Labeling of Bruton’s Tyrosine Kinase (BTK) Inhibitor [11C]BIO-2008846 in Three Different Positions and Measurement in NHP Using PET
Abstract
:1. Introduction
2. Results and Discussions
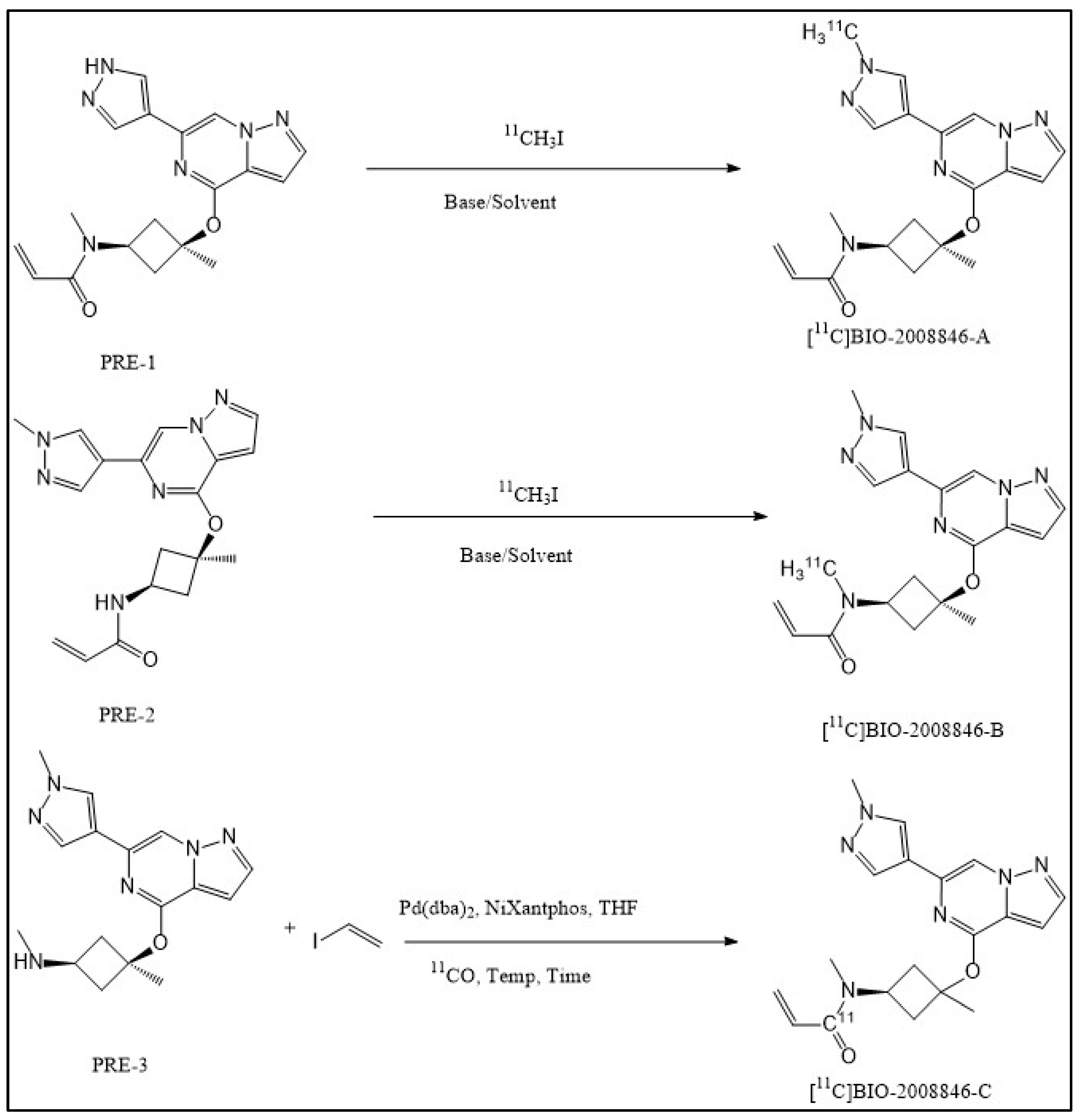
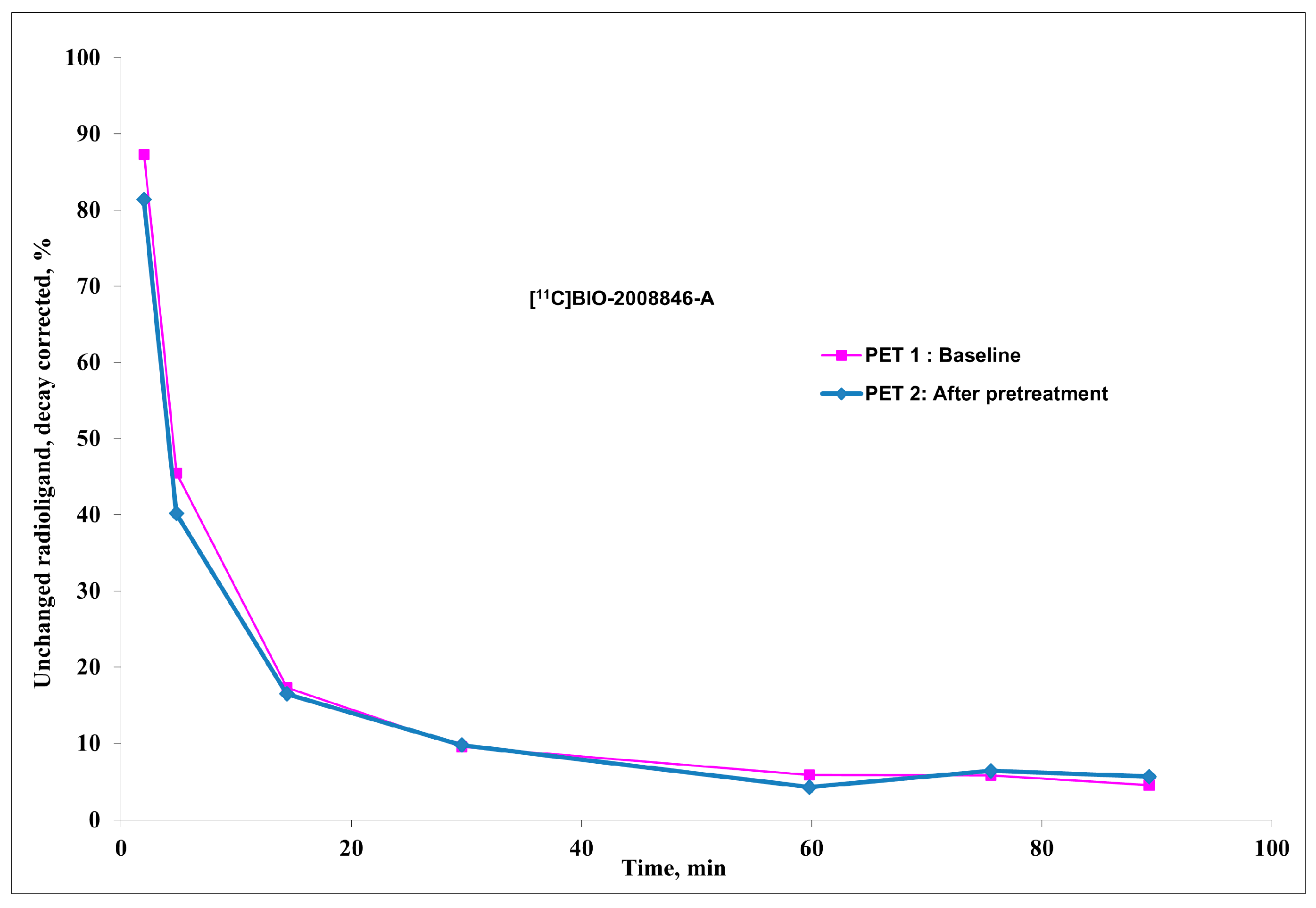
Radiochemistry
3. Materials and Methods
3.1. Radiochemistry
General
3.2. Synthesis of 11C-Methyliodide (11C-CH3I)
3.3. Synthesis of N-methyl-N-((1s,3s)-3-methyl-3-((6-(1-[11C]methyl-1H-pyrazol-4-yl)pyrazolo [1,5-a]pyrazin-4-yl)oxy)cyclobutyl) acrylamide ([11C]BIO-2008846-A) and N-[11C]methyl-N-((1s,3s)-3-methyl-3-((6-(1-methyl-1H-pyrazol-4-yl)pyrazolo [1,5-a]pyrazin-4-yl)oxy)cyclobutyl) acrylamide ([11C]BIO-2008846-B)
3.4. Synthesis of [11C]Carbon Monoxide ([11C]CO)
3.5. Synthesis of N-methyl-N-((1s,3s)-3-Methyl-3-((6-(1-Methyl-1H-Myrazol-4-yl)Pyrazolo [1,5-a]Pyrazin-4-yl)oxy)cyclobutyl)([11C]CO)acrylamide ([11C]BIO-2008846-C)
3.6. Study Design in Non-Human Primates (NHP), PET Procedure and Quantification
3.7. Radiometabolite Analysis and Protein Binding in Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woyach, J.A.; Bojnik, E.; Ruppert, A.S.; Stefanovski, M.R.; Goettl, V.M.; Smucker, K.A.; Smith, L.L.; Dubovsky, J.A.; Towns, W.H.; MacMurray, J.; et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood 2014, 123, 1207–1213. [Google Scholar] [CrossRef]
