Disruption of Cell Membranes and Redox Homeostasis as an Antibacterial Mechanism of Dielectric Barrier Discharge Plasma against Fusarium oxysporum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bactericidal Effect of DBD on F. oxysporum
2.2. Morphological Changes Induced in Fungal Spores by DBD
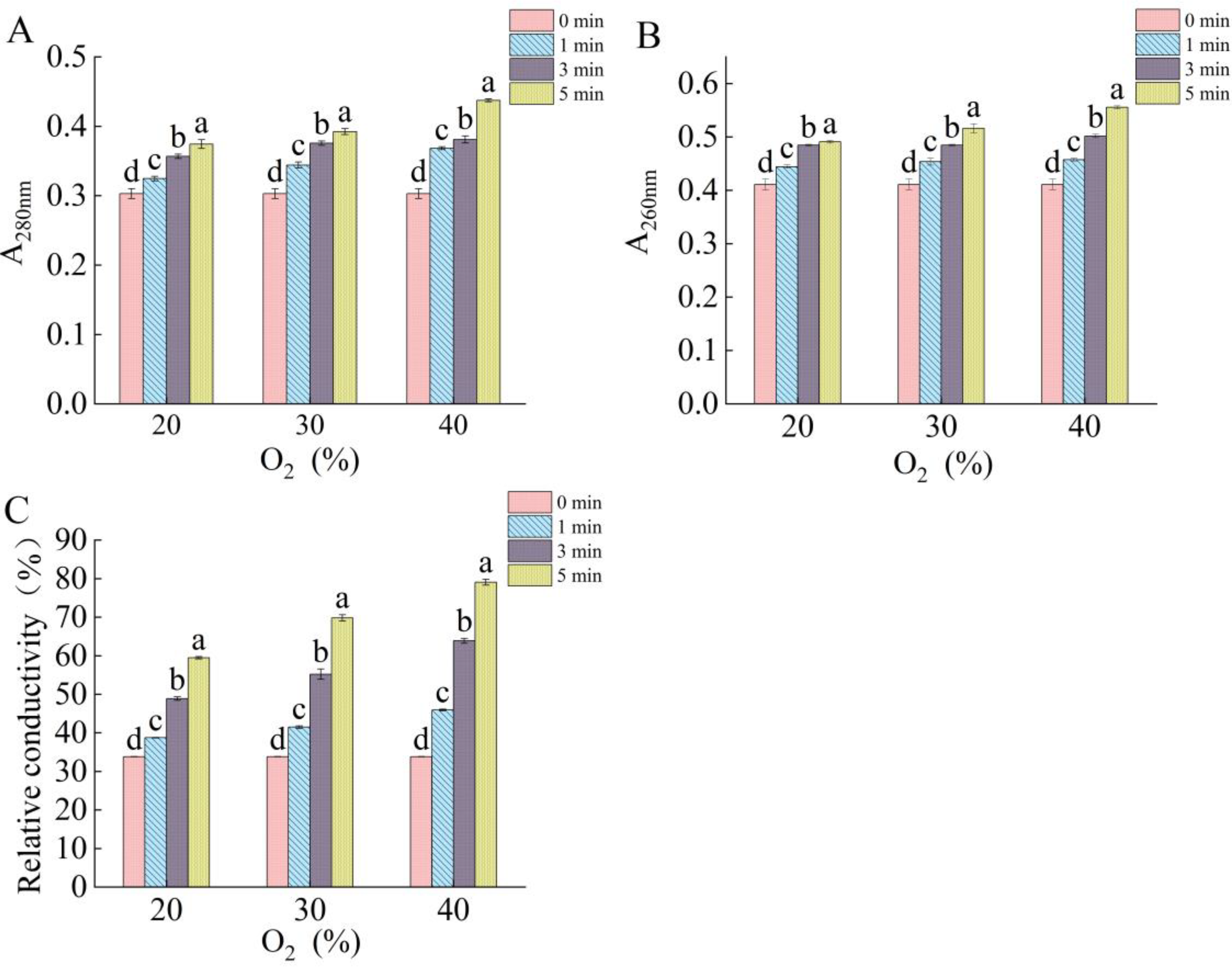
2.3. Effect of DBD Plasma on Membrane Integrity of Fungal Spores
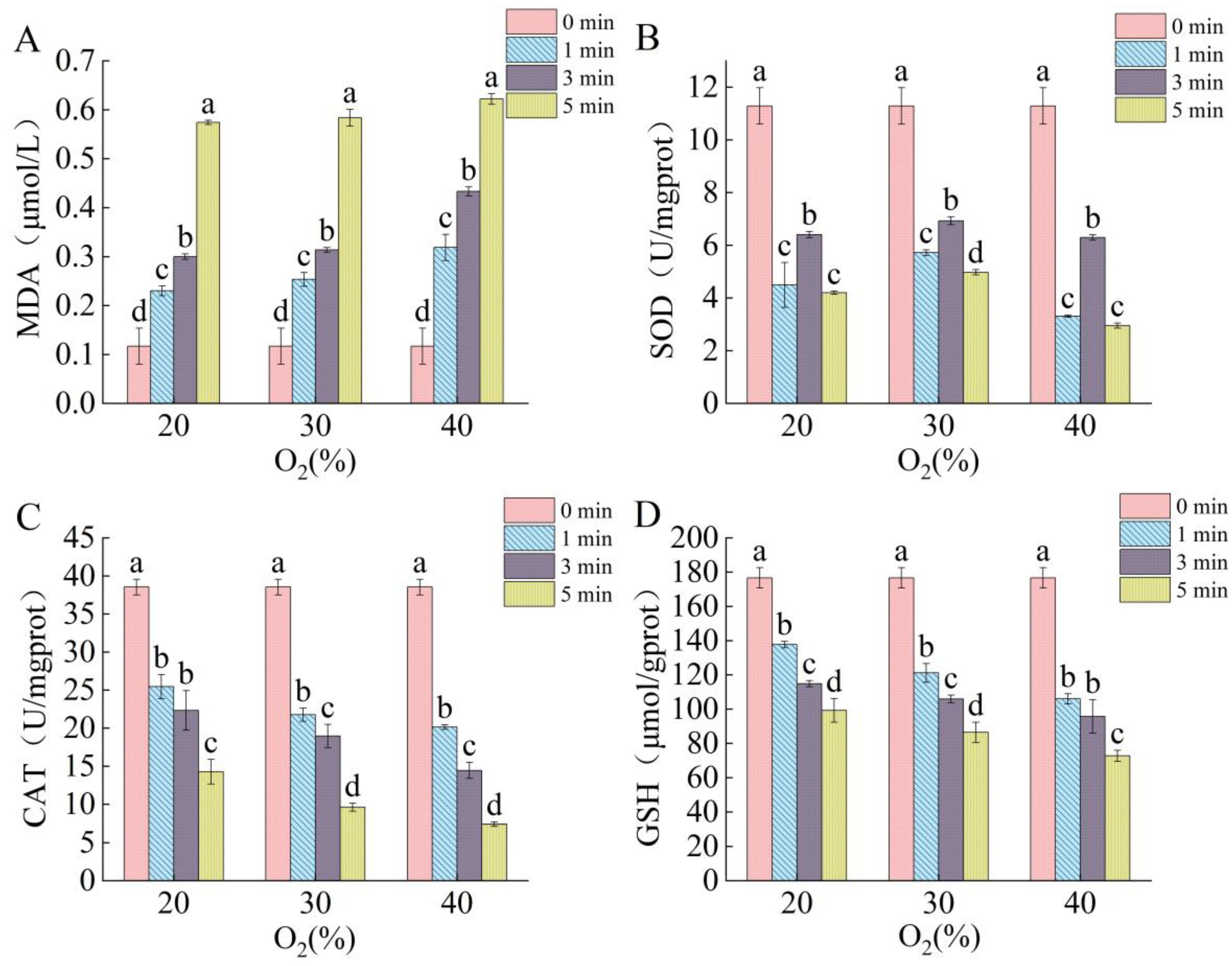
2.4. Effects of DBD Plasma on Intraspore ROS Levels
2.5. Effects of DBD Plasma on Contents of Redox System-Related Components
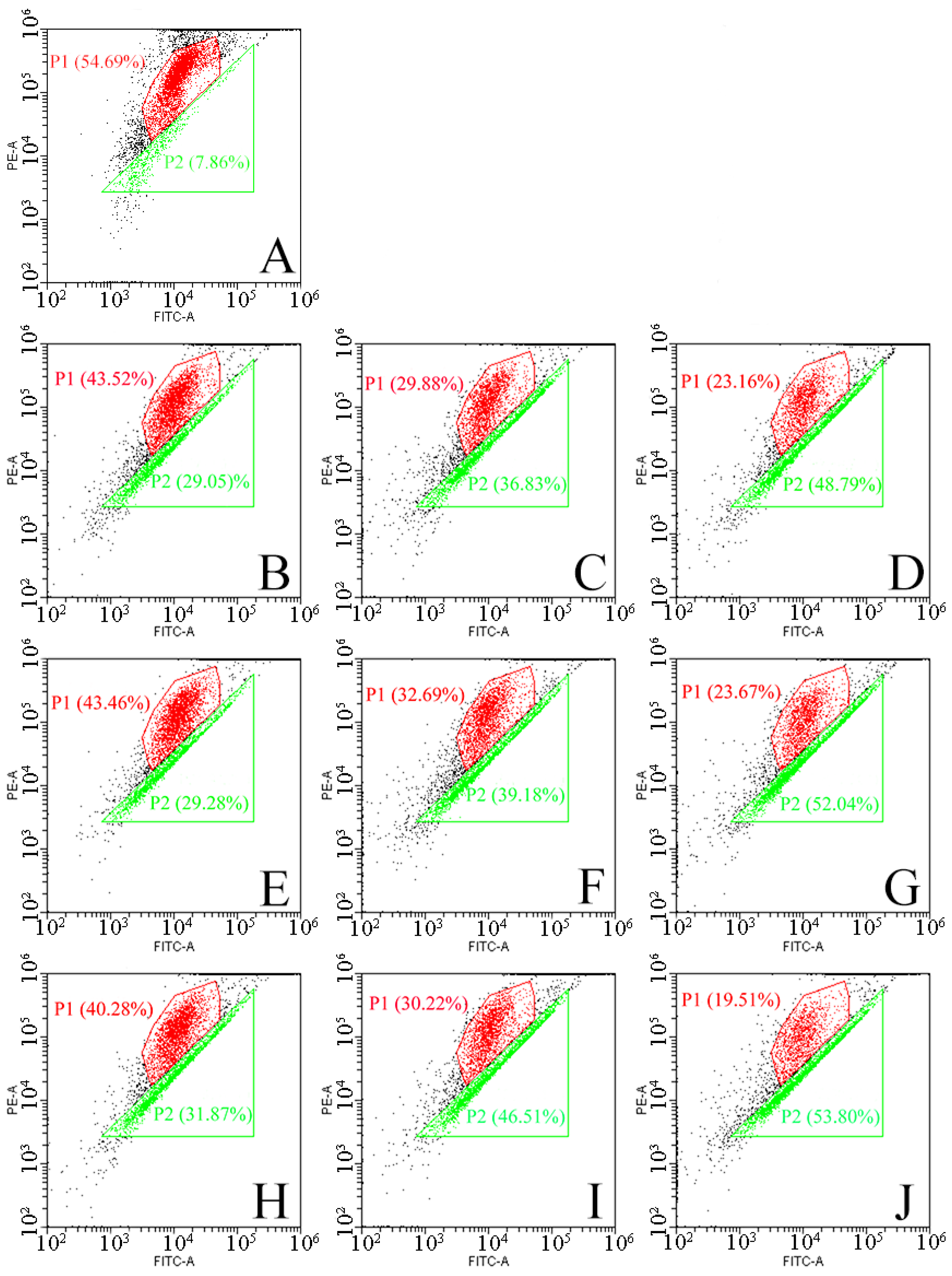
2.6. Effect of DBD Plasma on Mitochondrial Membrane Potential
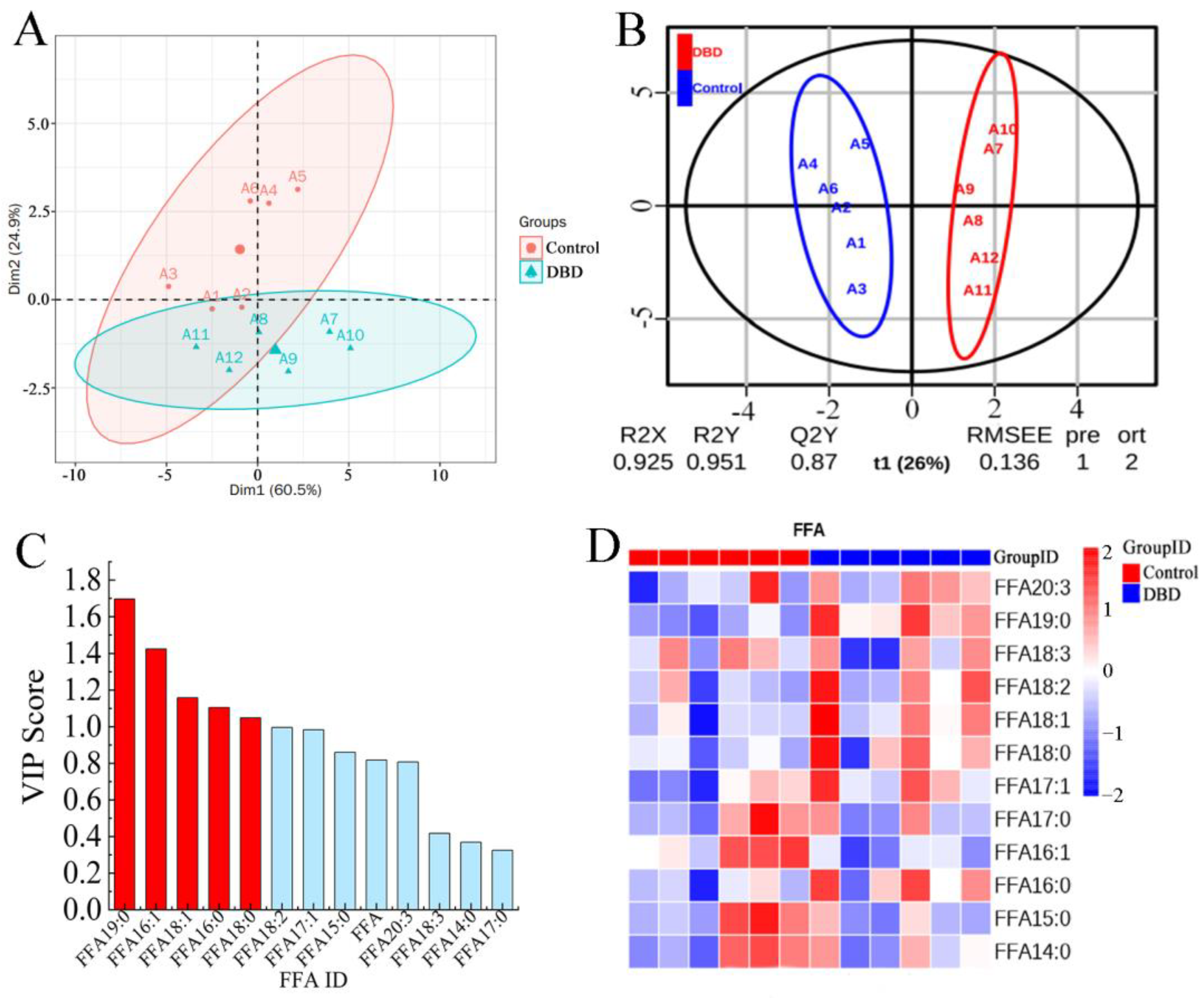
2.7. Effect of DBD Plasma Treatment on Free Fatty Acids
3. Materials and Methods
3.1. Reagents
3.2. Preparation of Spore Suspensions
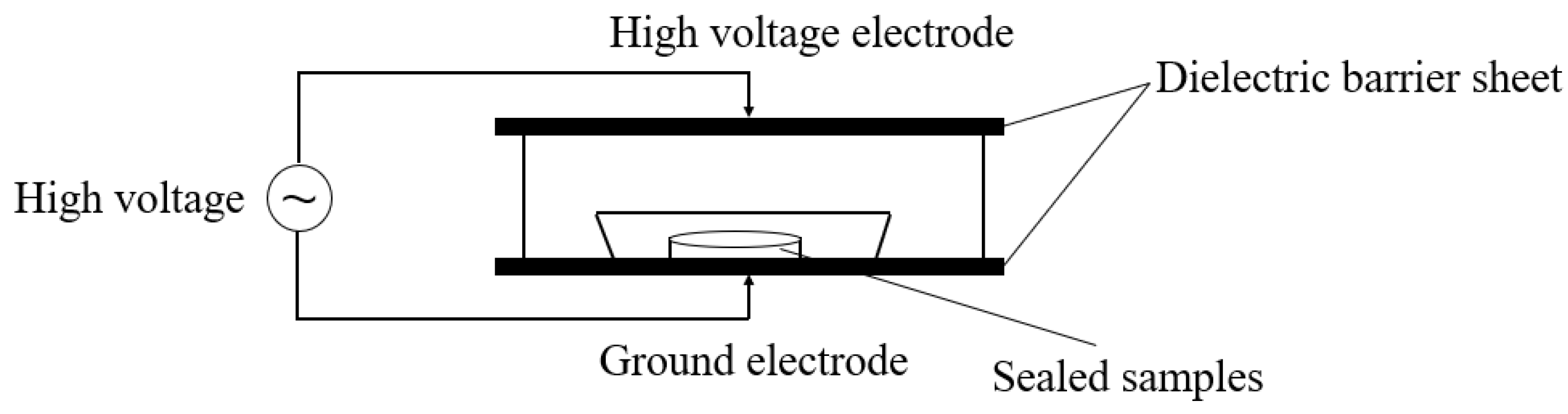
3.3. DBD Plasma Equipment and Treatment Conditions
3.4. Bactericidal Effect of DBD Plasma on Fusarium Acuminatum
3.5. Field Emission Transmission Electron Microscope (FETEM)
3.6. Effects of DBD Plasma on Cell Membrane Integrity
3.7. Determination of ROS Level
3.8. Lipid Peroxidation Assay
3.9. Determination of SOD and CAT Activity and GSH Content
3.10. Detection of Mitochondrial Membrane Potential
3.11. Metabolism of Free Fatty Acids
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corbo, M.R.; Bevilacqua, A.; Campaniello, D.; Ciccarone, C.; Sinigaglia, M. Use of high pressure homogenization as a mean to control the growth of foodborne moulds in tomato juice. Food Control 2010, 21, 1507–1511. [Google Scholar] [CrossRef]
