Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role
Abstract
:1. Introduction
2. Results
2.1. Expression of TREK-1 (Variant 2) and TREK-2 in Rat Articular Cartilage Is Evidenced by RT-PCR Assays
| Channel | NCBI RefSeq | Product | Primer | Primer Sequence | Primer |
|---|---|---|---|---|---|
| Name | Acces. Num. | Size | Sense | Size | |
| TREK-1.1 | NM_172041.2 | 678 | Forward | GAAAAGGAGCGTCTACCTGG | 20 |
| Reverse | AGGACACAGCCAAACAGGATG | 21 | |||
| TREK-1.2 | NM_172042.2 | 470 | Forward | CCGGCTATACCGCAGGAGTG | 20 |
| Reverse | ACCTTCAGTTCGTGGGGAGAT | 21 | |||
| TREK-2 | NM_023096.2 | 653 | Forward | GGTGCAAACACCCAACCAAG | 20 |
| Reverse | GAGTTTCCTACCGGGCTGAC | 20 | |||
| TRAAK | NM_053804.3 | 503 | Forward | CCTGGAAGGTTGGACTCTGC | 20 |
| Reverse | ATTGCCGTAGCCGATGGTAG | 20 |

2.2. The Expression of TREK-1 Proteins in Rat Articular Cartilage Is Demonstrated by Western Blot and Immunohistochemistry Procedures
2.3. Freshly Isolated Chondrocytes Exhibit Ionic Currents Associated with the Presence of TREK (1/2) Channels
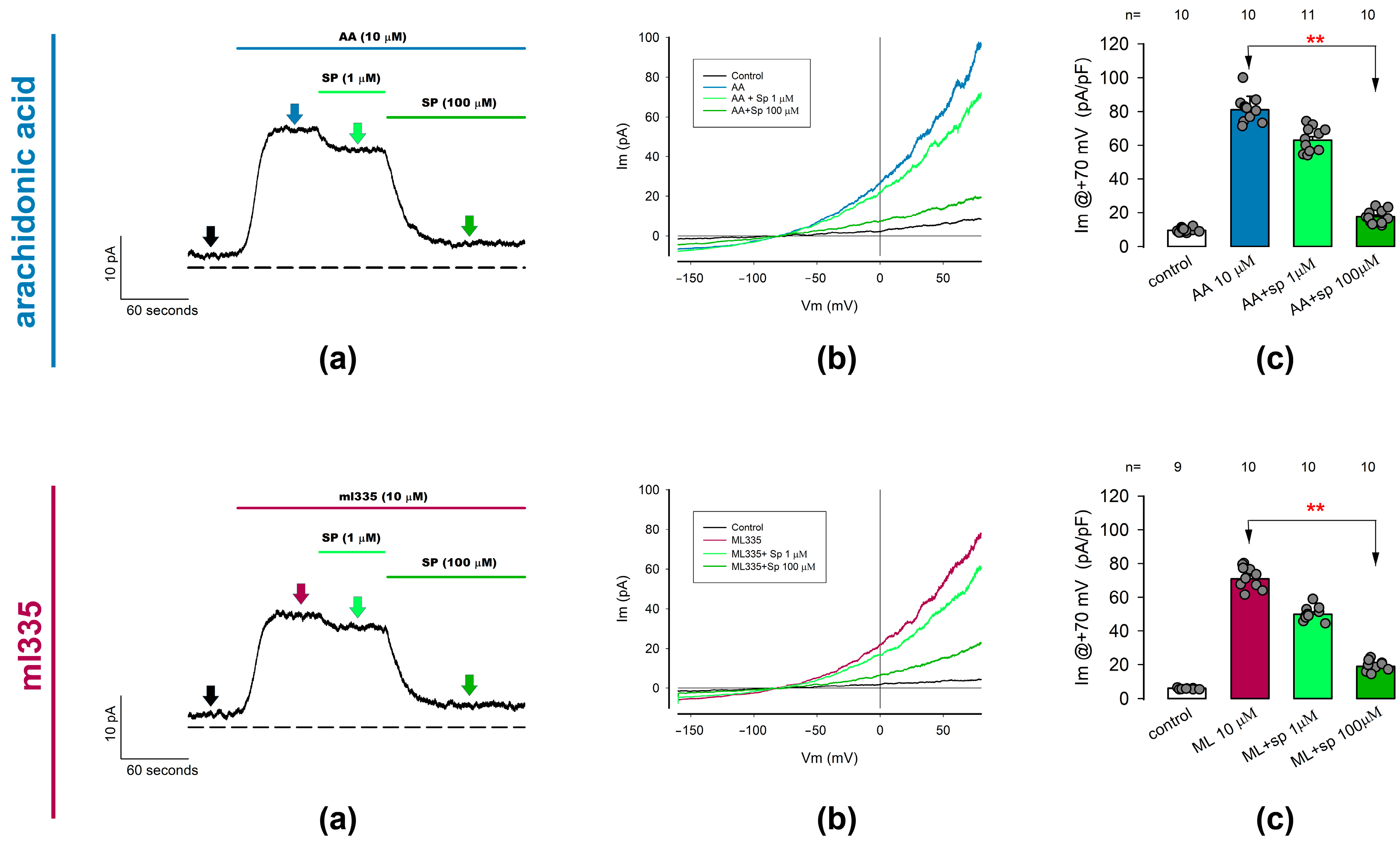
2.3.1. Ion Currents Recorded from Freshly Dissociated Chondrocytes Are Induced by TREK Channel Activators Arachidonic Acid and ml335
2.3.2. ITREK Currents Are Notoriously Inhibited by Spadin, a Specific Inhibitor of TREK-1 Channels
2.4. Hypo-Osmolar Shock Induces Activation of Ion Currents Attributable to TREK-1 Channels
3. Discussion
3.1. Implications for Cartilage Health and Disease
3.2. Limitations and Future Directions
4. Materials and Methods
4.1. RT-PCR Assays
4.2. Western Blot Assays
4.3. Immunofluorescence of Cartilage Slices
Confocal Microscopy
4.4. Recording of Ion Currents from Freshly Dissociated Chondrocytes
4.4.1. Cell Culture of Freshly Dissociated Articular Chondrocytes
4.4.2. Whole-Cell Patch Clamp Assays
4.4.3. Measurement of Membrane Capacitance
4.5. Solutions
4.6. Analysis of Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Ulrich-Vinther, M.; Maloney, M.D.; Schwarz, E.M.; Rosier, R.; O’Keefe, R.J. Articular Cartilage Biology. J. Am. Acad. Orthop. Surg. 2003, 11, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Strecanska, M.; Danisovic, L.; Ziaran, S.; Cehakova, M. The Role of Extracellular Matrix and Hydrogels in Mesenchymal Stem Cell Chondrogenesis and Cartilage Regeneration. Life 2022, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A. The Mechanical Environment of Chondrocytes in Articular Cartilage. Biorheology 2006, 43, 537–545. [Google Scholar]
- Hopewell, B.; Urban, J.P.G. Adaptation of Articular Chondrocytes to Changes in Osmolality. Biorheology 2003, 40, 73–77. [Google Scholar]
- Borghetti, P.; Della Salda, L.; De Angelis, E.; Maltarello, M.C.; Petronini, P.G.; Cabassi, E.; Marcato, P.S.; Maraldi, N.M.; Borghetti, A.F. Adaptive Cellular Response to Osmotic Stress in Pig Articular Chondrocytes. Tissue Cell 1995, 27, 173–183. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage: Tissue Design and Chondrocyte-Matrix Interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar]
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The Cellular Physiology of Articular Cartilage. Exp. Physiol. 1996, 81, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.J.; Browning, J.A.; Urban, J.P. Chondrocyte Regulation by Mechanical Load. Biorheology 2000, 37, 67–74. [Google Scholar]