- Woyach, J.A.; Johnson, A.J. Targeted therapies in CLL: Mechanisms of resistance and strategies for management. Blood 2015, 126, 471–477. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Shi, Y.; Qian, H.; Liu, P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia 2021, 35, 312–332. [Google Scholar] [CrossRef]
- Ran, F.; Liu, Y.; Wang, C.; Xu, Z.; Zhang, Y.; Liu, Y.; Zhao, G.; Ling, Y. Review of the development of BTK inhibitors in overcoming the clinical limitations of ibrutinib. Eur. J. Med. Chem. 2022, 229, 114009. [Google Scholar] [CrossRef]
- Herman, S.E.; Gordon, A.L.; Hertlein, E.; Ramanunni, A.; Zhang, X.; Jaglowski, S.; Flynn, J.; Jones, J.; Blum, K.A.; Buggy, J.J.; et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011, 117, 6287–6296. [Google Scholar] [CrossRef]
- Brullo, C.; Villa, C.; Tasso, B.; Russo, E.; Spallarossa, A. Btk inhibitors: A medicinal chemistry and drug delivery perspective. Int. J. Mol. Sci. 2021, 22, 7641. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wang, D.; Zhang, J.; Zhang, T. Emerging small-molecule inhibitors of the Bruton’s tyrosine kinase (BTK): Current development. Eur. J. Med. Chem. 2021, 217, 113329. [Google Scholar] [CrossRef]