- Gilardi, G.; Pintore, I.; Gullino, M.L.; Garibaldi, A. Occurence of Fusarium Equiseti as a contaminant of Diplotaxis tenuifolia seeds. J. Plant Pathol. 2017, 99, 245–248. [Google Scholar]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Ren, J.; Tong, J.; Li, P.; Huang, X.; Dong, P.; Ren, M. Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant Pathol. 2021, 113, 101601. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. J. Adv. Res. 2022, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Jiang, C.; Schaudinn, C.; Jaramillo, D.E.; Webster, P.; Costerton, J.W. In vitro antimicrobial effect of a cold plasma jet against Enterococcus faecalis biofilms. ISRN Dent. 2012, 2012, 295736. [Google Scholar] [PubMed]
- Zhao, L.; Wang, J.; Sheng, X.; Li, S.; Yan, W.; Qian, J.; Zhang, J.; Raghavan, V. Non-thermal plasma inhibited the growth and aflatoxins production of Aspergillus flavus, degraded aflatoxin B1 and its potential mechanisms. Chem. Eng. J. 2023, 475, 146017. [Google Scholar] [CrossRef]
- Xu, H.; Fang, C.; Shao, C.; Li, L.; Huang, Q. Study of the synergistic effect of singlet oxygen with other plasma-generated ROS in fungi inactivation during water disinfection. Sci. Total Environ. 2022, 838, 156576. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Yun, G. The sterilization of Escherichia coli by dielectric-barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 7065–7070. [Google Scholar] [CrossRef]
