Inner Structure of the Lateral Geniculate Complex of Adult and Newborn Acomys cahirinus
Abstract
:1. Introduction
2. Results
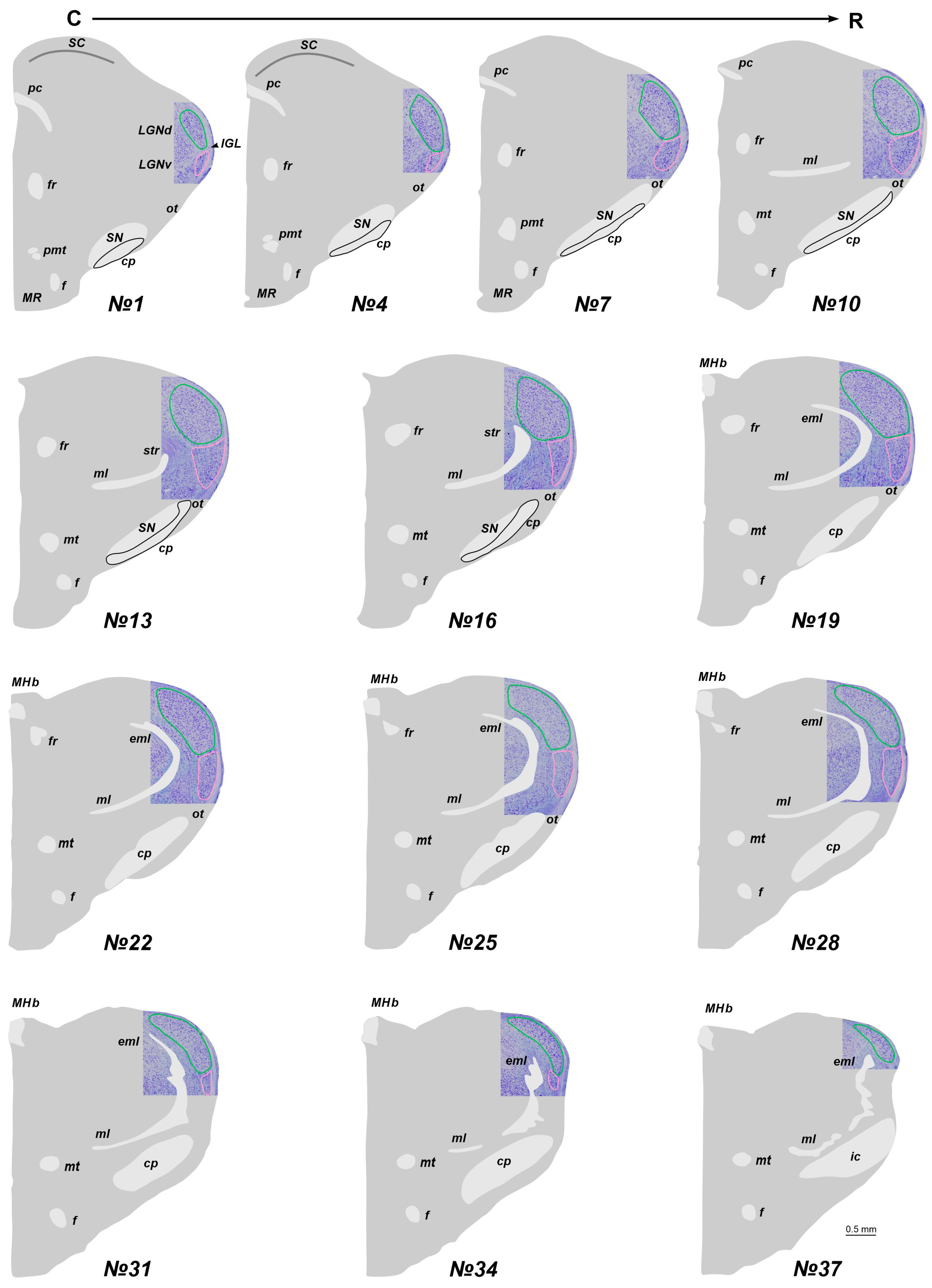
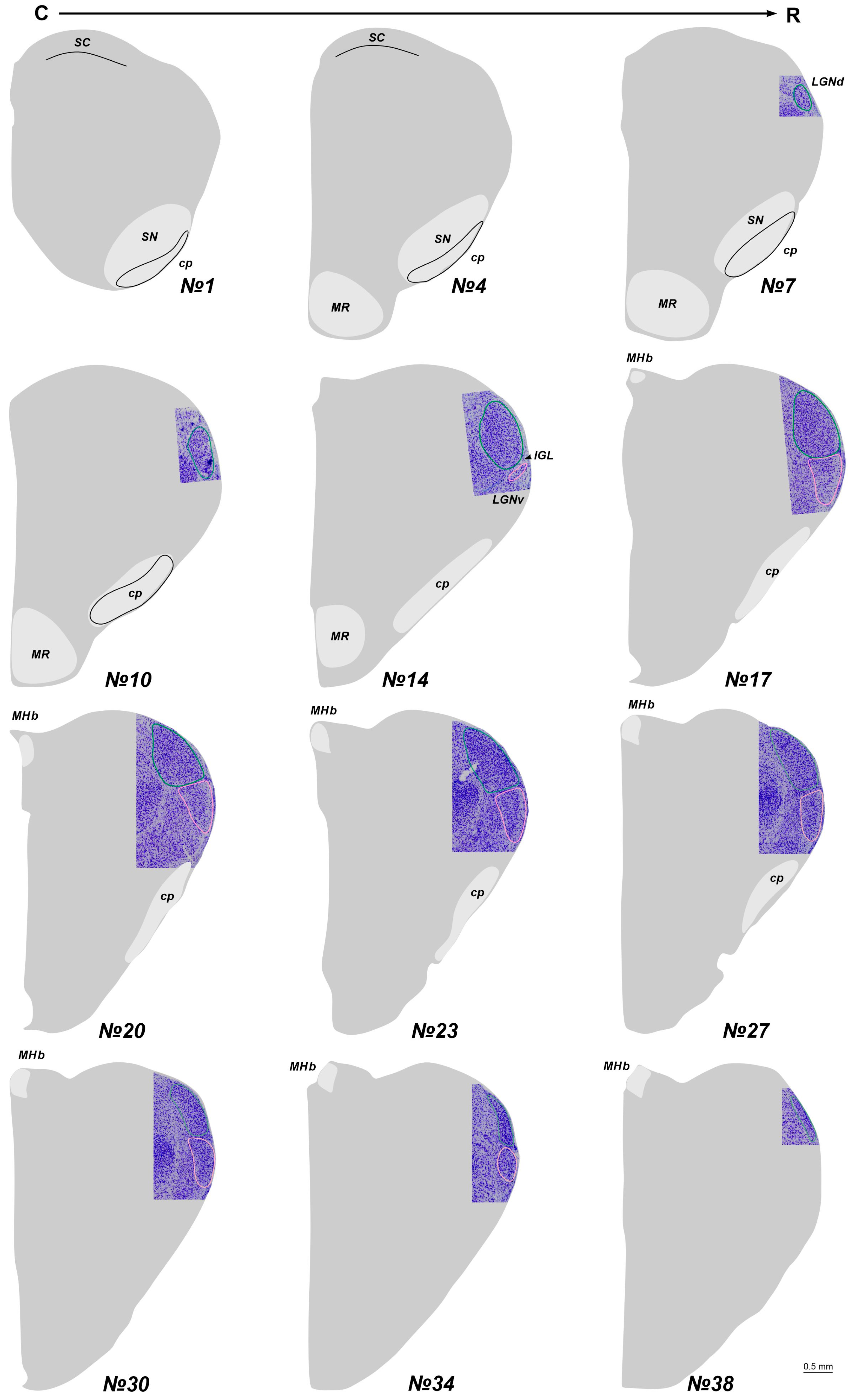
2.1. The Location of the LGN in Stereotaxic Levels
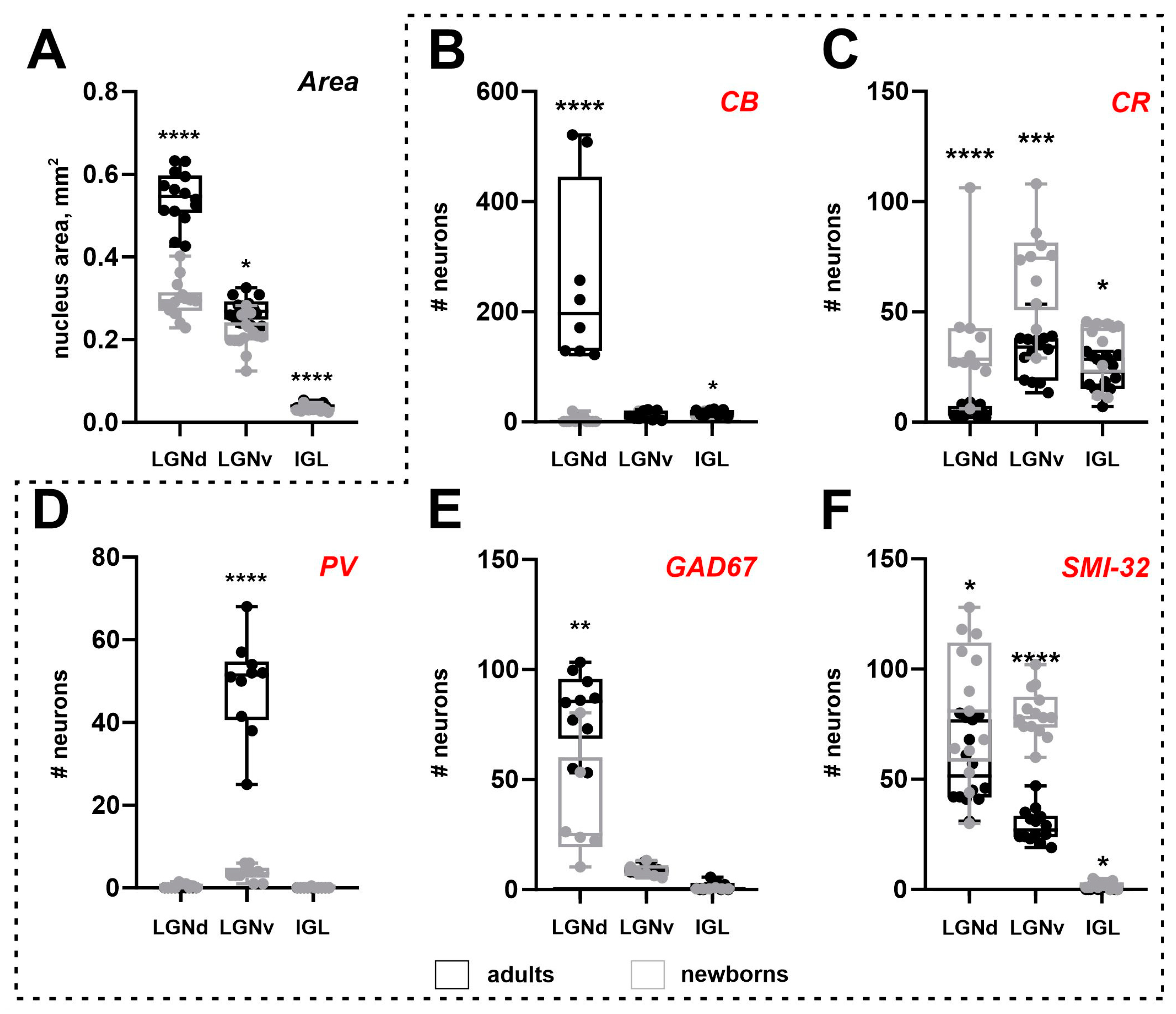
2.2. Area of the Lateral Geniculate Complex
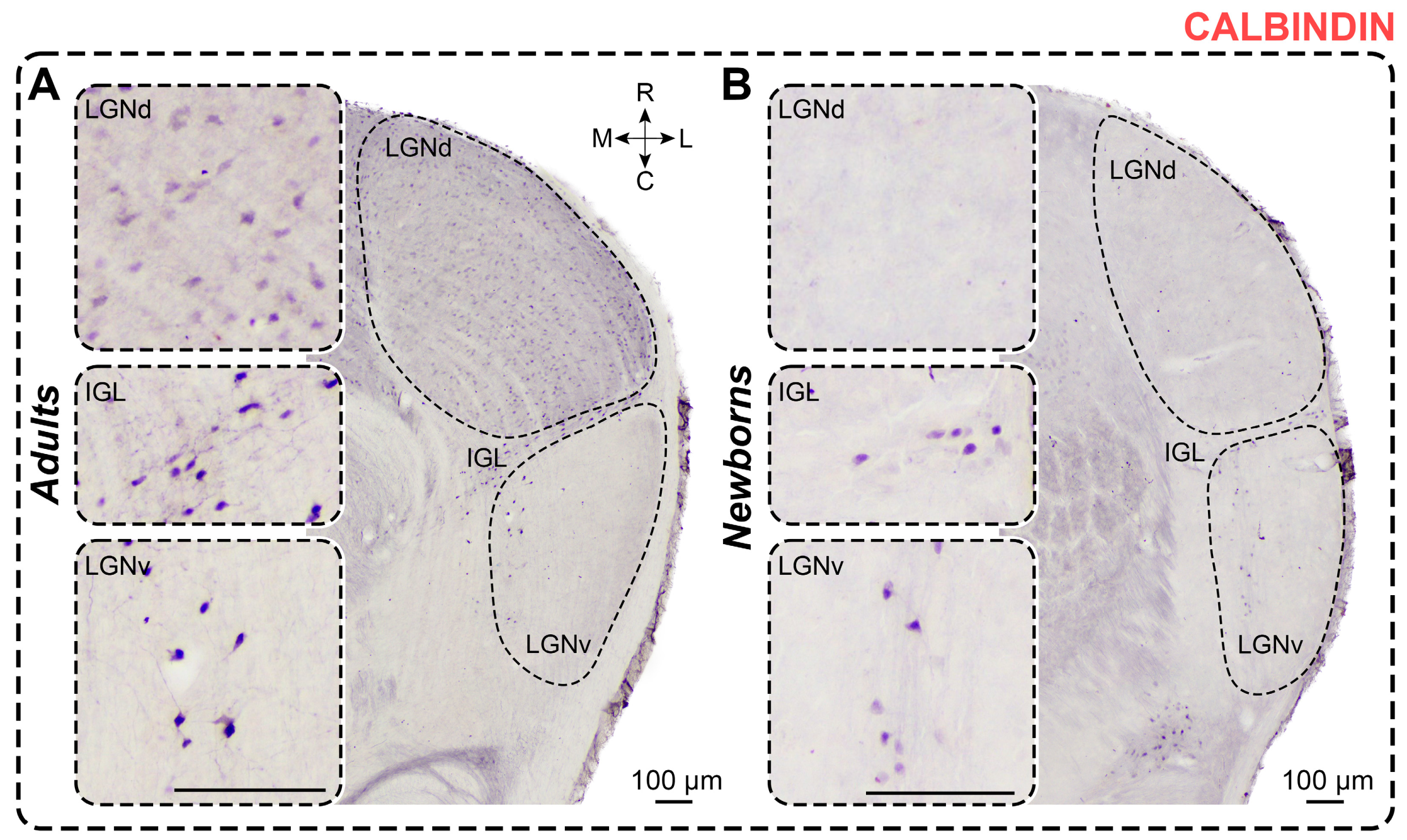
2.3. Calbindin Staining
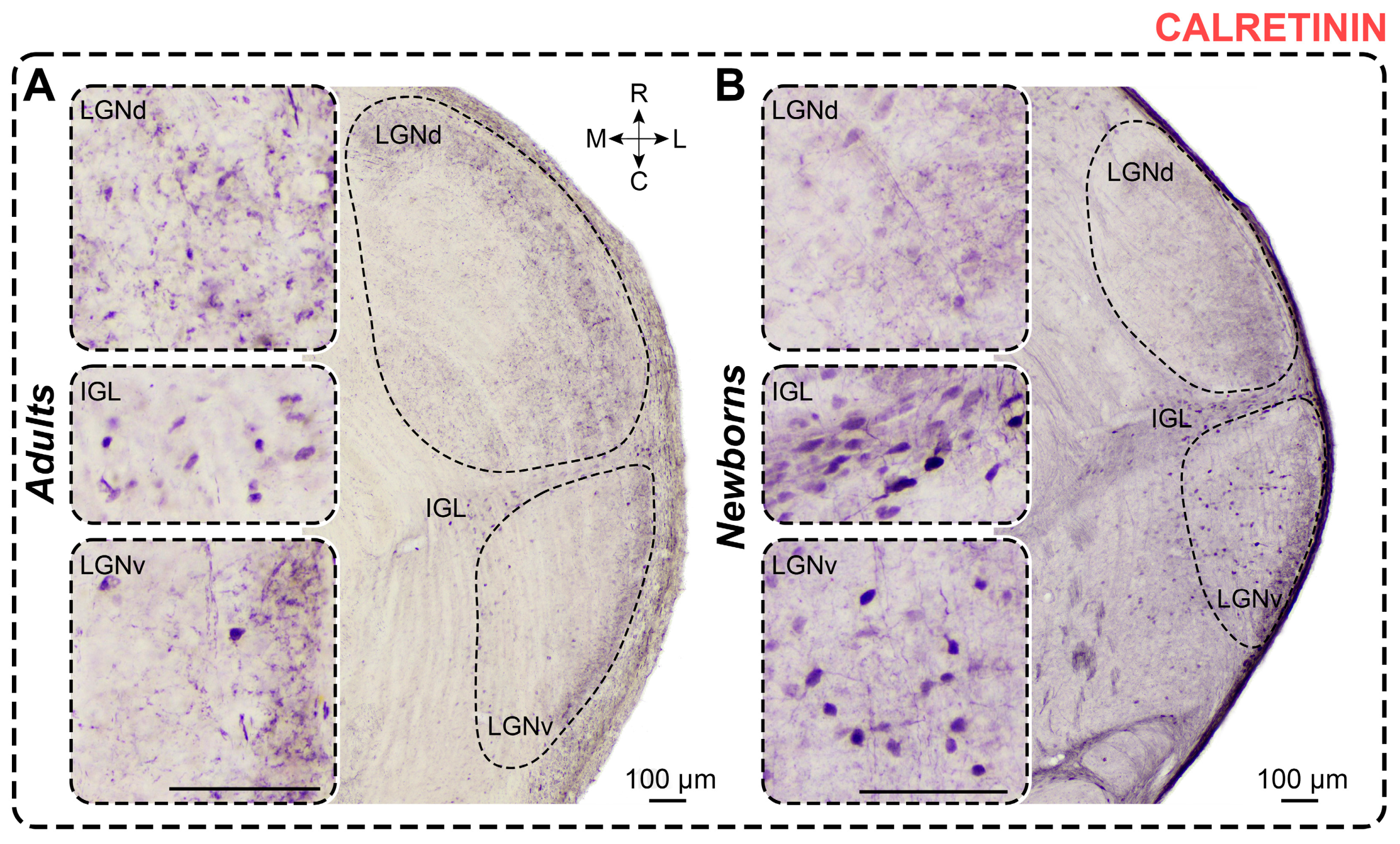
2.4. Calretinin Staining
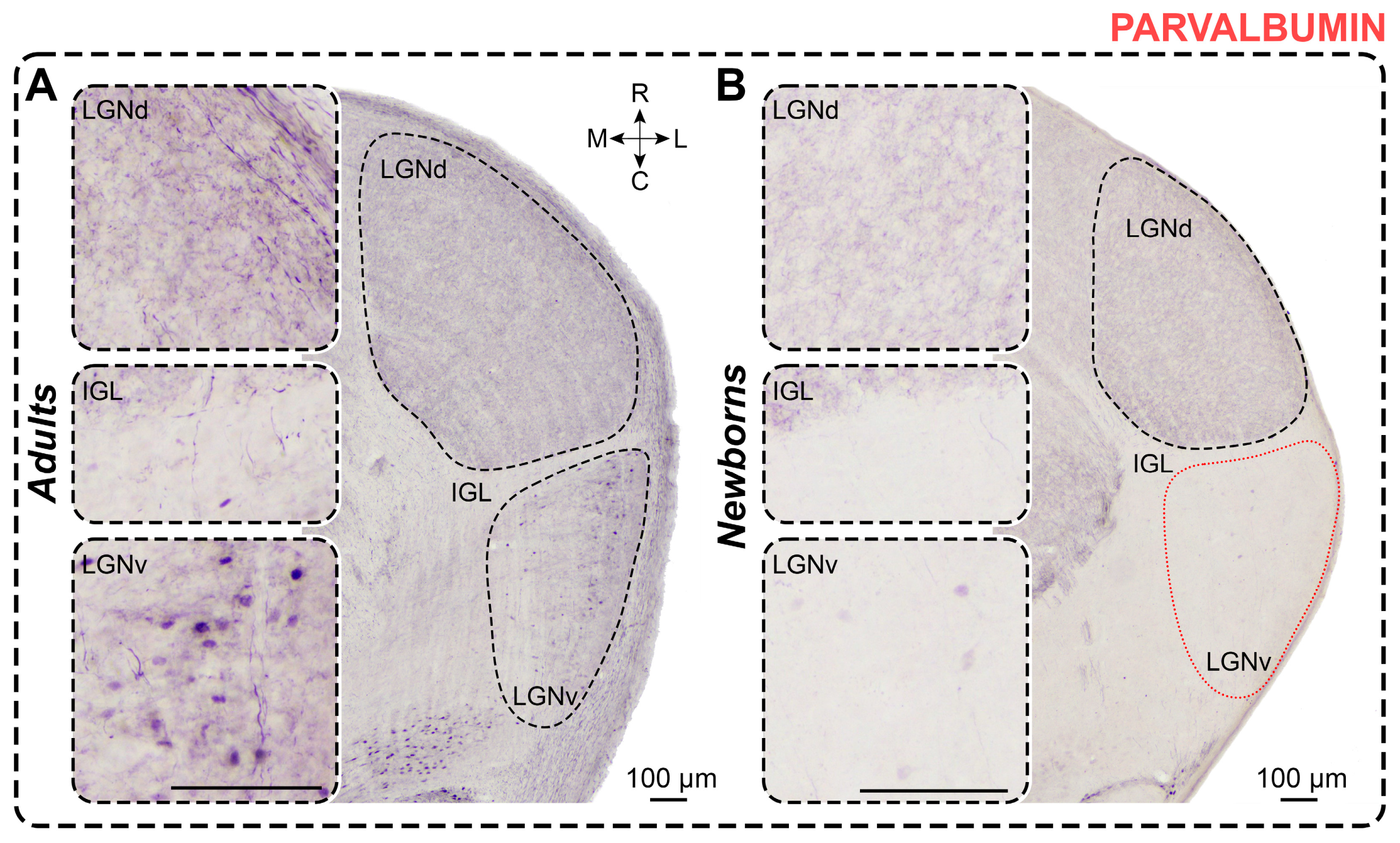
2.5. Parvalbumin Staining
2.6. Glutamic Acid Decarboxylase Staining
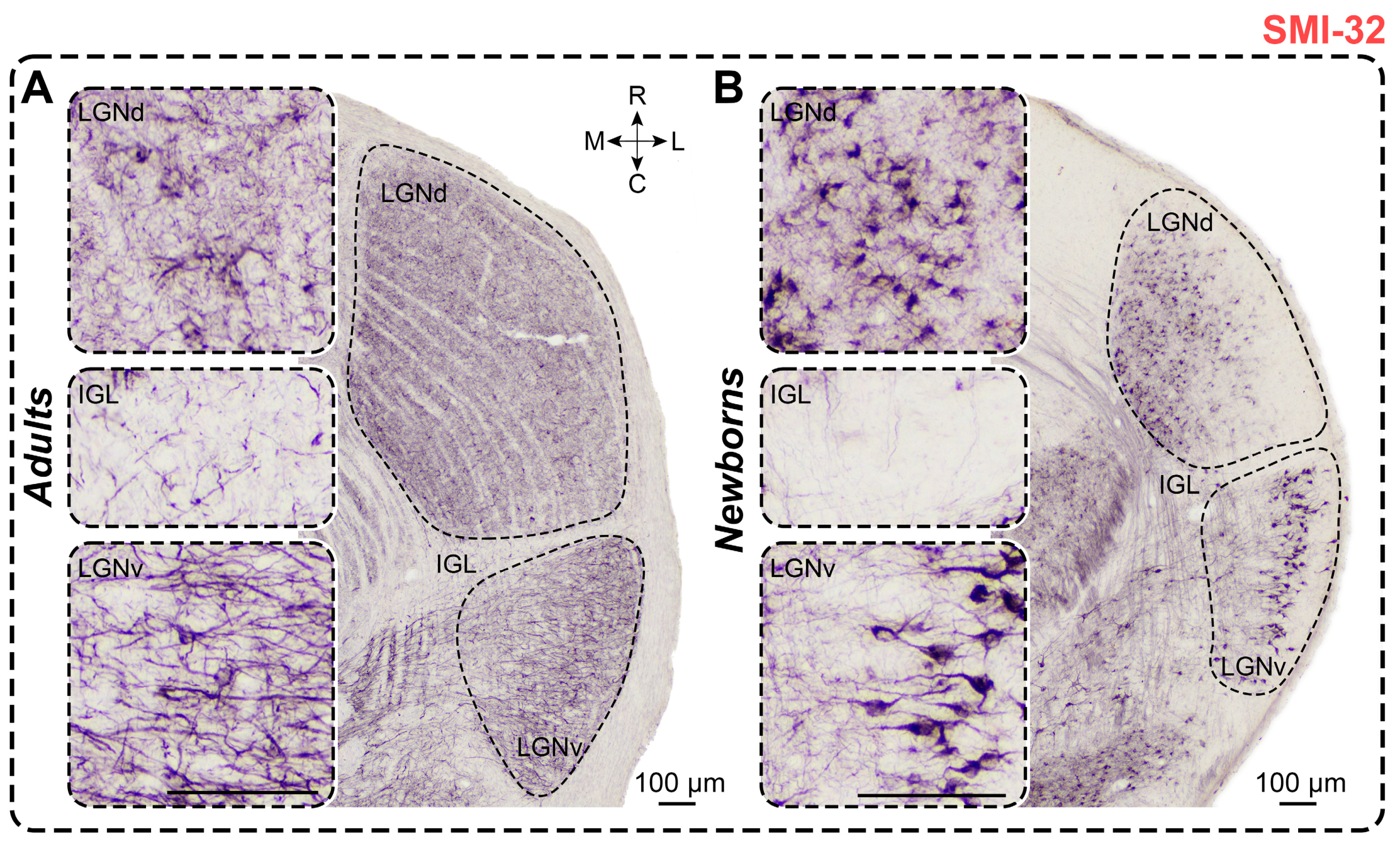
2.7. Non-Phosphorylated Heavy-Chain Neurofilament Staining
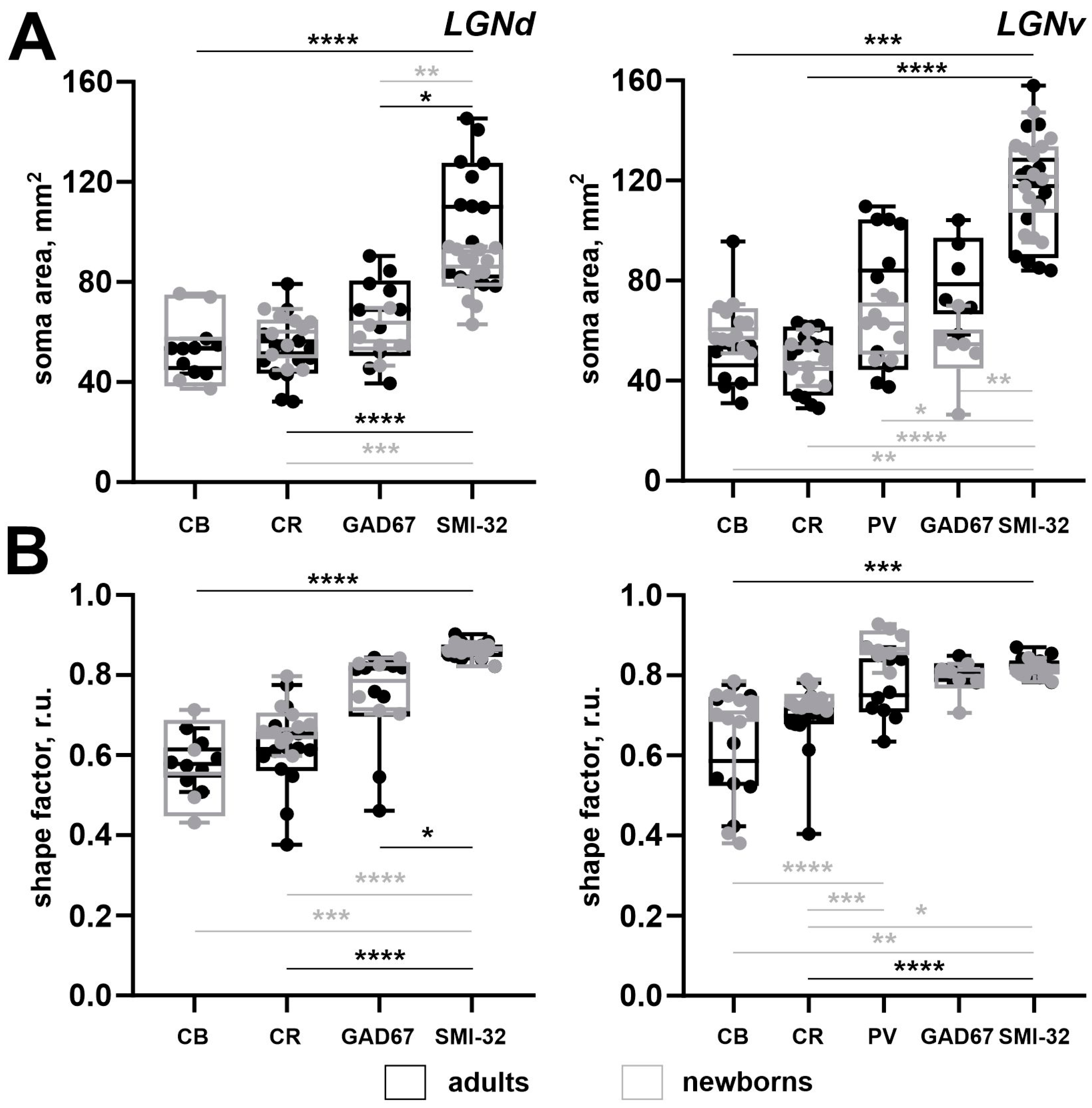
2.8. Comparison of Different Staining
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Perfusion and Histological Slices Preparing
4.3. Immunohistochemistry
4.4. Image Processing
4.5. Statistics
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matias Santos, D.; Rita, A.M.; Casanellas, I.; Brito Ova, A.; Araújo, I.M.; Power, D.; Tiscornia, G. Ear Wound Regeneration in the African Spiny Mouse Acomys cahirinus. Regeneration 2016, 3, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Maden, M.; Brant, J.O.; Rubiano, A.; Sandoval, A.G.W.; Simmons, C.; Mitchell, R.; Collin-Hooper, H.; Jacobson, J.; Omairi, S.; Patel, K. Perfect Chronic Skeletal Muscle Regeneration in Adult Spiny Mice, Acomys cahirinus. Sci. Rep. 2018, 8, 8920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-X.; Harn, H.I.-C.; Ou, K.-L.; Lei, M.; Chuong, C.-M. Comparative Regenerative Biology of Spiny (Acomys cahirinus) and Laboratory (Mus musculus) Mouse Skin. Exp. Dermatol. 2019, 28, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.G.W.; Maden, M. Regeneration in the Spiny Mouse, Acomys, a New Mammalian Model. Curr. Opin. Genet. Dev. 2020, 64, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, E.; Operschall, P. Die Funktionelle Reife der Neurohypophyse bei Neonaten Nestfl Uchtern und Nesthockern. Rev. Suisse Zool. 1962, 69, 297–301. [Google Scholar] [CrossRef]
- Brunjes, P.C. A Stereological Study of Neocortical Maturation in the Precocial Mouse, Acomys cahirinus. Brain Res. 1985, 351, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Felch, D.L.; Van Hooser, S.D. Molecular Compartmentalization of Lateral Geniculate Nucleus in the Gray Squirrel (Sciurus carolinensis). Front. Neuroanat. 2012, 6, 12. [Google Scholar] [CrossRef]
- Fiuza, F.P.; Queiroz, J.P.G.; Aquino, A.C.Q.; Câmara, D.A.; Brandão, L.E.M.; Lima, R.H.; Cavalcanti, J.R.L.P.; Engelberth, R.C.G.J.; Cavalcante, J.S. Aging Alters Daily and Regional Calretinin Neuronal Expression in the Rat Non-Image Forming Visual Thalamus. Front. Aging Neurosci. 2021, 13, 613305. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Arckens, L.; Vandesande, F.; Orban, G.A.; Heizmann, C.W.; Pochet, R. Calcium Binding Proteins as Molecular Markers for Cat Geniculate Neurons. Exp. Brain Res. 1991, 83, 513–520. [Google Scholar] [CrossRef]
