Evaluating the Therapeutic Effect of Hispidin on Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
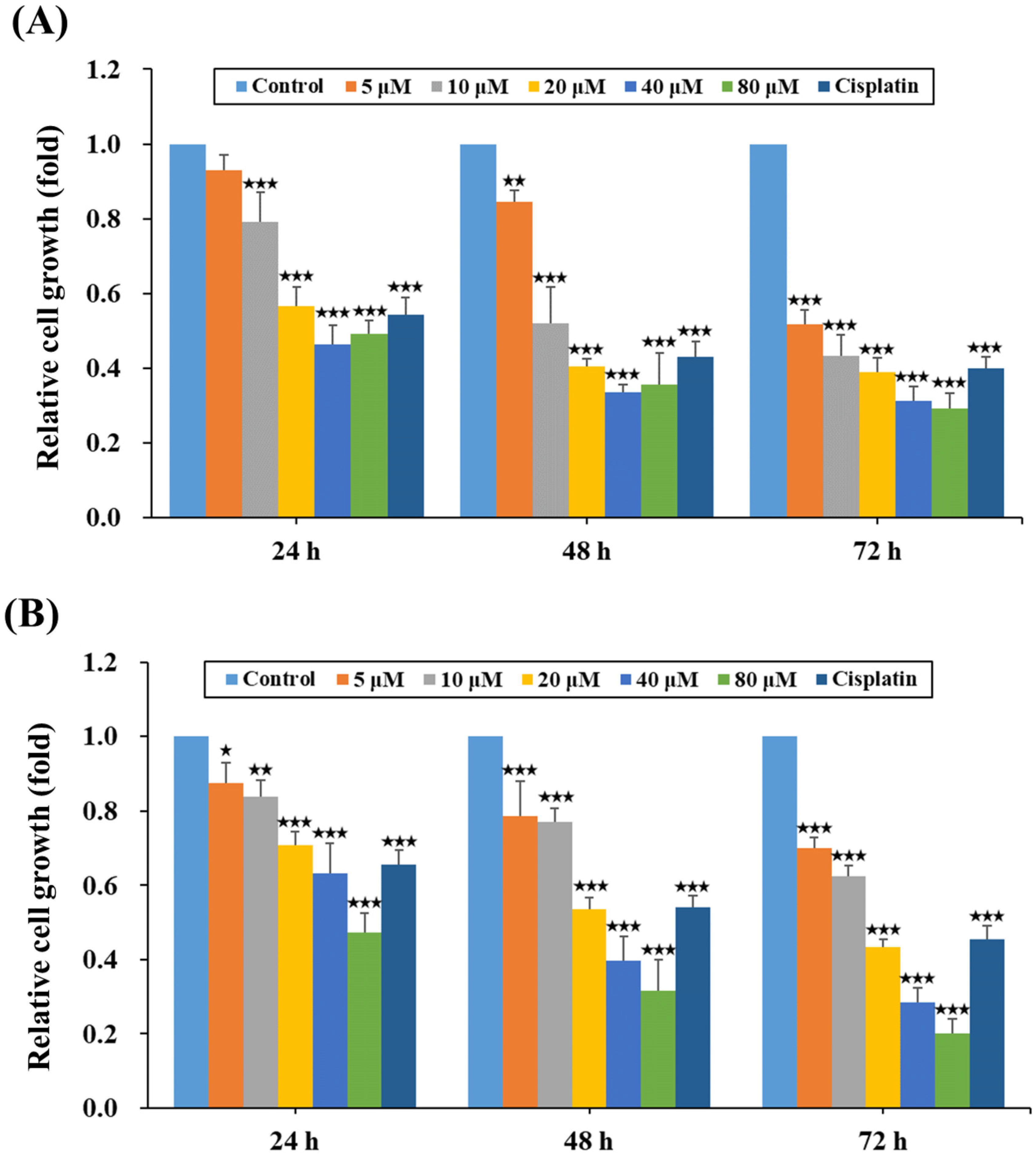
2.1. HPD Attenuated the Growth of PCa Cells
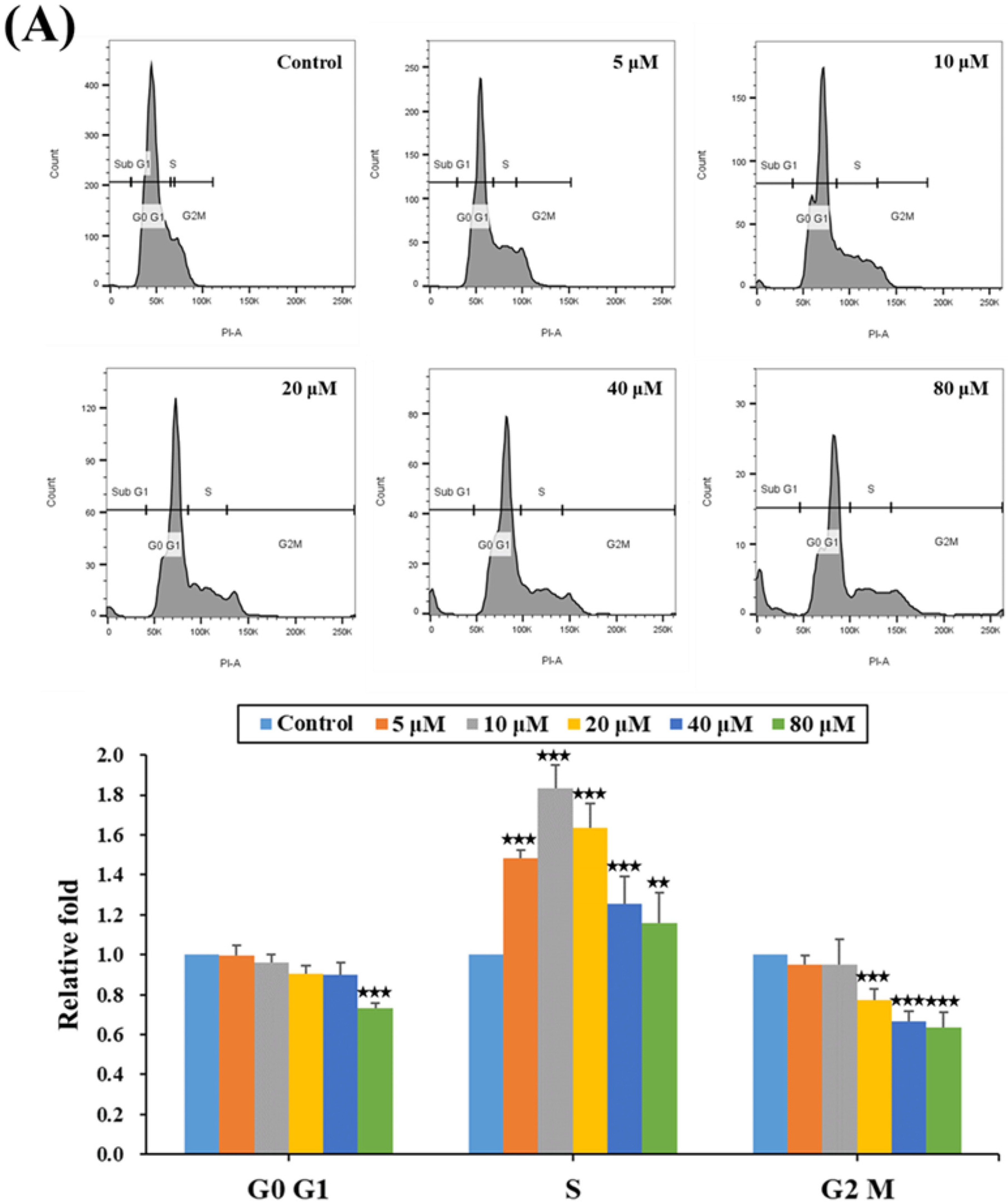
2.2. HPD Impeded Cell Cycle Progression at the S Phase in PCa Cells
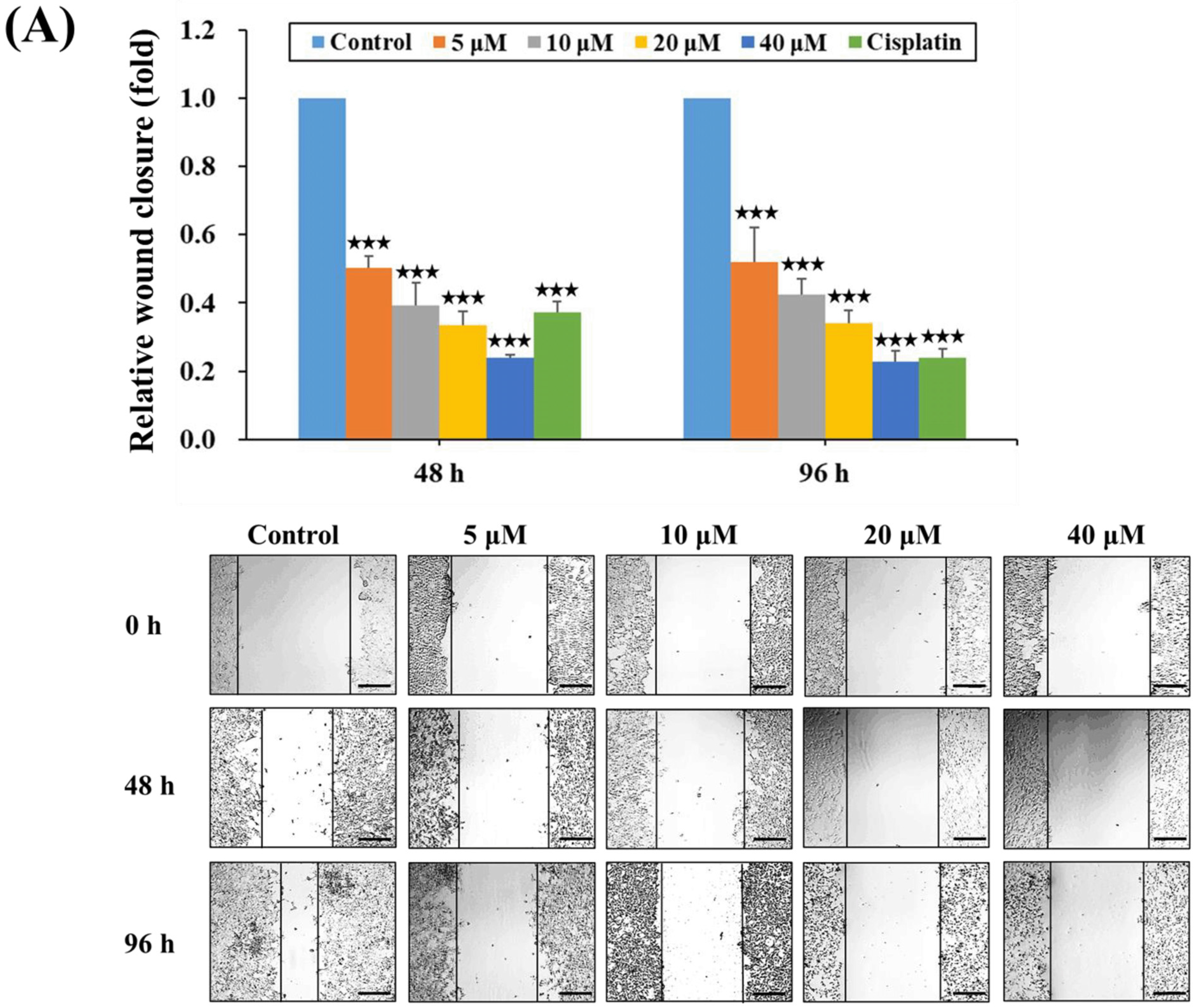
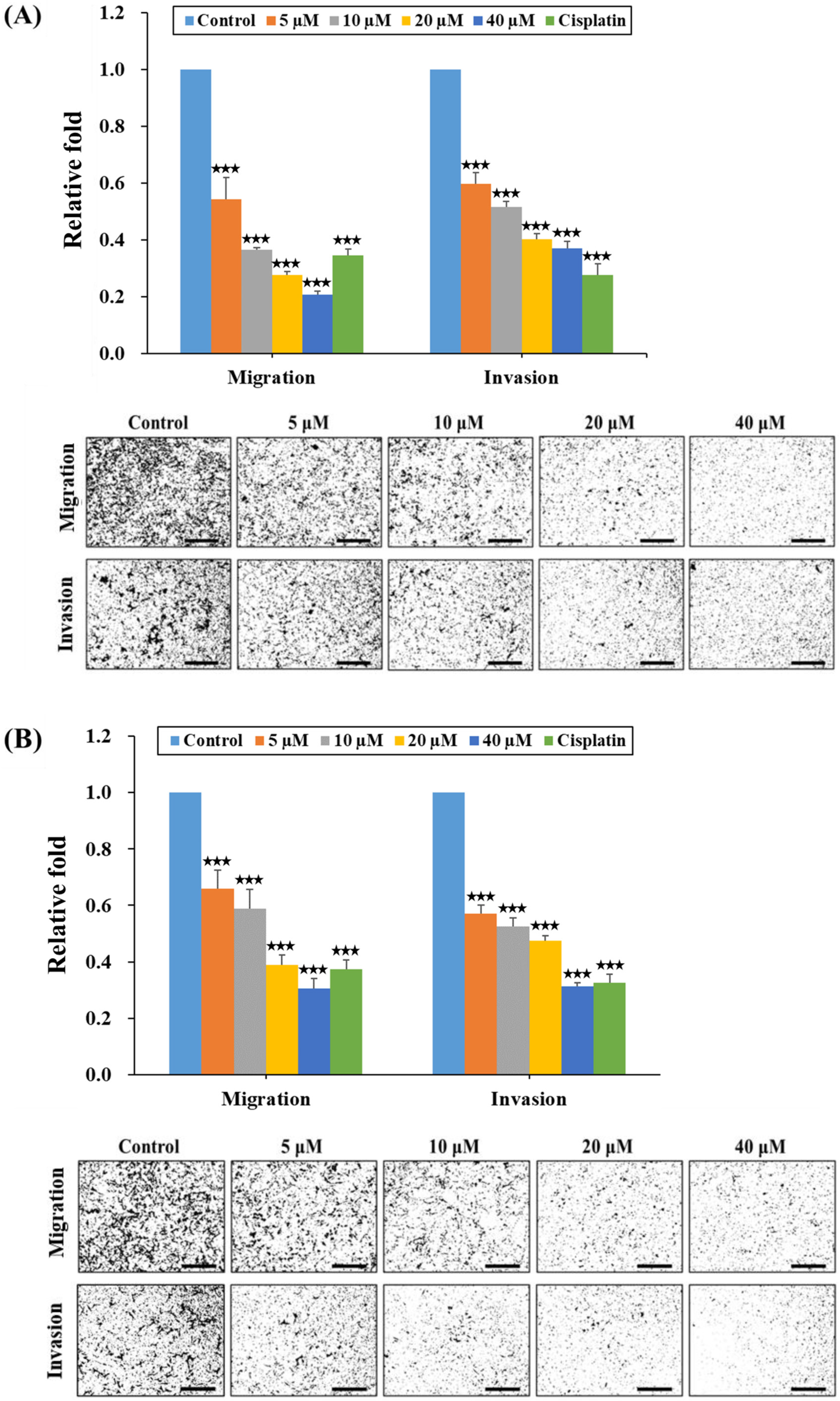
2.3. HPD Suppressed Migration and Invasion of PCa Cells
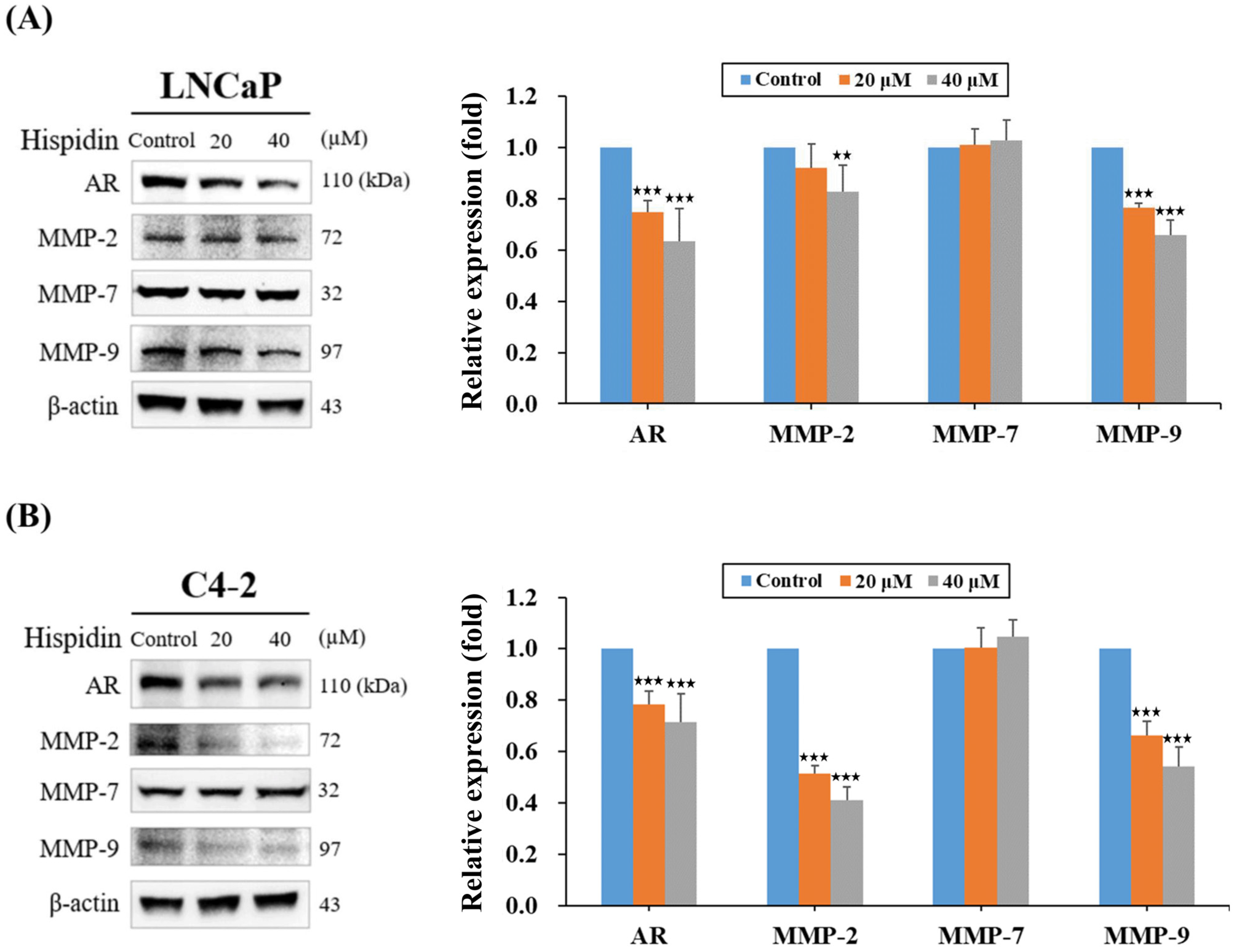
2.4. HPD Reduced Protein Expression Related to Cell Growth and Cancer Progression in PCa Cells
2.5. HPD Induced Cell Death via Activation of Caspase-Dependent Apoptosis in PCa Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Cell Growth Assay
4.3. Cell Migration Assay
4.4. Cell Progression Assays In Vitro
4.5. Investigation of Cell Cycle Arrest
4.6. Apoptosis Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
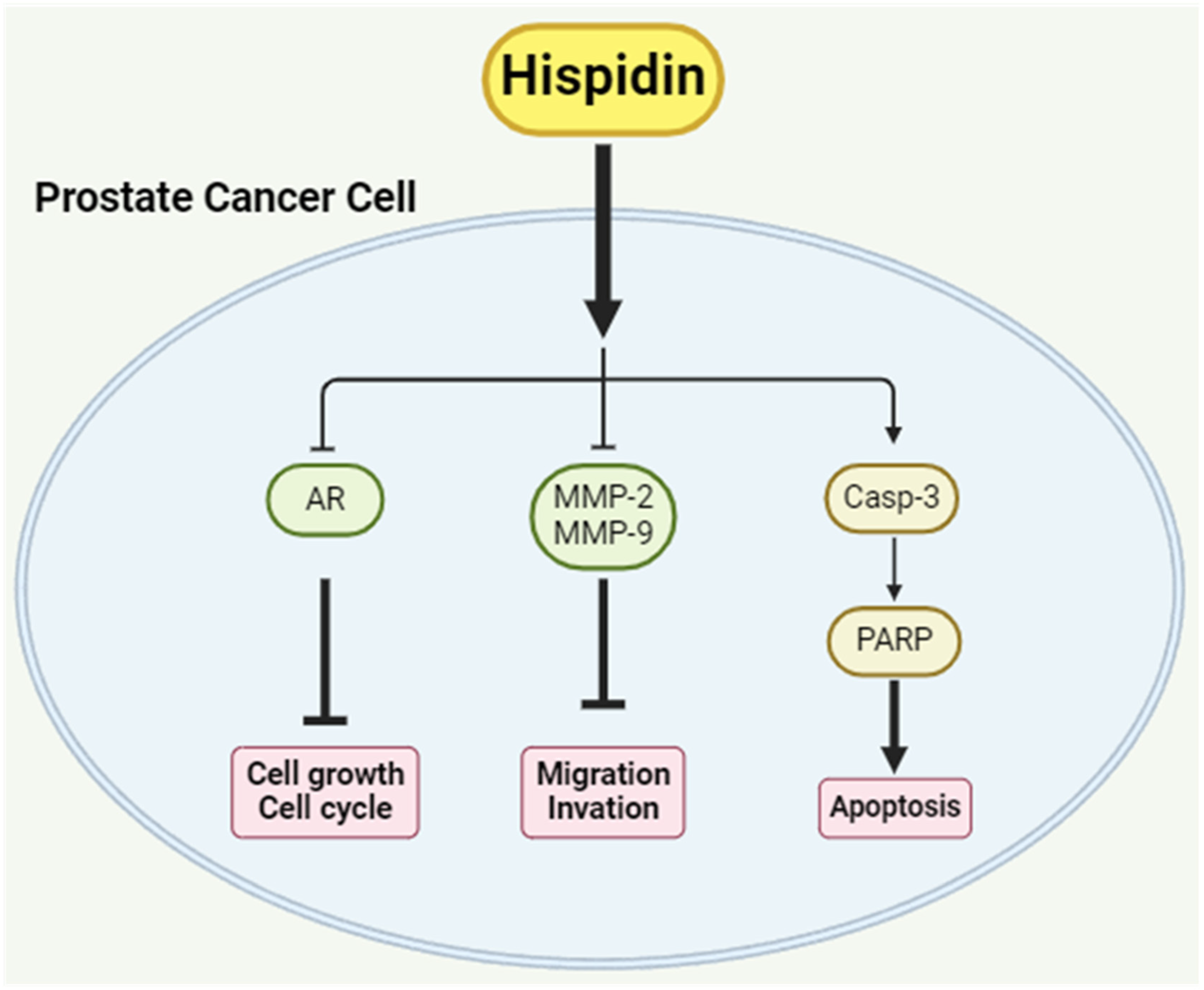
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bracarda, S.; de Cobelli, O.; Greco, C.; Prayer-Galetti, T.; Valdagni, R.; Gatta, G.; de Braud, F.; Bartsch, G. Cancer of the prostate. Crit. Rev. Oncol. Hematol. 