Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation
Abstract
1. Introduction
2. Results
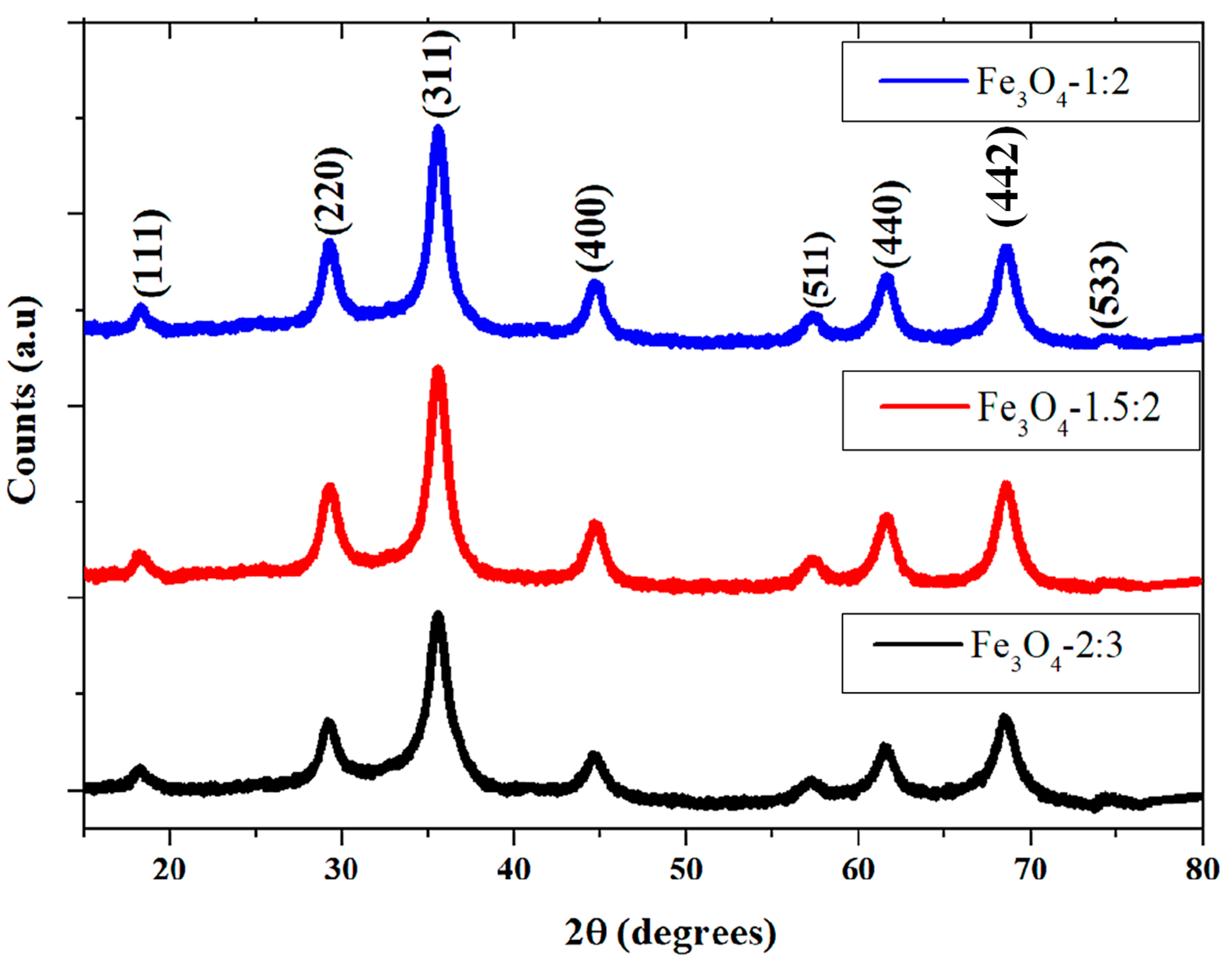
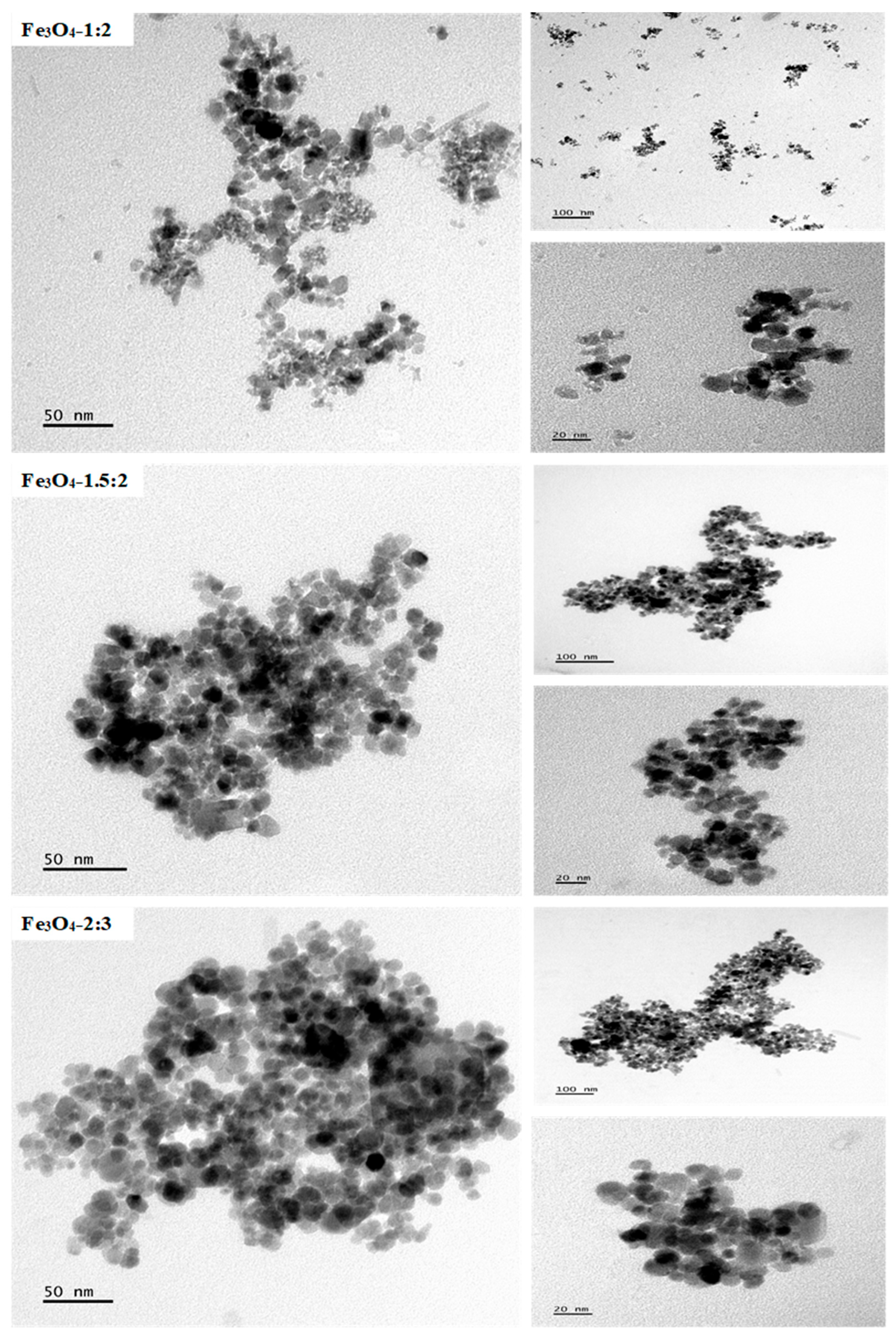
2.1. Morphological Studies of the Iron Oxide