- Arckens, L.; Rosier, A.; Heizmann, C.W.; Orban, G.A.; Vandesande, F. Partial Colocalization of the GABAA Receptor with Parvalbumin and Calbindin D-28K in Neurons of the Visual Cortex and the Dorsal Lateral Geniculate Nucleus of the Cat. J. Chem. Neuroanat. 1994, 8, 1–10. [Google Scholar] [CrossRef]
- Yan, Y.H.; Winarto, A.; Mansjoer, I.; Hendrickson, A. Parvalbumin, Calbindin, and Calretinin Mark Distinct Pathways during Development of Monkey Dorsal Lateral Geniculate Nucleus. J. Neurobiol. 1996, 31, 189–209. [Google Scholar] [CrossRef]
- Goodchild, A.K.; Martin, P.R. The Distribution of Calcium-Binding Proteins in the Lateral Geniculate Nucleus and Visual Cortex of a New World Monkey, the Marmoset, Callithrix Jacchus. Vis. Neurosci. 1998, 15, 625–642. [Google Scholar] [CrossRef]
- Erlander, M.G.; Tobin, A.J. The Structural and Functional Heterogeneity of Glutamic Acid Decarboxylase: A Review. Neurochem. Res. 1991, 16, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Lee, Y.; Lee, G.H. The Regulation of Glutamic Acid Decarboxylases in GABA Neurotransmission in the Brain. Arch. Pharm. Res. 2019, 42, 1031–1039. [Google Scholar] [CrossRef]
- Sternberger, L.A.; Sternberger, N.H. Monoclonal Antibodies Distinguish Phosphorylated and Nonphosphorylated Forms of Neurofilaments in Situ. Proc. Natl. Acad. Sci. USA 1983, 80, 6126–6130. [Google Scholar] [CrossRef]
- Bickford, M.E.; Guido, W.; Godwin, D.W. Neurofilament Proteins in Y-Cells of the Cat Lateral Geniculate Nucleus: Normal Expression and Alteration with Visual Deprivation. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.A.; Rosa, M.G.P. Neurofilament Protein Expression in the Geniculostriate Pathway of a New World Monkey (Callithrix jacchus). Exp. Brain Res. 2003, 150, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Yaun, A.; Cusick, C.G. Neurochemical Subdivisions of the Inferior Pulvinar in Macaque Monkeys. J. Comp. Neurol. 1995, 363, 545–562. [Google Scholar] [CrossRef]
- Greenberg, G. Depth Perception in Mongolian Gerbils (Meriones unguiculatus) and Spiny Mice (Acomys russatus and A. cahirinus). J. Comp. Psychol. 1986, 100, 81–84. [Google Scholar] [CrossRef]
- Goldman, M.; Skolnick, A.J.; Hernandez, T.P.; Tobach, E. Distance Perception in the Spiny Mouse Acomys cahirinus: Vertical Jumping. Percept. Mot. Skills 1992, 75, 883–895. [Google Scholar] [CrossRef]
- Chevret, P.; Denys, C.; Jaeger, J.J.; Michaux, J.; Catzeflis, F.M. Molecular Evidence That the Spiny Mouse (Acomys) Is More Closely Related to Gerbils (Gerbillinae) than to True Mice (Murinae). Proc. Natl. Acad. Sci. USA 1993, 90, 3433–3436. [Google Scholar] [CrossRef]