- Li, Y.; Yi, C.; Li, J.; Yi, R.; Wang, H. Experimental research on the sterilization of Escherichia Coli and Bacillus Subtilis in drinking water by dielectric barrier discharge. Plasma Sci. Technol. 2016, 18, 173–178. [Google Scholar] [CrossRef]
- Hong, Y.F.; Kang, J.G.; Lee, H.Y.; Uhm, H.S.; Moon, E.; Park, Y.H. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett. Appl. Microbiol. 2009, 48, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yun, H.; Jung, S.; Jung, Y.; Jung, H.; Choe, W.; Jo, C. Effect of atmospheric pressure plasma on inactivation of pathogens inoculated onto bacon using two different gas compositions. Food Microbiol. 2011, 28, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef]
- Jun, A.H.; Il, K.K.; Geunyoung, K.; Eunpyo, M.; Sik, Y.S.; Jong-Soo, L.; Boris, Z. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Lin, C.-M.; Patel, A.K.; Chiu, Y.-C.; Hou, C.-Y.; Kuo, C.-H.; Dong, C.-D.; Chen, H.-L. The application of novel rotary plasma jets to inhibit the aflatoxin-producing Aspergillus flavus and the spoilage fungus, Aspergillus niger on peanuts. Innov. Food Sci. Emerg. Technol. 2022, 78, 102994. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Aspergillus decontamination in hazelnuts: Evaluation of atmospheric and low-pressure plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 54, 235–242. [Google Scholar] [CrossRef]