2005, 56, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, G.; Kato, T.; Uemura, M.; Nonomura, N. Diagnosing and Prognosing Bone Metastasis in Prostate Cancer: Clinical Utility of Blood Biomarkers. Anticancer Res. 2023, 43, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Aus, G.; Bolla, M.; Joniau, S.; Matveev, V.B.; Schmid, H.P.; Zattoni, F. EAU guidelines on prostate cancer. Eur. Urol. 2008, 53, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Personalised medicine for cancer: From drug development into clinical practice. Expert. Opin. Pharmacother. 2005, 6, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, C.C.; Sheu, S.J.; Huang, I.H.; Chen, C.C. Optimized production and safety evaluation of hispidin-enriched Sanghuangporus sanghuang mycelia. Food Sci. Nutr. 2020, 8, 1864–1873. [Google Scholar] [CrossRef]
- Tian, L.W.; Feng, Y.; Tran, T.D.; Shimizu, Y.; Pfeifer, T.; Vu, H.T.; Quinn, R.J. Achyrodimer F, a tyrosyl-DNA phosphodiesterase I inhibitor from an Australian fungus of the family Cortinariaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4007–4010. [Google Scholar] [CrossRef]
- Yousfi, M.; Djeridane, A.; Bombarda, I.; Duhem, B.; Gaydou, E.M. Isolation and characterization of a new hispolone derivative from antioxidant extracts of Pistacia atlantica. Phytother. Res. 2009, 23, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, R.; Zhang, J.; Bu, Q.; Wang, W.; Liu, Y.; Li, Q.; Guo, Y.; Zhang, L.; Yang, Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019, 9, 16172. [Google Scholar] [CrossRef] [PubMed]