- Gustavsen, C.R.; Kvicerova, J.; Dickinson, H.; Heller, R.S. Acomys, the Closest Relatives to Gerbils, Do Express Pdx-1 Protein and Have Similar Islet Morphology to Gerbils. Islets 2009, 1, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Satorre, J.; Cano, J.; Reinoso-Suárez, F. Quantitative Cellular Changes during Postnatal Development of the Rat Dorsal Lateral Geniculate Nucleus. Anat. Embryol. 1986, 174, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Rübsamen, R.; Gutowski, M.; Langkau, J.; Dörrscheidt, G.J. Growth of Central Nervous System Auditory and Visual Nuclei in the Postnatal Gerbil (Meriones unguiculatus). J. Comp. Neurol. 1994, 346, 289–305. [Google Scholar] [CrossRef]
- Heumann, D.; Rabinowicz, T. Postnatal Development of the Dorsal Lateral Geniculate Nucleus in the Normal and Enucleated Albino Mouse. Exp. Brain Res. 1980, 38, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Satriotomo, I.; Miki, T.; Lee, K.-Y.; Yokoyama, T.; Touge, T.; Matsumoto, Y.; Li, H.-P.; Kuriyama, S.; Takeuchi, Y. Effects of Monocular Enucleation on Calbindin-D 28k and c-Fos Expression in the Lateral Geniculate Nucleus in Rats. Okajimas Folia Anat. Jpn. 2005, 82, 9–18. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Q.; Chen, Y.; Hu, H.; Gao, L.; Takahata, T. Three-Dimensional Topography of Eye-Specific Domains in the Lateral Geniculate Nucleus of Pigmented and Albino Rats. Cereb. Cortex 2023, 33, 9599–9615. [Google Scholar] [CrossRef]
- Grubb, M.S.; Thompson, I.D. Biochemical and Anatomical Subdivision of the Dorsal Lateral Geniculate Nucleus in Normal Mice and in Mice Lacking the Beta2 Subunit of the Nicotinic Acetylcholine Receptor. Vis. Res. 2004, 44, 3365–3376. [Google Scholar] [CrossRef]
- Horowitz, S.S.; Blanchard, J.H.; Morin, L.P. Intergeniculate Leaflet and Ventral Lateral Geniculate Nucleus Afferent Connections: An Anatomical Substrate for Functional Input from the Vestibulo-Visuomotor System. J. Comp. Neurol. 2004, 474, 227–245. [Google Scholar] [CrossRef]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain Atlas of the Mongolian Gerbil (Meriones unguiculatus) in CT/MRI-Aided Stereotaxic Coordinates. Brain Struct. Funct. 2016, 221, 1–272. [Google Scholar] [CrossRef]
- Resende, N.R.; Soares Filho, P.L.; Peixoto, P.P.A.; Silva, A.M.; Silva, S.F.; Soares, J.G.; do Nascimento, E.S.; Cavalcante, J.C.; Cavalcante, J.S.; Costa, M.S.M.O. Nuclear Organization and Morphology of Cholinergic Neurons in the Brain of the Rock Cavy (Kerodon rupestris). J. Chem. Neuroanat. 2018, 94, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Bhagwandin, A.; Gravett, N.; Bennett, N.C.; Manger, P.R. Distribution of Parvalbumin, Calbindin and Calretinin Containing Neurons and Terminal Networks in Relation to Sleep Associated Nuclei in the Brain of the Giant Zambian Mole-Rat (Fukomys mechowii). J. Chem. Neuroanat. 2013, 52, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gravett, N.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Distribution of Orexin-A Immunoreactive Neurons and Their Terminal Networks in the Brain of the Rock Hyrax, Procavia capensis. J. Chem. Neuroanat. 