- Kinnunen, T.; Chamberlain, N.; Morbach, H.; Cantaert, T.; Lynch, M.; Preston-Hurlburt, P.; Herold, K.C.; Hafler, D.A.; O’Connor, K.C.; Meffre, E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Investig. 2013, 123, 2737–2741. [Google Scholar] [CrossRef]
- Aalipour, A.; Advani, R.H. Bruton tyrosine kinase inhibitors: A promising novel targeted treatment for B cell lymphomas. Br. J. Haematol. 2013, 163, 436–443. [Google Scholar] [CrossRef]
- Bernard-Gauthier, V.; Bailey, J.J.; Berke, S.; Schirrmacher, R. Recent advances in the development and application of radiolabeled kinase inhibitors for PET imaging. Molecules 2015, 20, 22000–22027. [Google Scholar] [CrossRef]
- Mayer, A.T.; Gambhir, S.S. The Immunoimaging Toolbox. J. Nucl. Med. 2018, 59, 1174–1182. [Google Scholar] [CrossRef]
- Sun, C.; Nierman, P.; Kendall, E.K.; Cheung, J.; Gulrajani, M.; Herman, S.E.; Pleyer, C.; Ahn, I.E.; Stetler-Stevenson, M.; Yuan, C.M. Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood J. Am. Soc. Hematol. 2020, 136, 93–105. [Google Scholar] [CrossRef]