- Kaupp, J.A.; Weber, J.F.; Waldman, S.D. Mechanical Stimulation of Chondrocyte-Agarose Hydrogels. J. Vis. Exp. 2012, e4229. [Google Scholar] [CrossRef]
- Salinas, D.; Mumey, B.M.; June, R.K. Physiological Dynamic Compression Regulates Central Energy Metabolism in Primary Human Chondrocytes. Biomech. Model. Mechanobiol. 2019, 18, 69–77. [Google Scholar] [CrossRef]
- Lee, D.A.; Noguchi, T.; Frean, S.P.; Lees, P.; Bader, D.L. The Influence of Mechanical Loading on Isolated Chondrocytes Seeded in Agarose Constructs. Biorheology 2000, 37, 149–161. [Google Scholar] [PubMed]
- Gilbert, S.J.; Blain, E.J. Chapter 4—Cartilage Mechanobiology: How Chondrocytes Respond to Mechanical Load. In Mechanobiology in Health and Disease; Verbruggen, S.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 99–126. ISBN 978-0-12-812952-4. [Google Scholar]
- Lane Smith, R.; Trindade, M.C.; Ikenoue, T.; Mohtai, M.; Das, P.; Carter, D.R.; Goodman, S.B.; Schurman, D.J. Effects of Shear Stress on Articular Chondrocyte Metabolism. Biorheology 2000, 37, 95–107. [Google Scholar] [PubMed]
- Ouyang, X.; Xie, Y.; Wang, G. Mechanical Stimulation Promotes the Proliferation and the Cartilage Phenotype of Mesenchymal Stem Cells and Chondrocytes Co-Cultured in Vitro. Biomed. Pharmacother. 2019, 117, 109146. [Google Scholar] [CrossRef]
- Guo, T.; Yu, L.; Lim, C.G.; Goodley, A.S.; Xiao, X.; Placone, J.K.; Ferlin, K.M.; Nguyen, B.-N.B.; Hsieh, A.H.; Fisher, J.P. Effect of Dynamic Culture and Periodic Compression on Human Mesenchymal Stem Cell Proliferation and Chondrogenesis. Ann. Biomed. Eng. 2016, 44, 2103–2113. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.; Rothrauff, B.B.; Zheng, A.; Lamb, A.; Yan, Y.; Lipa, K.E.; Lei, G.; Lin, H. Mechanotransduction Pathways in Articular Chondrocytes and the Emerging Role of Estrogen Receptor-α. Bone Res. 2023, 11, 13. [Google Scholar] [CrossRef]
- Anderson, D.E.; Johnstone, B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Castro-Viñuelas, R.; Viudes-Sarrión, N.; Rojo-García, A.V.; Monteagudo, S.; Lories, R.J.; Jonkers, I. Mechanical Loading Rescues Mechanoresponsiveness in a Human Osteoarthritis Explant Model despite Wnt Activation. Osteoarthr. Cartil. 2024. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage-An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, P.; Hu, B.; Xiao, Y.; Su, T.; Luo, X.; Tu, M.; Cai, G. Excessive Mechanical Loading Promotes Osteoarthritis Development by Upregulating Rcn2. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2024, 1870, 167251. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Penton, D.; Peyronnet, R.; Arhatte, M.; Moro, C.; Picard, N.; Kurt, B.; Patel, A.; Honoré, E.; Demolombe, S. Piezo1-Dependent Regulation of Urinary Osmolarity. Pflug. Arch. 2016, 468, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs Mediate Neuronal Sensing of Blood Pressure and the Baroreceptor Reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Jan, L.Y.; Jan, Y.-N. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu. Rev. Neurosci. 2020, 43, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Machura, Ł.; Dworakowska, B.; Grzywna, Z.J. Mechanosensitivity of the BK Channels in Human Glioblastoma Cells: Kinetics and Dynamical Complexity. J. Membr. Biol. 2018, 251, 667–679. [Google Scholar] [CrossRef]