2011, 41, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Giolli, R.A.; Creel, D.J. The Primary Optic Projections in Pigmented and Albino Guinea Pigs: An Experimental Degeneration Study. Brain Res. 1973, 55, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, P.G. A Study of the Lateral Geniculate Body in the Diurnal Ground Squirrel, Citellus Tridecemlineatus Tridecemlineatus and in the Nocturnal Guinea Pig. Anat. Rec. 1963, 147, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Kanaseki, T.; Takimoto, T. The Comparative Anatomy of the Ventral Nucleus of the Lateral Geniculate Body in Mammals. J. Comp. Neurol. 1963, 121, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Dwarika, S.; Maseko, B.C.; Ihunwo, A.O.; Fuxe, K.; Manger, P.R. Distribution and Morphology of Putative Catecholaminergic and Serotonergic Neurons in the Brain of the Greater Canerat, Thryonomys swinderianus. J. Chem. Neuroanat. 2008, 35, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Bickford, M.E.; Zhou, N.; Krahe, T.E.; Govindaiah, G.; Guido, W. Retinal and Tectal “Driver-Like” Inputs Converge in the Shell of the Mouse Dorsal Lateral Geniculate Nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 10523–10534. [Google Scholar] [CrossRef] [PubMed]
- Okigawa, S.; Yamaguchi, M.; Ito, K.N.; Takeuchi, R.F.; Morimoto, N.; Osakada, F. Cell Type- and Layer-Specific Convergence in Core and Shell Neurons of the Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2021, 529, 2099–2124. [Google Scholar] [CrossRef]
- Huberman, A.D.; Wei, W.; Elstrott, J.; Stafford, B.K.; Feller, M.B.; Barres, B.A. Genetic Identification of an On-Off Direction-Selective Retinal Ganglion Cell Subtype Reveals a Layer-Specific Subcortical Map of Posterior Motion. Neuron 2009, 62, 327–334. [Google Scholar] [CrossRef]
- Kay, J.N.; De la Huerta, I.; Kim, I.-J.; Zhang, Y.; Yamagata, M.; Chu, M.W.; Meister, M.; Sanes, J.R. Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 7753–7762. [Google Scholar] [CrossRef]
- Marshel, J.H.; Kaye, A.P.; Nauhaus, I.; Callaway, E.M. Anterior-Posterior Direction Opponency in the Superficial Mouse Lateral Geniculate Nucleus. Neuron 2012, 76, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, D.M.; El-Danaf, R.N.; Huberman, A.D.; Niell, C.M. Diverse Visual Features Encoded in Mouse Lateral Geniculate Nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martín, A.; El-Danaf, R.N.; Osakada, F.; Sriram, B.; Dhande, O.S.; Nguyen, P.L.; Callaway, E.M.; Ghosh, A.; Huberman, A.D. A Dedicated Circuit Links Direction-Selective Retinal Ganglion Cells to the Primary Visual Cortex. Nature 2014, 507, 358–361. [Google Scholar] [CrossRef]
- Kerschensteiner, D.; Guido, W. Organization of the Dorsal Lateral Geniculate Nucleus in the Mouse. Vis. Neurosci. 2017, 34, E008. [Google Scholar] [CrossRef]
- Martersteck, E.M.; Hirokawa, K.E.; Evarts, M.; Bernard, A.; Duan, X.; Li, Y.; Ng, L.; Oh, S.W.; Ouellette, B.; Royall, J.J.; et al. Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep. 2017, 18, 2058–2072. [Google Scholar] [CrossRef]