- Fernandez, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int. J. Antimicrob. Agents 2014, 43, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative dna damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Churpita, O.; Zablotskii, V.; Deyneka, I.G.; Meshkovskii, I.K.; Jaeger, A.; Sykova, E.; Kubinova, S.; Dejneka, A. Non-thermal plasma mills bacteria: Scanning electron microscopy observations. Appl. Phys. Lett. 2015, 106, 053703. [Google Scholar] [CrossRef]
- Lia, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the induction of nitric oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, L.; Jia, L.; Zou, B.; Dai, R.; Li, X.; Jia, F. Inactivation effects, kinetics and mechanisms of air- and nitrogen-based cold atmospheric plasma on Pseudomonas aeruginosa. Innov. Food Sci. Emerg. Technol. 2022, 79, 103051. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Du, M.; Wang, Y.; Ju, S.; Ma, R.; Jiao, Z. Subcellular mechanism of microbial inactivation during water disinfection by cold atmospheric-pressure plasma. Water Res. 2021, 188, 116513. [Google Scholar] [CrossRef] [PubMed]
- Šimončicová, J.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Lakatoš, B.; Kryštofová, S.; Hoppanová, L.; Palušková, V.; Hudecová, D.; Ďurina, P.; et al. Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2018, 102, 6647–6658. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhu, B.; Xue, X.; Hu, S.; Cheng, C. Study on immediate and long-term growth inhibition of Microcystis aeruginosa by non-thermal plasma. Chem. Eng. J. 2022, 429, 132397. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Tian, L.; Zhang, Q.; Guan, Y.; Chen, L.; Liu, G.; Yu, H.Q.; Tian, Y.; Huang, Q. Raman micro-spectroscopy monitoring of cytochrome c redox state in Candida utilis during cell death under low-temperature plasma-induced oxidative stress. Analyst 2020, 145, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zuo, X.; Guo, F.; Li, Y.; Xie, Y. Paeonol inhibits Aspergillus flavus via disrupting ergosterol biosynthesis, redox metabolism, and aflatoxin biosynthesis on rice. LWT-Food Sci. Technol. 2022, 163, 113587. [Google Scholar] [CrossRef]
- Lv, X.; Cheng, J.-H. Evaluation of the effects of cold plasma on cell membrane lipids and oxidative injury of Salmonella typhimurium. Molecules 2022, 27, 640. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qu, G.; Gan, Y.; Zhang, Z.; Li, R.; Wang, T. Elimination of Microcystis aeruginosa in water via dielectric barrier discharge plasma: Efficacy, mechanism and toxin release. J. Hazard. Mater. 2022, 422, 126956. [Google Scholar] [CrossRef]
- Long, H.; Pu, L.; Xu, W.; Nan, M.; Oyom, W.; Prusky, D.; Bi, Y.; Xue, H. Inactivation of Penicillium expansum spores in apple juice by contact glow discharge electrolysis and its related mechanism. Innov. Food Sci. Emerg. Technol. 2022, 80, 103100. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Malina, C.; Larsson, C.; Nielsen, J. Yeast mitochondria: An overview of mitochondrial biology and the potential of mitochondrial systems biology. FEMS Yeast Res. 2018, 18, foy040. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Yoon, A.M.; Kim, Y.-M.; Lee, M.-Y.; Lee, J.R. Antifungal action of Arabidopsis thaliana TCP21 via induction of oxidative stress and apoptosis. Antioxidants 2023, 12, 1767. [Google Scholar] [CrossRef]
- Gottlieb, R.A. Role of mitochondria in apoptosis. Exp. Physiol. 2000, 88, 85–90. [Google Scholar]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Montie, T.C.; Kelly-Wintenberg, K.; Roth, J.R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Yi, F.; Wang, J.; Xiang, Y.; Yun, Z.; Pan, Y.; Jiang, Y.; Zhang, Z. Physiological and quality changes in fresh-cut mango fruit as influenced by cold plasma. Postharvest Biol. Technol. 2022, 194, 112105. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: Inoculation and accelerated shelf-life studies. Food Control 2019, 106, 106678. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yu, S.; Jiang, X.; Wu, F.; Zhou, J.; Mao, D.; Wang, H.; Tao, Y.; Liu, Y.; Jin, F. Celastrus orbiculatus Thunb. extract targeting DJ-1 inhibits non-small cell lung cancer invasion and metastasis through mitochondrial-induced ROS accumulation. J. Ethnopharmacol. 2024, 318, 116944. [Google Scholar] [CrossRef] [PubMed]
- Kissova, I.; Deffieu, M.; Samokhvalov, V.; Velours, G.; Bessoule, J.