- Wangun, H.V.; Härtl, A.; Tam Kiet, T.; Hertweck, C. Inotilone and related phenylpropanoid polyketides from Inonotus sp. and their identification as potent COX and XO inhibitors. Org. Biomol. Chem. 2006, 4, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Mothana, R.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Jeon, S.Y.; Lee, H.J.; Kim, S.I.; Song, K.S. A beta-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Dyshlovoy, S.A.; von Amsberg, G.; Yurchenko, E.A.; Afiyatullov, S.S. Unique prostate cancer-toxic polyketides from marine sediment-derived fungus Isaria felina. J. Antibiot. 2017, 70, 856–858. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, F.C.; Kuo, C.C.; Chu, H.T.; Li, T.J.; Chen, C.C. Pilot Study: Nutritional and Preclinical Safety Investigation of Fermented Hispidin-Enriched Sanghuangporus sanghuang Mycelia: A Promising Functional Food Material to Improve Sleep. Front. Nutr. 2021, 8, 788965. [Google Scholar] [CrossRef]
- Lv, L.X.; Zhou, Z.X.; Zhou, Z.; Zhang, L.J.; Yan, R.; Zhao, Z.; Yang, L.Y.; Bian, X.Y.; Jiang, H.Y.; Li, Y.D.; et al. Hispidin induces autophagic and necrotic death in SGC-7901 gastric cancer cells through lysosomal membrane permeabilization by inhibiting tubulin polymerization. Oncotarget 2017, 8, 26992–27006. [Google Scholar] [CrossRef]