- Zheng, N.; Catlett, I.M.; Taylor, K.; Gu, H.; Pattoli, M.A.; Neely, R.J.; Li, W.; Allentoff, A.; Yuan, X.; Ciccimaro, E. Determination of real time in vivo drug receptor occupancy for a covalent binding drug as a clinical pharmacodynamic biomarker by immunocapture-LC-MS/MS. Anal. Chem. 2019, 91, 8443–8452. [Google Scholar] [CrossRef]
- Dahl, K.; Turner, T.; Vasdev, N. Radiosynthesis of a Bruton’s tyrosine kinase inhibitor, [(11) C]Tolebrutinib, via palladium-NiXantphos-mediated carbonylation. J. Label. Comp. Radiopharm. 2020, 63, 482–487. [Google Scholar] [CrossRef]
- Lindberg, A.; Boyle, A.J.; Tong, J.; Harkness, M.B.; Garcia, A.; Tran, T.; Zhai, D.; Liu, F.; Donnelly, D.J.; Vasdev, N. Radiosynthesis of [11C] ibrutinib via Pd-mediated [11C] CO carbonylation: Preliminary PET imaging in experimental autoimmune encephalomyelitis mice. Front. Nucl. Med. 2021, 1, 772289. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Preshlock, S.; Kaur, T.; Tran, T.; Wilson, T.C.; Mhanna, K.; Henderson, B.D.; Batalla, D.; Scott, P.J.; Shao, X. Synthesis of radiopharmaceuticals via “in-loop” 11C-carbonylation as exemplified by the radiolabeling of inhibitors of Bruton’s tyrosine kinase. Front. Nucl. Med. 2022, 1, 820235. [Google Scholar] [CrossRef]