-J.; Manon, S.; Camougrand, N. Lipid oxidation and autophagy in yeast. Free Radic. Biol. Med. 2006, 41, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Li, J.; Sun, H.; Mao, W.; Lu, Z.; Zhao, Q.; Han, C.; Gong, X.; Jiang, B.; Chua, G.H.; et al. Quantitative lipidomics and spatial ms-imaging uncovered neurological and systemic lipid metabolic pathways underlying troglomorphic adaptations in cave-dwelling fish. Mol. Biol. Evol. 2022, 39, msac050. [Google Scholar] [CrossRef]
- Lam, S.M.; Zhang, C.; Wang, Z.; Ni, Z.; Zhang, S.; Yang, S.; Huang, X.; Mo, L.; Li, J.; Lee, B.; et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 2021, 3, 909–922. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Sun, J.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Pei, J.; He, R.; Chen, W. Disruption of Cell Membranes and Redox Homeostasis as an Antibacterial Mechanism of Dielectric Barrier Discharge Plasma against Fusarium oxysporum. Int. J. Mol. Sci. 2024, 25, 7875. https://doi.org/10.3390/ijms25147875
Yu S, Sun J, Chen H, Chen W, Zhong Q, Zhang M, Pei J, He R, Chen W. Disruption of Cell Membranes and Redox Homeostasis as an Antibacterial Mechanism of Dielectric Barrier Discharge Plasma against Fusarium oxysporum. International Journal of Molecular Sciences. 2024; 25(14):7875. https://doi.org/10.3390/ijms25147875
Chicago/Turabian StyleYu, Shiqian, Jiajin Sun, Haiming Chen, Weijun Chen, Qiuping Zhong, Ming Zhang, Jianfei Pei, Rongrong He, and Wenxue Chen. 2024. "Disruption of Cell Membranes and Redox Homeostasis as an Antibacterial Mechanism of Dielectric Barrier Discharge Plasma against Fusarium oxysporum" International Journal of Molecular Sciences 25, no. 14: 7875. https://doi.org/10.3390/ijms25147875