- Chandimali, N.; Huynh, D.L.; Jin, W.Y.; Kwon, T. Combination Effects of Hispidin and Gemcitabine via Inhibition of Stemness in Pancreatic Cancer Stem Cells. Anticancer Res. 2018, 38, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, Y.M.; Park, S.R.; Kim, D.H.; Lim, B.O. Anticancer activity of hispidin via reactive oxygen species-mediated apoptosis in colon cancer cells. Anticancer Res. 2014, 34, 4087–4093. [Google Scholar] [PubMed]
- Agoulnik, I.U.; Weigel, N.L. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J. Cell Biochem. 2006, 99, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, B.; Dai, R.; Wang, R. The Role of Androgen Receptor in Cross Talk Between Stromal Cells and Prostate Cancer Epithelial Cells. Front. Cell Dev. Biol. 2021, 9, 729498. [Google Scholar] [CrossRef] [PubMed]
- El-Chaer, W.K.; Moraes, C.F.; Nóbrega, O.T. Diagnosis and Prognosis of Prostate Cancer from Circulating Matrix Metalloproteinases and Inhibitors. J. Aging Res. 2018, 2018, 7681039. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.; Li, N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Funct. 2021, 12, 1856–1881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Hao, Y.Y.; Lee, D.H.; Guo, X.Y.; Sun, H.N.; Kwon, T. Hispidin Increases Cell Apoptosis and Ferroptosis in Prostate Cancer Cells Through Phosphatidylinositol-3-Kinase and Mitogen-activated Protein Kinase Signaling Pathway. Anticancer Res. 2024, 44, 2533–2544. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, W.; Zhou, R.; Lv, G.; Sun, M.; Zhao, Y.; Zheng, W. Production of hispidin polyphenols from medicinal mushroom Sanghuangporus vaninii in submerged cultures. Chin. Herb. Med. 2023, 15, 594–602. [Google Scholar] [CrossRef]
- Benarous, K.; Serseg, T.; Mermer, A.; Tahmasebifar, A.; Boulebd, H.; Linani, A. Exploring the Anti-Cancer Potential of Hispidin: A Comprehensive in Silico and in Vitro Study on Human Osteosarcoma Saos2 Cells. Chem. Biodivers. 2024, 21, e202301833. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Samiani, S.; Khanghah, O.J.; Gholipour, E.; Najafi, F.; Zeinalzadeh, E.; Samadi, P.; Sarvarian, P.; Pourvahdani, S.; Kelaye, S.K.; Hamblin, M.R.; et al. Cell cycle involvement in cancer therapy; WEE1 kinase, a potential target as therapeutic strategy. Mutat. Res. 2022, 824, 111776. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G. The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol. Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Formenti, S.C.; Chen-Kiang, S.; Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 2020, 20, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The history of matrix metalloproteinases: Milestones, myths, and misperceptions. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H919–H930. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.; Stearns, M.E. Evidence for increased activated metalloproteinase 2 (MMP-2a) expression associated with human prostate cancer progression. Oncol. Res. 1996, 8, 69–75. [Google Scholar]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, B.B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol. Investig. 2011, 40, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Pego, E.R.; Fernández, I.; Núñez, M.J. Molecular basis of the effect of MMP-9 on the prostate bone metastasis: A review. Urol. Oncol. 2018, 36, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Justulin, L.A., Jr.; Della-Coleta, H.H.; Taboga, S.R.; Felisbino, S.L. Matrix metalloproteinase (MMP)-2 and MMP-9 activity and localization during ventral prostate atrophy and regrowth. Int. J. Androl. 2010, 33, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.F.; Jiang, W.P.; Basavaraj, P.; Huang, S.Y.; Ruangsai, P.; Wu, J.B.; Huang, G.J.; Huang, W.C. Cell suspension culture extract of Eriobotrya japonica attenuates growth and induces apoptosis in prostate cancer cells via targeting SREBP-1/FASN-driven metabolism and AR. Phytomedicine 2021, 93, 153806. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, P.; Ruangsai, P.; Hsieh, P.F.; Jiang, W.P.; Bau, D.T.; Huang, G.J.; Huang, W.C. Alpinumisoflavone Exhibits the Therapeutic Effect on Prostate Cancer Cells by Repressing AR and Co-Targeting FASN- and HMGCR-Mediated Lipid and Cholesterol Biosynthesis. Life 2022, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, P.; Hsieh, P.F.; Jiang, W.P.; Bau, D.T.; Huang, G.J.; Huang, W.C. Elucidation of scandenolone as anti-cancer activity through impairment of the metabolic and signaling vulnerabilities in prostate cancer. Biomed. Pharmacother. 2023, 164, 114948. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Agarwal, A.; Mahfouz, R.Z.; Sharma, R.K.; Sarkar, O.; Mangrola, D.; Mathur, P.P. Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod. Biol. Endocrinol. 2009, 7, 143. [Google Scholar] [CrossRef]
- Fang, C.W.; Yang, J.S.; Chiang, J.H.; Shieh, P.C.; Tsai, F.J.; Tsai, C.W.; Chang, W.S. Metformin induces autophagy of cisplatin-resistant human gastric cancer cells in addition to apoptosis. Biomedicine 2023, 13, 14–23. [Google Scholar] [CrossRef]