- Skaddan, M.B.; Wooten, D.W.; Wilcox, K.C.; Voorbach, M.J.; Reuter, D.R.; Jia, Z.J.; Foster-Duke, K.D.; Hickson, J.A.; Vaidyanathan, S.; Reed, A.D. [18F] BTK-1: A Novel Positron Emission Tomography Tracer for Imaging Bruton’s Tyrosine Kinase. Mol. Imaging Biol. 2022, 24, 830–841. [Google Scholar] [CrossRef]
- Skaddan, M.; Wilcox, K.; Reuter, D.; Jia, Z.; Foster-Duke, K.; Hickson, J.; Wooten, D.; Reed, A.; Tovcimak, A.; Guo, Q. Development of [18F] ABBV-BTK1: A novel BTK PET tracer. J. Nucl. Med. 2019, 60 (Suppl. 1), 1025. [Google Scholar]
- Li, K.; Wang, M.; Akoglu, M.; Pollard, A.C.; Klecker, J.B.; Alfonso, P.; Corrionero, A.; Prendiville, N.; Qu, W.; Parker, M.F.L.; et al. Synthesis and Preclinical Evaluation of a Novel Fluorine-18-Labeled Tracer for Positron Emission Tomography Imaging of Bruton’s Tyrosine Kinase. ACS Pharmacol. Transl. Sci. 2023, 6, 410–421. [Google Scholar] [CrossRef]
- Himmelbauer, M.K.; Bajrami, B.; Basile, R.; Capacci, A.; Chen, T.Y.; Choi, C.K.; Gilfillan, R.; de Turiso, F.G.L.; Gu, C.G.; Hoemberger, M.; et al. Discovery and Preclinical Characterization of BIIB129, a Covalent, Selective, and Brain-Penetrant BTK Inhibitor for the Treatment of Multiple Sclerosis. J. Med. Chem. 2024, 67, 8122–8140. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Bolin, M.; Datta, P.; Arakawa, R.; Forsberg Moren, A.; Khani Maynaq, Y.; Lin, E.; Genung, N.; Hering, H.; Guckian, K.; et al. Development of a Novel [(11)C]CO-Labeled Positron Emission Tomography Radioligand [(11)C]BIO-1819578 for the Detection of O-GlcNAcase Enzyme Activity. Acs Chem. Neurosci. 2023, 14, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Ichise, M.; Toyama, H.; Innis, R.B.; Carson, R.E. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J. Cereb. Blood Flow. Metab. 2002, 22, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Nakao, R.; Amini, N.; Abdel-Rehim, M.; Schou, M.; Halldin, C. Sample preparation techniques for radiometabolite analysis of positron emission tomography radioligands; trends, progress, limitations and future prospects. Trac-Trend Anal. Chem. 2019, 110, 1–7. [Google Scholar] [CrossRef]
- Elkjaer, M.L.; Waede, M.R.; Kingo, C.; Damsbo, K.; Illes, Z. Expression of Bruton s tyrosine kinase in different type of brain lesions of multiple sclerosis patients and during experimental demyelination. Front. Immunol. 2023, 14, 1264128. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Truong, P.; Halldin, C. In-target produced [11C]methane: Increased specific radioactivity. Appl. Radiat. Isot. 2009, 67, 106–110. [Google Scholar] [CrossRef]