- Krahe, T.E.; El-Danaf, R.N.; Dilger, E.K.; Henderson, S.C.; Guido, W. Morphologically Distinct Classes of Relay Cells Exhibit Regional Preferences in the Dorsal Lateral Geniculate Nucleus of the Mouse. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17437–17448. [Google Scholar] [CrossRef]
- Huberman, A.D.; Manu, M.; Koch, S.M.; Susman, M.W.; Lutz, A.B.; Ullian, E.M.; Baccus, S.A.; Barres, B.A. Architecture and Activity-Mediated Refinement of Axonal Projections from a Mosaic of Genetically Identified Retinal Ganglion Cells. Neuron 2008, 59, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, U.; Govindaiah, G.; Somaiya, R.D.; Ha, R.V.; Wei, J.C.; Guido, W.; Fox, M.A. Diverse GABAergic Neurons Organize into Subtype-Specific Sublaminae in the Ventral Lateral Geniculate Nucleus. J. Neurochem. 2021, 159, 479–497. [Google Scholar] [CrossRef]
- Moore, R.Y.; Card, J.P. Intergeniculate Leaflet: An Anatomically and Functionally Distinct Subdivision of the Lateral Geniculate Complex. J. Comp. Neurol. 1994, 344, 403–430. [Google Scholar] [CrossRef]
- Moore, R.Y.; Weis, R.; Moga, M.M. Efferent Projections of the Intergeniculate Leaflet and the Ventral Lateral Geniculate Nucleus in the Rat. J. Comp. Neurol. 2000, 420, 398–418. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; Cozzi, B.; Møller, M. Efferent Projections from the Lateral Geniculate Nucleus to the Pineal Complex of the Mongolian Gerbil (Meriones unguiculatus). Cell Tissue Res. 1991, 264, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cant, N.B.; Benson, C.G. Multiple Topographically Organized Projections Connect the Central Nucleus of the Inferior Colliculus to the Ventral Division of the Medial Geniculate Nucleus in the Gerbil, Meriones unguiculatus. J. Comp. Neurol. 2007, 503, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kastner, R.; Meller, D.; Eysel, U.T. Immunohistochemical Changes of Neuronal Calcium-Binding Proteins Parvalbumin and Calbindin-D-28k Following Unilateral Deafferentation in the Rat Visual System. Exp. Neurol. 1992, 117, 230–246. [Google Scholar] [CrossRef]
- Okoyama, S.; Moriizumi, T. Onset of Calbindin-D 28K and Parvalbumin Expression in the Lateral Geniculate Complex and Olivary Pretectal Nucleus during Postnatal Development of the Rat. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2001, 19, 655–661. [Google Scholar] [CrossRef]
- Frassoni, C.; Bentivoglio, M.; Spreafico, R.; Sánchez, M.P.; Puelles, L.; Fairen, A. Postnatal Development of Calbindin and Parvalbumin Immunoreactivity in the Thalamus of the Rat. Brain Res. Dev. Brain Res. 1991, 58, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Arai, R.; Kani, K.; Jacobowitz, D.M. Immunohistochemical Localization of Calretinin in the Rat Lateral Geniculate Nucleus and Its Retino-Geniculate Projection. Brain Res. 1992, 596, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Winsky, L.; Arai, M.; Jacobowitz, D.M. Immunohistochemical Localization of Calretinin in the Rat Hindbrain. J. Comp. Neurol. 1991, 310, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Winsky, L.; Montpied, P.; Arai, R.; Martin, B.M.; Jacobowitz, D.M. Calretinin Distribution in the Thalamus of the Rat: Immunohistochemical and in Situ Hybridization Histochemical Analyses. Neuroscience 1992, 50, 181–196. [Google Scholar] [CrossRef]