- Zhao, H.; Sokabe, M. Tuning the Mechanosensitivity of a BK Channel by Changing the Linker Length. Cell Res. 2008, 18, 871–878. [Google Scholar] [CrossRef]
- Tavernarakis, N.; Driscoll, M. Degenerins. At the Core of the Metazoan Mechanotransducer? Ann. N. Y. Acad. Sci. 2001, 940, 28–41. [Google Scholar] [CrossRef]
- Drummond, H.A.; Gebremedhin, D.; Harder, D.R. Degenerin/Epithelial Na+ Channel Proteins: Components of a Vascular Mechanosensor. Hypertension 2004, 44, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Gomis, A. TRP Channels and Mechanical Transduction. In TRP Channels in Sensory Transduction; Madrid, R., Bacigalupo, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 141–163. ISBN 978-3-319-18705-1. [Google Scholar]
- Plant, T.D. TRPs in Mechanosensing and Volume Regulation. Handb. Exp. Pharmacol. 2014, 223, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W. TRPV Channels’ Role in Osmotransduction and Mechanotransduction. Handb. Exp. Pharmacol. 2007, 473–487. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo Proteins Are Pore-Forming Subunits of Mechanically Activated Channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Volkers, L.; Mechioukhi, Y.; Coste, B. Piezo Channels: From Structure to Function. Pflug. Arch. 2015, 467, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically-Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Lazdunski, M. Molecular and Functional Properties of Two-Pore-Domain Potassium Channels. Am. J. Physiol. Renal Physiol. 2000, 279, F793–F801. [Google Scholar] [CrossRef] [PubMed]
- Sorum, B.; Docter, T.; Panico, V.; Rietmeijer, R.A.; Brohawn, S.G. Tension Activation of Mechanosensitive Two-Pore Domain K+ Channels TRAAK, TREK-1, and TREK-2. Nat. Commun. 2024, 15, 3142. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G. How Ion Channels Sense Mechanical Force: Insights from Mechanosensitive K2P Channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 2015, 1352, 20–32. [Google Scholar] [CrossRef]
- Douguet, D.; Honoré, E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell 2019, 179, 340–354. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, a Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, N.; Liu, J.-W.; Zeng, B.; Chen, G.-L. TMEM63 Mechanosensitive Ion Channels: Activation Mechanisms, Biological Functions and Human Genetic Disorders. Biochem. Biophys. Res. Commun. 2023, 683, 149111. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The Chondrocyte Channelome: A Narrative Review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Jolley, R.; Lewis, R.; Fallman, R.; Mobasheri, A. The Emerging Chondrocyte Channelome. Front. Physiol. 2010, 1, 135. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Yoshino, M.; Nagao, M.; Ishii, S.; Yabu, H. Voltage-Gated Ionic Channels in Cultured Rabbit Articular Chondrocytes. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 115, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A. Expression of Voltage Dependent Potassium Currents in Freshly Dissociated Rat Articular Chondrocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2006, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Cannon, S.D.; Wuthier, R.E. Characterization of a Delayed Rectifier Potassium Current in Chicken Growth Plate Chondrocytes. Am. J. Physiol. 1992, 262, C1335–C1340. [Google Scholar] [CrossRef] [PubMed]