- Varrone, A.; Sjoholm, N.; Eriksson, L.; Gulyas, B.; Halldin, C.; Farde, L. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur. J. Nucl. Med. Mol. I 2009, 36, 1639–1650. [Google Scholar] [CrossRef]
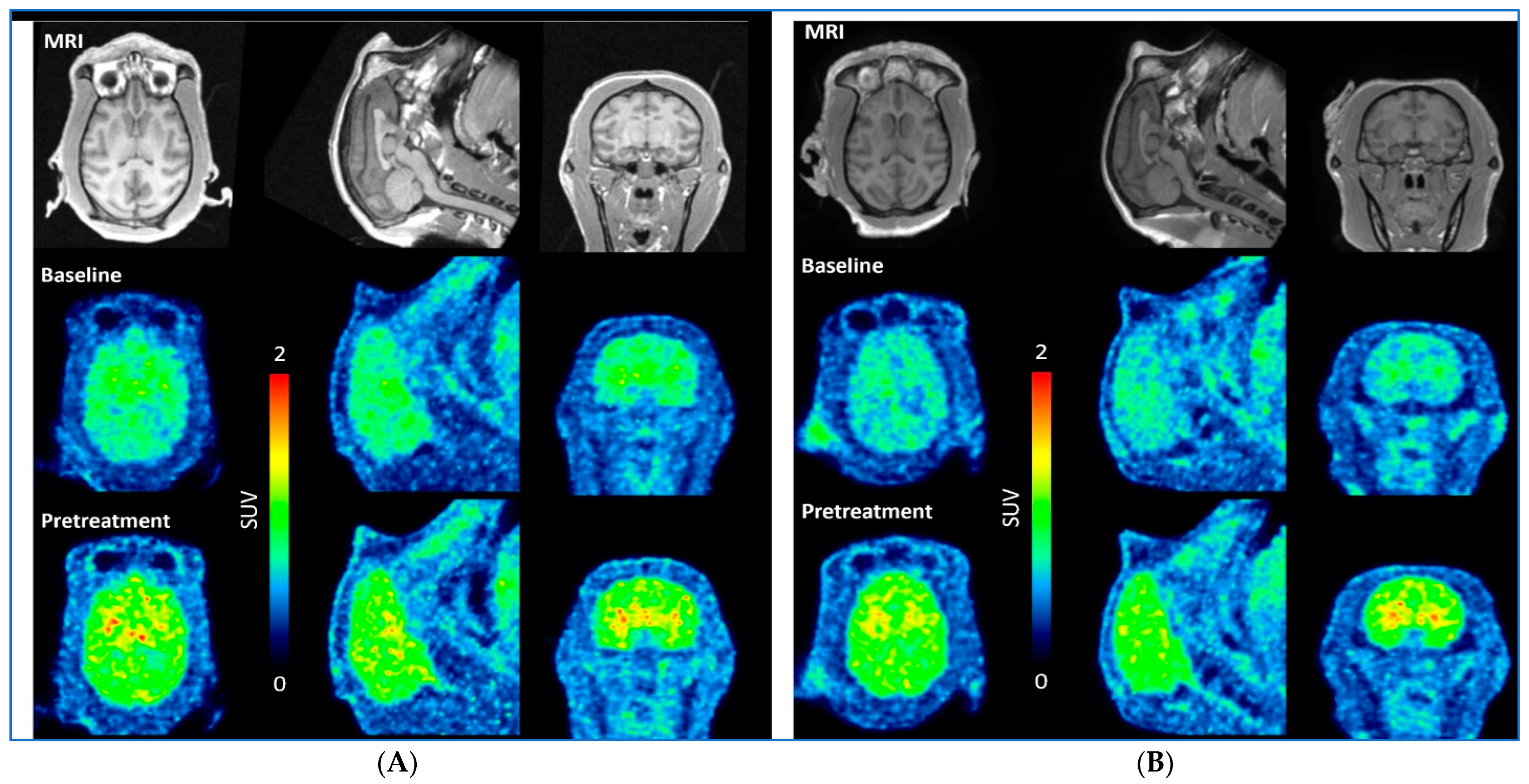
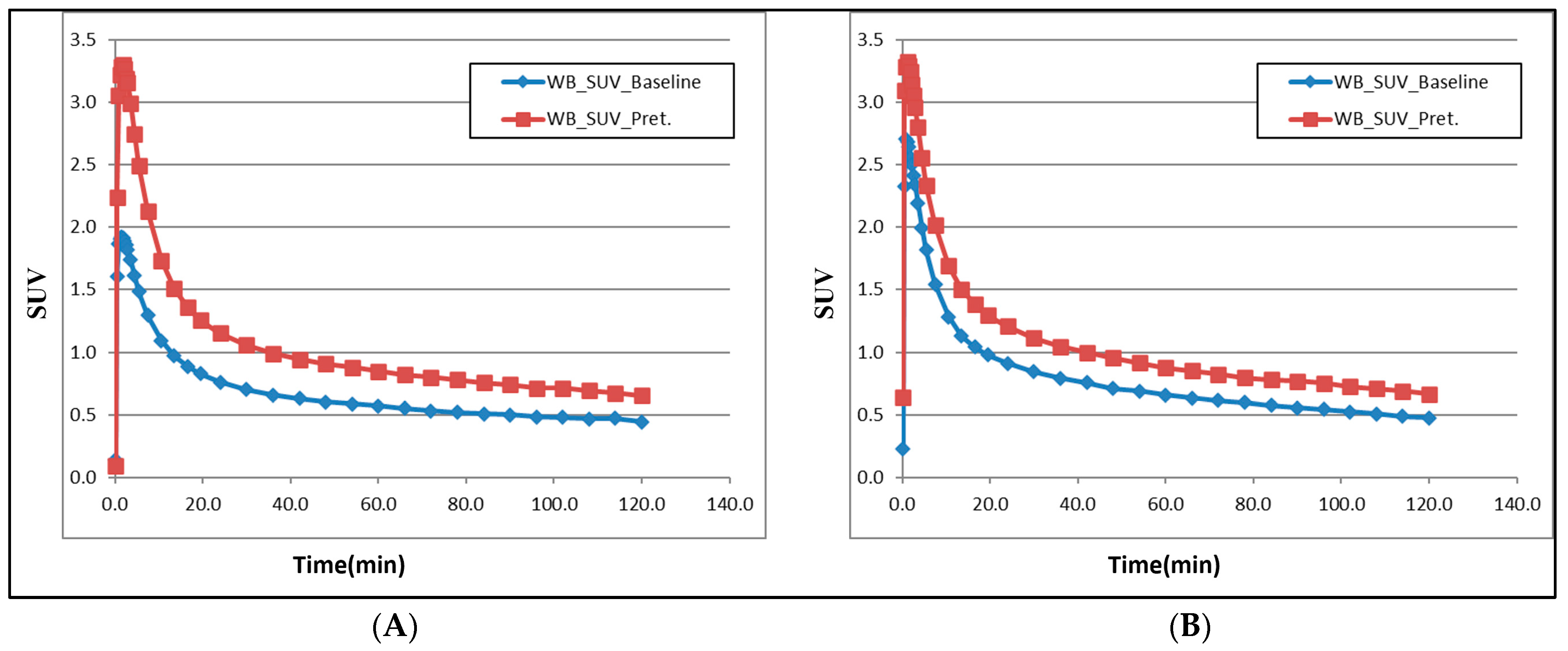
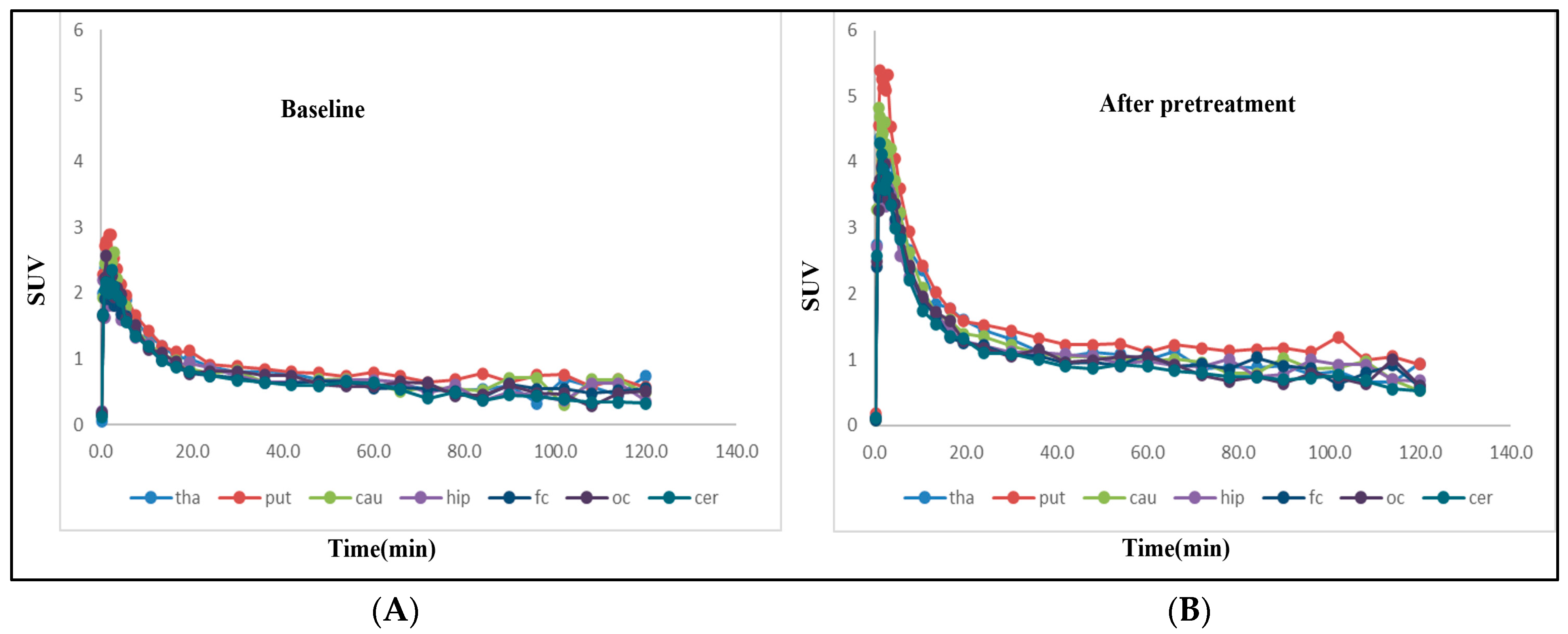

| Radioligand | Weight (kg) | Injected Radioactivity (MBq) | Molar Activity (GBq/µmol) | Injected Mass (µg) | Condition | Dose mg/kg |
|---|---|---|---|---|---|---|
| [11C]BIO-2008846-A | 7.1 | 157 | 558 | 0.1 | Baseline | - |
| 160 | 452 | 0.13 | Pretreat | 1 | ||
| [11C]BIO-2008846-A | 8.4 | 160 | 359 | 0.17 | Baseline | - |
| 118 | 518 | 0.08 | Pretreat | 1 |
| NHP1 | NHP2 | ||||
|---|---|---|---|---|---|
| Region of Interest | VT at Baseline | VT after Pretreatment | Region of Interest | VT at Baseline | VT after Pretreatment |
| PUT | 7.2 | 12.5 | PUT | 7.7 | 11.9 |
| CAU | 7.1 | 6.7 | CAU | 8.4 | 8.7 |
| FC | 5.5 | 7.2 | FC | 5.6 | 7.0 |
| OCC | 3.8 | 5.9 | OCC | 5.2 | 8.3 |
| HIP | 4.0 | 7.1 | HIP | 5.7 | 8.4 |
| CER | 3.0 | 5.2 | CER | 3.8 | 6.2 |
| THA | 5.9 | 6.5 | THA | 10.8 | 11.6 |
| WB | 4.3 | 5.8 | WB | 5.8 | 8.0 |
| PET Experiments | Membrane Binding (Non-Specific): | Protein Binding (%) | Free Fraction (%) |
|---|---|---|---|