- Frassoni, C.; Arcelli, P.; Selvaggio, M.; Spreafico, R. Calretinin Immunoreactivity in the Developing Thalamus of the Rat: A Marker of Early Generated Thalamic Cells. Neuroscience 1998, 83, 1203–1214. [Google Scholar] [CrossRef]
- Merkulyeva, N.; Mikhalkin, A.; Kostareva, A.; Vavilova, T. Transient Neurochemical Features of the Perigeniculate Neurons during Early Postnatal Development of the Cat. J. Comp. Neurol. 2022, 530, 3193–3208. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Yamada, Y.; Imamura, K.; Mataga, N.; Watanabe, Y.; Yamamoto, M. Effects of Monocular Enucleation on Parvalbumin in Rat Visual System during Postnatal Development. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2535–2545. [Google Scholar]
- Gonzalez, D.; Satriotomo, I.; Miki, T.; Lee, K.-Y.; Yokoyama, T.; Touge, T.; Matsumoto, Y.; Li, H.-P.; Kuriyama, S.; Takeuchi, Y. Changes of Parvalbumin Immunoreactive Neurons and GFAP Immunoreactive Astrocytes in the Rat Lateral Geniculate Nucleus Following Monocular Enucleation. Neurosci. Lett. 2006, 395, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Arai, R.; Jacobowitz, D.M.; Deura, S. Distribution of Calretinin, Calbindin-D28k, and Parvalbumin in the Rat Thalamus. Brain Res. Bull. 1994, 33, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Giolli, R.A.; Peterson, G.M.; Ribak, C.E.; McDonald, H.M.; Blanks, R.H.; Fallon, J.H. GABAergic Neurons Comprise a Major Cell Type in Rodent Visual Relay Nuclei: An Immunocytochemical Study of Pretectal and Accessory Optic Nuclei. Exp. Brain Res. 1985, 61, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.L.; Isackson, P.J.; Gall, C.M.; Jones, E.G. Contrasting Patterns in the Localization of Glutamic Acid Decarboxylase and Ca2+/Calmodulin Protein Kinase Gene Expression in the Rat Central Nervous System. Neuroscience 1992, 46, 825–849. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Carrillo, G.L.; Govindaiah, G.; Monavarfeshani, A.; Bircher, J.S.; Su, J.; Guido, W.; Fox, M.A. Nuclei-Specific Differences in Nerve Terminal Distribution, Morphology, and Development in Mouse Visual Thalamus. Neural Dev. 2014, 9, 16. [Google Scholar] [CrossRef]
- Langel, J.; Ikeno, T.; Yan, L.; Nunez, A.A.; Smale, L. Distributions of GABAergic and Glutamatergic Neurons in the Brains of a Diurnal and Nocturnal Rodent. Brain Res. 2018, 1700, 152–159. [Google Scholar] [CrossRef]