- Mozrzymas, J.W.; Visintin, M.; Vittur, F.; Ruzzier, F. Potassium Channels of Pig Articular Chondrocytes Are Blocked by Propofol. Biochem. Biophys. Res. Commun. 1994, 202, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Duncan, N.A.; Giles, W.R.; Clark, R.B. A Voltage-Dependent K+ Current Contributes to Membrane Potential of Acutely Isolated Canine Articular Chondrocytes. J. Physiol. 2004, 557, 93–104. [Google Scholar] [CrossRef]
- Mobasheri, A.; Gent, T.C.; Womack, M.D.; Carter, S.D.; Clegg, P.D.; Barrett-Jolley, R. Quantitative Analysis of Voltage-Gated Potassium Currents from Primary Equine (Equus Caballus) and Elephant (Loxodonta Africana) Articular Chondrocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R172–R180. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lewis, R.; Maxwell, J.E.J.; Hill, C.; Womack, M.; Barrett-Jolley, R. Characterization of a Stretch-Activated Potassium Channel in Chondrocytes. J. Cell. Physiol. 2010, 223, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Alicknavitch, M.; Farach-Carson, M.C. Expression of Voltage Sensitive Calcium Channel (VSCC) L-Type Cav1.2 (alpha1C) and T-Type Cav3.2 (alpha1H) Subunits during Mouse Bone Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 234, 54–62. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Danks, T.A.; Wilkins, R.J. Mechanisms Involved in the Increase in Intracellular Calcium Following Hypotonic Shock in Bovine Articular Chondrocytes. Gen. Physiol. Biophys. 2003, 22, 487–500. [Google Scholar] [PubMed]
- Mobasheri, A.; Trujillo, E.; Bell, S.; Carter, S.D.; Clegg, P.D.; Martín-Vasallo, P.; Marples, D. Aquaporin Water Channels AQP1 and AQP3, Are Expressed in Equine Articular Chondrocytes. Vet. J. 2004, 168, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tsuga, K.; Tohse, N.; Yoshino, M.; Sugimoto, T.; Yamashita, T.; Ishii, S.; Yabu, H. Chloride Conductance Determining Membrane Potential of Rabbit Articular Chondrocytes. J. Membr. Biol. 2002, 185, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Suzuki, Y.; Imaizumi, Y. Physiological and Pathological Functions of Cl− Channels in Chondrocytes. Biol. Pharm. Bull. 2018, 41, 1145–1151. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction Pathways in the Regulation of Cartilage Chondrocyte Homoeostasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef] [PubMed]
- Servin-Vences, M.R.; Richardson, J.; Lewin, G.R.; Poole, K. Mechanoelectrical Transduction in Chondrocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 Channels Confers High-Strain Mechanosensitivity to Articular Cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef]
- Sánchez, J.C.; López-Zapata, D.F.; Wilkins, R.J. TRPV4 Channels Activity in Bovine Articular Chondrocytes: Regulation by Obesity-Associated Mediators. Cell Calcium 2014, 56, 493–503. [Google Scholar] [CrossRef]
- Ponce, A.; Jimenez-Peña, L.; Tejeda-Guzman, C. The Role of Swelling-Activated Chloride Currents (I(CL,Swell)) in the Regulatory Volume Decrease Response of Freshly Dissociated Rat Articular Chondrocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2012, 30, 1254–1270. [Google Scholar] [CrossRef] [PubMed]