| Baseline (PET1) NHP1 | 0.63 | 79.68 | 20.32 |
| After Pretreatment (PET2) NHP1 | 0.47 | 66.16 | 33.84 |
| Baseline (PET1) NHP2 | 0.71 | 69.20 | 30.80 |
| After Pretreatment (PET2) NHP2 | 0.55 | 62.28 | 37.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, S.; Datta, P.; Morén, A.F.; Khani, Y.; Martarello, L.; Kaliszczak, M.; Halldin, C. Labeling of Bruton’s Tyrosine Kinase (BTK) Inhibitor [11C]BIO-2008846 in Three Different Positions and Measurement in NHP Using PET. Int. J. Mol. Sci. 2024, 25, 7870. https://doi.org/10.3390/ijms25147870
Nag S, Datta P, Morén AF, Khani Y, Martarello L, Kaliszczak M, Halldin C. Labeling of Bruton’s Tyrosine Kinase (BTK) Inhibitor [11C]BIO-2008846 in Three Different Positions and Measurement in NHP Using PET. International Journal of Molecular Sciences. 2024; 25(14):7870. https://doi.org/10.3390/ijms25147870
Chicago/Turabian StyleNag, Sangram, Prodip Datta, Anton Forsberg Morén, Yasir Khani, Laurent Martarello, Maciej Kaliszczak, and Christer Halldin. 2024. "Labeling of Bruton’s Tyrosine Kinase (BTK) Inhibitor [11C]BIO-2008846 in Three Different Positions and Measurement in NHP Using PET" International Journal of Molecular Sciences 25, no. 14: 7870. https://doi.org/10.3390/ijms25147870