- Ohara, P.T.; Lieberman, A.R.; Hunt, S.P.; Wu, J.Y. Neural Elements Containing Glutamic Acid Decarboxylase (GAD) in the Dorsal Lateral Geniculate Nucleus of the Rat; Immunohistochemical Studies by Light and Electron Microscopy. Neuroscience 1983, 8, 189–211. [Google Scholar] [CrossRef]
- Gabbott, P.L.; Somogyi, J.; Stewart, M.G.; Hamori, J. GABA-Immunoreactive Neurons in the Rat Dorsal Lateral Geniculate Nucleus: Light Microscopical Observations. Brain Res. 1985, 346, 171–175. [Google Scholar] [CrossRef]
- Gabbott, P.L.; Somogyi, J.; Stewart, M.G.; Hámori, J. A Quantitative Investigation of the Neuronal Composition of the Rat Dorsal Lateral Geniculate Nucleus Using GABA-Immunocytochemistry. Neuroscience 1986, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Amadeo, A.; Arcelli, P.; Frassoni, C.; Spreafico, R. Postnatal Development of GABA-Immunoreactive Terminals in the Reticular and Ventrobasal Nuclei of the Rat Thalamus: A Light and Electron Microscopic Study. Neuroscience 1997, 76, 503–515. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.K.; Speciale, S.G.; Parnavelas, J.G. The Development of Glutamic Acid Decarboxylase in the Visual Cortex and the Dorsal Lateral Geniculate Nucleus of the Rat. Brain Res. 1981, 217, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.G.M.; Rosado De Castro, P.H.; Fiorani, M.; Nascimento-Silva, S.; Gattass, R. Distribution of Neurofilament Proteins in the Lateral Geniculate Nucleus, Primary Visual Cortex, and Area MT of Adult Cebus Monkeys. J. Comp. Neurol. 2008, 508, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mikhalkin, A.; Nikitina, N.; Merkulyeva, N. Heterochrony of Postnatal Accumulation of Nonphosphorylated Heavy-Chain Neurofilament by Neurons of the Cat Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2021, 529, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.; van der List, D.; Wang, G.-Y.; Chalupa, L.M. Morphological Properties of Mouse Retinal Ganglion Cells. Neuroscience 2006, 140, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bleckert, A.; Schwartz, G.W.; Turner, M.H.; Rieke, F.; Wong, R.O.L. Visual Space Is Represented by Nonmatching Topographies of Distinct Mouse Retinal Ganglion Cell Types. Curr. Biol. CB 2014, 24, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.; Li, C.; Do, Q.; Burke, E.G.; Heynderickx, S.; Farrow, K. A Projection Specific Logic to Sampling Visual Inputs in Mouse Superior Colliculus. eLife 2019, 8, e50697. [Google Scholar] [CrossRef] [PubMed]
- Kutcher, M.R.; Duffy, K.R. Cytoskeleton Alteration Correlates with Gross Structural Plasticity in the Cat Lateral Geniculate Nucleus. Vis. Neurosci. 2007, 24, 775–785. [Google Scholar] [CrossRef]
- Brunjes, P.C. Olfactory Bulb Maturation in Acomys cahirinus: Is Neural Growth Similar in Precocial and Altricial Murids? Dev. Brain Res. 1983, 8, 335–341. [Google Scholar] [CrossRef]
- Shkorbatova, P.Y.; Veshchitskii, A.A.; Mikhalkin, A.A.; Nikitina, N.I.; Belyaev, A.V.; Merkulyeva, N.S. Development of Acomys Cahirinus in the Laboratory Conditions. J. Evol. Biochem. Physiol. in press.