- Stampoultzis, T.; Guo, Y.; Nasrollahzadeh, N.; Karami, P.; Pioletti, D.P. Mimicking Loading-Induced Cartilage Self-Heating in Vitro Promotes Matrix Formation in Chondrocyte-Laden Constructs with Different Mechanical Properties. ACS Biomater. Sci. Eng. 2023, 9, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dyachenko, V.; Zuzarte, M.; Putzke, C.; Preisig-Müller, R.; Isenberg, G.; Daut, J. The Stretch-Activated Potassium Channel TREK-1 in Rat Cardiac Ventricular Muscle. Cardiovasc. Res. 2006, 69, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Duprat, F.; Lesage, F.; Reyes, R.; Romey, G.; Heurteaux, C.; Lazdunski, M. Cloning, Functional Expression and Brain Localization of a Novel Unconventional Outward Rectifier K+ Channel. EMBO J. 1996, 15, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honoré, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A Mammalian Two Pore Domain Mechano-Gated S-like K+ Channel. EMBO J. 1998, 17, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Arrigoni, C.; Mori, T.; Sekioka, Y.; Bryant, C.; Clark, K.A.; Minor, D.L. K2P2.1 (TREK-1)-Activator Complexes Reveal a Cryptic Selectivity Filter Binding Site. Nature 2017, 547, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.; Minor, D.L. The Polysite Pharmacology of TREK K2P Channels. Adv. Exp. Med. Biol. 2021, 1349, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Baggetta, A.M.; Bayliss, D.A.; Czirják, G.; Enyedi, P.; Goldstein, S.A.N.; Lesage, F.; Daniel, L.; Minor, J.; Plant, L.D.; Sepúlveda, F. Two-Pore Domain Potassium Channels (K2P) in GtoPdb v.2023.1. IUPHARBPS Guide Pharmacol. CITE 2023, 2023. [Google Scholar] [CrossRef]
- Moha Ou Maati, H.; Veyssiere, J.; Labbal, F.; Coppola, T.; Gandin, C.; Widmann, C.; Mazella, J.; Heurteaux, C.; Borsotto, M. Spadin as a New Antidepressant: Absence of TREK-1-Related Side Effects. Neuropharmacology 2012, 62, 278–288. [Google Scholar] [CrossRef]
- Mazella, J.; Pétrault, O.; Lucas, G.; Deval, E.; Béraud-Dufour, S.; Gandin, C.; El-Yacoubi, M.; Widmann, C.; Guyon, A.; Chevet, E.; et al. Spadin, a Sortilin-Derived Peptide, Targeting Rodent TREK-1 Channels: A New Concept in the Antidepressant Drug Design. PLoS Biol. 2010, 8, e1000355. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Chen, Q. Mechanoregulation of Chondrocyte Proliferation, Maturation, and Hypertrophy: Ion-Channel Dependent Transduction of Matrix Deformation Signals. Exp. Cell Res. 2000, 256, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-Y.; Jin, Y.; Ma, X.-H.; Wang, C.-Y.; Guo, Y.; Zhou, D. The Potential Role of Mechanically Sensitive Ion Channels in the Physiology, Injury, and Repair of Articular Cartilage. J. Orthop. Surg. 2020, 28, 2309499020950262. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B.; Kondo, C.; Belke, D.D.; Giles, W.R. Two-Pore Domain K+ Channels Regulate Membrane Potential of Isolated Human Articular Chondrocytes. J. Physiol. 2011, 589, 5071–5089. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Lewis, R.; Ferreira-Mendes, A.; Rufino, A.; Dart, C.; Barrett-Jolley, R. Potassium Channels in Articular Chondrocytes. Channels 2012, 6, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Mathie, A.; Clarke, C.E. Background Potassium Channels Move into Focus. J. Physiol. 2002, 542, 334. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hughes, S.; El Haj, A.; Maffulli, N. Expression of the Two Pore Domain Potassium Channel TREK-1 in Human Intervertebral Disc Cells. Curr. Stem Cell Res. Ther. 2012, 7, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Magra, M.; Hughes, S.; El Haj, A.J.; Maffulli, N. VOCCs and TREK-1 Ion Channel Expression in Human Tenocytes. Am. J. Physiol. Cell Physiol. 2007, 292, C1053–C1060. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-Extracellular Matrix Mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between Mechanotransduction and Metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Amado, I.N.; O’Brien, F.J.; Kennedy, O.D. The Role of Mechanobiology in Bone and Cartilage Model Systems in Characterizing Initiation and Progression of Osteoarthritis. APL Bioeng. 2022, 6, 011501. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Liu, Z.; Geng, B.; Teng, Y.; Liu, X.; Yi, Q.; Yu, D.; Chen, X.; Zhao, D.; et al. Mechanosensory and Mechanotransductive Processes Mediated by Ion Channels in Articular Chondrocytes: Potential Therapeutic Targets for Osteoarthritis. Channels 2021, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Hu, W.; Li, Y.; Yao, X.; Li, J.; Li, X.; Zhang, J.; Wu, Y.; Kang, F.; Dong, S. Mechanotransduction in Subchondral Bone Microenvironment and Targeted Interventions for Osteoarthritis. Mechanobiol. Med. 2024, 2, 100043. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Cereijido, M.; Jimenez, L.; Hinojosa, L.; Castillo, A.; Martínez-Rendon, J.; Ponce, A. Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell-Cell Contacts. Int. J. Mol. Sci. 2022, 23, 3257. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Bolivar, J.; Vega, J.; Cereijido, M. Synthesis of Plasma Membrane and Potassium Channels in Epithelial (MDCK) Cells. Cell. Physiol. Biochem. 1991, 1, 195–204. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]



| Extracellular Solutions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | NaGln | TEACl | KGln | CaCl2 | MgCl2 | Glucose | HEPES | Mannitol | 4AP |
| 330 mOsm | 52.3 | 20 | 5 | 1.8 | 1 | 5 | 10 | 150 | 5 |
| 280 mOsm | 53.3 | 20 | 5 | 1.8 | 1 | 5 | 10 | 100 | 5 |
| 180 mOsm | 53.3 | 20 | 5 | 1.8 | 1 | 5 | 10 | 0 | 5 |
| Intracellular solution | |||||||||
| IS | 5 | 10 | 115 | 1 | 1 | 0 | 10 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, A.; Ogazon del Toro, A.; Jimenez, L.; Roldan, M.L.; Shoshani, L. Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role. Int. J. Mol. Sci. 2024, 25, 7848. https://doi.org/10.3390/ijms25147848
Ponce A, Ogazon del Toro A, Jimenez L, Roldan ML, Shoshani L. Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role. International Journal of Molecular Sciences. 2024; 25(14):7848. https://doi.org/10.3390/ijms25147848
Chicago/Turabian StylePonce, Arturo, Alejandro Ogazon del Toro, Lidia Jimenez, Maria Luisa Roldan, and Liora Shoshani. 2024. "Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role" International Journal of Molecular Sciences 25, no. 14: 7848. https://doi.org/10.3390/ijms25147848
APA StylePonce, A., Ogazon del Toro, A., Jimenez, L., Roldan, M. L., & Shoshani, L. (2024). Osmotically Sensitive TREK Channels in Rat Articular Chondrocytes: Expression and Functional Role. International Journal of Molecular Sciences, 25(14), 7848. https://doi.org/10.3390/ijms25147848