- Lin, W.-C.; Deng, J.-S.; Huang, S.-S.; Wu, S.-H.; Lin, H.-Y.; Huang, G.-J. Evaluation of antioxidant, anti-inflammatory and anti-proliferative activities of ethanol extracts from different varieties of Sanghuang species. RSC Adv. 2017, 7, 7780–7788. [Google Scholar] [CrossRef]
- Huang, S.Y.; Huang, G.J.; Hsieh, P.F.; Wu, H.C.; Huang, W.C. Osajin displays potential antiprostate cancer efficacy via impairment of fatty acid synthase and androgen receptor expression. Prostate 2019, 79, 1543–1552. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.-C.; Basavaraj, P.; Tsai, J.-C.; Viehoever, J.; Hsieh, B.-Y.; Li, X.-Y.; Huang, G.-J.; Huang, W.-C. Evaluating the Therapeutic Effect of Hispidin on Prostate Cancer Cells. Int. J. Mol. Sci. 2024, 25, 7857. https://doi.org/10.3390/ijms25147857
Chan K-C, Basavaraj P, Tsai J-C, Viehoever J, Hsieh B-Y, Li X-Y, Huang G-J, Huang W-C. Evaluating the Therapeutic Effect of Hispidin on Prostate Cancer Cells. International Journal of Molecular Sciences. 2024; 25(14):7857. https://doi.org/10.3390/ijms25147857
Chicago/Turabian StyleChan, Kai-Cheng, Praveenkumar Basavaraj, Jui-Chen Tsai, Jonathan Viehoever, Bing-Yan Hsieh, Xin-Yu Li, Guan-Jhong Huang, and Wen-Chin Huang. 2024. "Evaluating the Therapeutic Effect of Hispidin on Prostate Cancer Cells" International Journal of Molecular Sciences 25, no. 14: 7857. https://doi.org/10.3390/ijms25147857





