material for an in vitro assay. ALTEX Altern. Anim. Exp. 2021, 38, 365–376. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. ISO/TC 194. ISO: Geneva, Switzerland, 2021.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Strategy |
|---|---|
| PubMed | (pyrogen test OR “monocyte activation test”) AND (medical device* OR biomaterial*) |
| Scopus | TITLE-ABS-KEY ((pyrogen AND test OR “monocyte activation test”) AND (medical AND device* OR biomaterial*)) |
| Web of Science | TS = (pyrogen) OR TS = (test) OR TS = (“monocyte activation test”) AND TS = (medical AND device*) OR TS = (biomaterial*) |
| Author/Year | Material | Controls | Protocol of Exposure | Test Matrix | Pro-Inflammatory Cytokine | Comparisons with Other Tests |
|---|---|---|---|---|---|---|
| Haishima et al., 2001 [33] | Surgical gloves and catheters | LPS E. coli O55:B5; (JPSE) E. coli UKT-B; PGN S. aureus | Extract 1 | MM6 cells | IL-1 β, IL-6, and TNF-α | LAL kinetic-chromogenic and RPT |
| Nakagawa et al., 2003 [34] | Natural biomaterial dressing (calcium alginate, collagen, quinine, and poly-L-leucine) | LPS E. coli 055:B5 | Extract | Fresh blood (human and rabbit); MM6-CA8 cells | TNF-α and IL-6 | LAL, RPT |
| Hasiwa et al., 2007 [35] | Steel and titanium implants; polystyrene and metal plates | LPS E. coli O113; E. coli O111:B4; LTA S. aureus (SaLTA); B. subtilis (BsLTA); Peptidoglycan (PGN): S. aureus (SaPGN); B. subtilis (BsPGN); E. coli (EcPGN) and Zymosan 1 | Direct contact and extract | Fresh and cryopreserved blood | IL-1β | LAL chromogenic endpoint |
| Mazzotti et al., 2007 [22] | Titanium aneurysm clip | LPS E. coli O-113 and Zymosan A | Direct contact | Fresh and cryopreserved blood | IL-1β | LAL |
| Banerjee and Mohanan, 2011 [37] | Uninformed polymeric biomaterials | LPS from E. coli 055:B5; LTA from B. subtilis; 2,4,6-trinitrophenol, and PHA | Extract | Fresh blood | IL-1β | RPT |
| Mohanan et al., 2011 [36] | Gelatin polymeric materials | Endotoxin 1 | Extract | Fresh blood | IL-1β | LAL and RPT |
| Harder et al., 2012 [38] | Titanium and zirconia dental implants | LPS 1 | Direct contact | Fresh blood | (TLR4; TLR9; IL-1β; NF-kB; TNF- α; FADD); IL-1β | RT-qPCR |
| Stang et al., 2014 [4] | Steel plates, cobalt-chromium stents, and ePTFE vascular grafts | LPS E. coli O113:H10:K e LTA S. aureus | Direct contact and extract | Fresh blood | IL-1β | LAL, MAT, and modified MAT |
| Trunk et al., 2019 [39] | Cotton-based medical devices (swab) | LPS E. coli O113:H10:K; and Zymosan S. cerevisiae | Direct contact | Fresh blood or PBMC | Il-1β, IL-6 | - |
| Werner et al., 2009 [32] | Intraocular lenses | E. coli O-111; P. putida; S. epidermidis | Extract | Fresh blood | IL-1β | LAL |
| Reference | Group I: Test Substance Identification | Group II: Test System | Group III: Study Design | Group IV: Study Results | Group V: Plausibility of Design and Data | Total | Reliability Categorization |
|---|---|---|---|---|---|---|---|
| Haishima et al., 2001 [33] | 1 | 3 | 6 | 2 | 2 | 14 | reliable with restriction |
| Nakagawa et al., 2003 [34] | 2 | 3 | 6 | 3 | 2 | 16 | reliable without restrictions |
| Hasiwa et al., 2007 [35] | 2 | 3 | 5 | 3 | 1 | 14 | reliable with restriction |
| Mazzotti, F. et al., 2007 [22] | 3 | 3 | 5 | 2 | 2 | 15 | reliable without restrictions |
| Banerjee and Mohanan, 2011 [37] | 0 | 3 | 6 | 2 | 2 | 13 | reliable with restriction |
| Mohanan et al., 2011 [36] | 0 | 2 | 6 | 2 | 2 | 12 | reliable with restriction |
| Harder et al., 2012 [38] | 2 | 2 | 5 | 2 | 2 | 13 | reliable with restriction |
| Stang et al., 2014 [4] | 2 | 3 | 6 | 2 | 2 | 15 | reliable without restrictions |
| Trunk et al., 2019 [39] | 2 | 3 | 6 | 3 | 2 | 16 | reliable without restrictions |
| Werner et al., 2009 [32] | 2 | 3 | 6 | 3 | 1 | 15 | reliable without restrictions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimenes, I.; Spoladore, J.; Paranhos, B.A.; Romasco, T.; Di Pietro, N.; Piattelli, A.; Mourão, C.F.; Gomes Alves, G. Assessment of Pyrogenic Response of Medical Devices and Biomaterials by the Monocyte Activation Test (MAT): A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7844. https://doi.org/10.3390/ijms25147844
Gimenes I, Spoladore J, Paranhos BA, Romasco T, Di Pietro N, Piattelli A, Mourão CF, Gomes Alves G. Assessment of Pyrogenic Response of Medical Devices and Biomaterials by the Monocyte Activation Test (MAT): A Systematic Review. International Journal of Molecular Sciences. 2024; 25(14):7844. https://doi.org/10.3390/ijms25147844
Chicago/Turabian StyleGimenes, Izabela, Janaína Spoladore, Bruno Andrade Paranhos, Tea Romasco, Natalia Di Pietro, Adriano Piattelli, Carlos Fernando Mourão, and Gutemberg Gomes Alves. 2024. "Assessment of Pyrogenic Response of Medical Devices and Biomaterials by the Monocyte Activation Test (MAT): A Systematic Review" International Journal of Molecular Sciences 25, no. 14: 7844. https://doi.org/10.3390/ijms25147844
APA StyleGimenes, I., Spoladore, J., Paranhos, B. A., Romasco, T., Di Pietro, N., Piattelli, A., Mourão, C. F., & Gomes Alves, G. (2024). Assessment of Pyrogenic Response of Medical Devices and Biomaterials by the Monocyte Activation Test (MAT): A Systematic Review. International Journal of Molecular Sciences, 25(14), 7844. https://doi.org/10.3390/ijms25147844