- Dickinson, H.; Walker, D. Managing a Colony of Spiny Mice (Acomys cahirinus) for Perinatal Research. ANZCCART News 2007, 20, 4–10. [Google Scholar]
- Allen Institute for Brain Science Allen Mouse Brain Atlas [Dataset]. Available Mousebrain-Maporg, 2004; in press.
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Gulf Professional Publishing: Houston, TX, USA, 2004; ISBN 9780125476409. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.A.; Moritz, K.M.; Walker, D.W.; Dickinson, H. Sexually Dimorphic Placental Development throughout Gestation in the Spiny Mouse (Acomys cahirinus). Placenta 2013, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]

| Adults | Newborns | |||||
|---|---|---|---|---|---|---|
| LGNd | LGNv | IGL | LGNd | LGNv | IGL | |
| Soma area, µm2 | ||||||
| CB | 50.9 ± 5.3 | 50.5 ± 20.1 | 53.1 ± 11.0 | 56.6 ± 20.6 ns | 61.5 ± 6.9 * | 59.6 ± 7.6 ns |
| CR | 51.7 ± 11.6 | 49.9 ± 12.6 | – | 57.8 ± 8.7 ns | 48.6 ± 6.9 ns | 47.6 ± 4.9 ns |
| PV | – | 76.4 ± 29.7 | – | – | 61.8 ± 10.0 ns | – |
| GAD67 | 66.8 ± 16.9 | 80.6 ± 17.1 | – | 57.5 ± 7.9 ns | 52.5 ± 14.2 ** | – |
| SMI-32 | 106.7 ± 23.8 | 115.1 ± 23.1 | – | 83.7 ± 9.7 * | 120.8 ± 16.2 ns | – |
| Maximal diameter, µm | ||||||
| CB | 11.8 ± 0.8 | 11.4 ± 2.0 | 12.6 ± 1.5 | 11.8 ± 2.4 ns | 12.3 ± 1.5 ns | 12.4 ± 0.9 ns |
| CR | 11.6 ± 1.9 | 11.2 ± 1.6 | 11.9 ± 1.7 | 11.6 ± 1.0 ns | 10.6 ± 0.8 ns | 11.0 ± 0.6 ns |
| PV | – | 12.9 ± 2.3 | – | – | 11.2 ± 1.2 ns | – |
| GAD67 | 12.7 ± 1.1 | 13.7 ± 1.4 | – | 12.0 ± 1.1 ns | 11.2 ± 1.6 * | – |
| SMI-32 | 14.7 ± 1.5 | 16.1 ± 1.7 | – | 13.2 ± 0.7 ** | 16.8 ± 1.3 ns | – |
| Minimal diameter, µm | ||||||
| CB | 7.3 ± 0.2 | 7.3 ± 1.7 | 6.9 ± 0.9 | 7.9 ± 1.8 ns | 8.1 ± 0.9 ns | 7.7 ± 0.7 * |
| CR | 7.3 ± 0.9 | 7.2 ± 1.2 | 7.1 ± 1.2 | 8.0 ± 0.7 ns | 7.1 ± 0.5 ns | 6.7 ± 0.3 ns |
| PV | – | 8.3 ± 1.5 | – | – | 7.4 ± 0.6 ns | – |
| GAD67 | 7.7 ± 0.9 | 8.2 ± 1.3 | – | 6.9 ± 0.5 * | 6.6 ± 0.9 * | – |
| SMI-32 | 9.9 ± 1.1 | 10.0 ± 0.9 | – | 8.7 ± 0.6 * | 10.2 ± 0.7 ns | – |
| Shape factor, r.u. | ||||||
| CB | 0.58 ± 0.05 | 0.61 ± 0.13 | 0.62 ± 0.05 | 0.56 ± 0.12 ns | 0.66 ± 0.14 ns | 0.65 ± 0.05 ns |
| CR | 0.60 ± 0.10 | 0.68 ± 0.09 | 0.67 ± 0.03 | 0.68 ± 0.05 * | 0.74 ± 0.03 ** | 0.70 ± 0.02 * |
| PV | – | 0.77 ± 0.08 | – | – | 0.87 ± 0.04 ** | – |
| GAD67 | 0.75 ± 0.13 | 0.81 ± 0.03 | – | 0.78 ± 0.06 ns | 0.79 ± 0.04 ns | – |
| SMI-32 | 0.86 ± 0.02 | 0.83 ± 0.02 | – | 0.87 ± 0.03 ns | 0.82 ± 0.02 ns | – |
| Adults | Newborns | |||||
|---|---|---|---|---|---|---|
| LGNd | LGNv | IGL | LGNd | LGNv | IGL | |
| Number of neurons | ||||||
| CB | 257 ± 166 | 12 ± 8 | 17 ± 5 | 3 ± 6 **** | 12 ± 4 ns | 12 ± 4 * |
| CR | 5 ± 2 | 31 ± 11 | 22 ± 8 | 37 ± 27 **** | 69 ± 23 *** | 35 ± 14 * |
| PV | – | 49 ± 12 | – | – | 4 ± 2 **** | – |
| GAD67 | 81 ± 17 | 9 ± 2 | 2 ± 2 | 36 ± 26 ** | 8 ± 3 ns | 0.1 ± 0.2 ns |
| SMI-32 | 56 ± 17 | 29 ± 8 | 0.8 ± 0.9 | 82 ± 31 * | 76 ± 8 **** | 2 ± 2 * |
| Cellular density | ||||||
| CB | 517 ± 394 | 40 ± 28 | – | 10 ± 17 **** | 43 ± 14 ns | – |
| CR | 10 ± 5 | 99 ± 32 | – | 124 ± 81 **** | 349 ± 109 **** | – |
| PV | – | 241 ± 74 | – | – | 1 ± 2 **** | – |
| GAD67 | 154 ± 36 | 28 ± 11 | – | 118 ± 85 ns | 52 ± 16 ** | – |
| SMI-32 | 107 ± 36 | 147 ± 40 | – | 330 ± 128 **** | 492 ± 90 **** | – |
| Antibody | Host | Clonality | Dilution | Manufacturer | Lot | RRID |
|---|---|---|---|---|---|---|
| Calbindin | Mouse | Monoclonal | 1:10,000 | Sigma-Aldrich (Burlington, MA, USA) | C9848 | AB_476894 |
| Calretinin | Rabbit | Polyclonal | 1:10,000 | Sigma-Aldrich (Burlington, MA, USA) | AB5054 | AB_2068506 |
| Parvalbumin | Rabbit | Polyclonal | 1:5000 | Abcam (Cambridge, UK) | ab11427 | AB_298032 |
| GAD67 | Mouse | Monoclonal | 1:2000 | Sigma-Aldrich (Burlington, MA, USA) | MAB5406 | AB_2278725 |
| SMI-32 | Mouse | Monoclonal | 1:5000 | BioLegend (San Diego, CA, USA) | 801701 | AB_2564642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkulyeva, N.; Mikhalkin, A.; Veshchitskii, A. Inner Structure of the Lateral Geniculate Complex of Adult and Newborn Acomys cahirinus. Int. J. Mol. Sci. 2024, 25, 7855. https://doi.org/10.3390/ijms25147855
Merkulyeva N, Mikhalkin A, Veshchitskii A. Inner Structure of the Lateral Geniculate Complex of Adult and Newborn Acomys cahirinus. International Journal of Molecular Sciences. 2024; 25(14):7855. https://doi.org/10.3390/ijms25147855
Chicago/Turabian StyleMerkulyeva, Natalia, Aleksandr Mikhalkin, and Aleksandr Veshchitskii. 2024. "Inner Structure of the Lateral Geniculate Complex of Adult and Newborn Acomys cahirinus" International Journal of Molecular Sciences 25, no. 14: 7855. https://doi.org/10.3390/ijms25147855