Nanoparticles
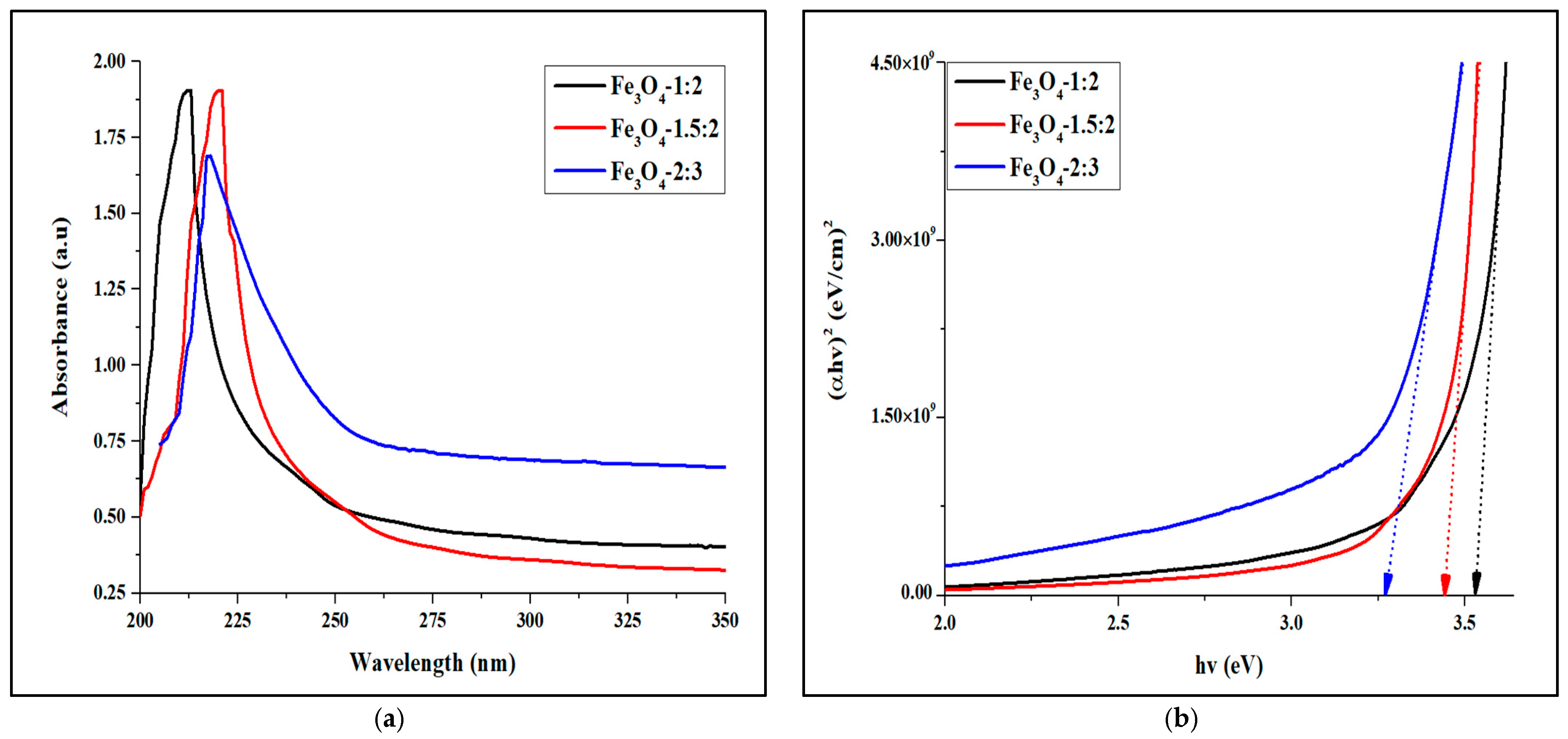
2.2. Optical Studies of the Magnetite Nanoparticles
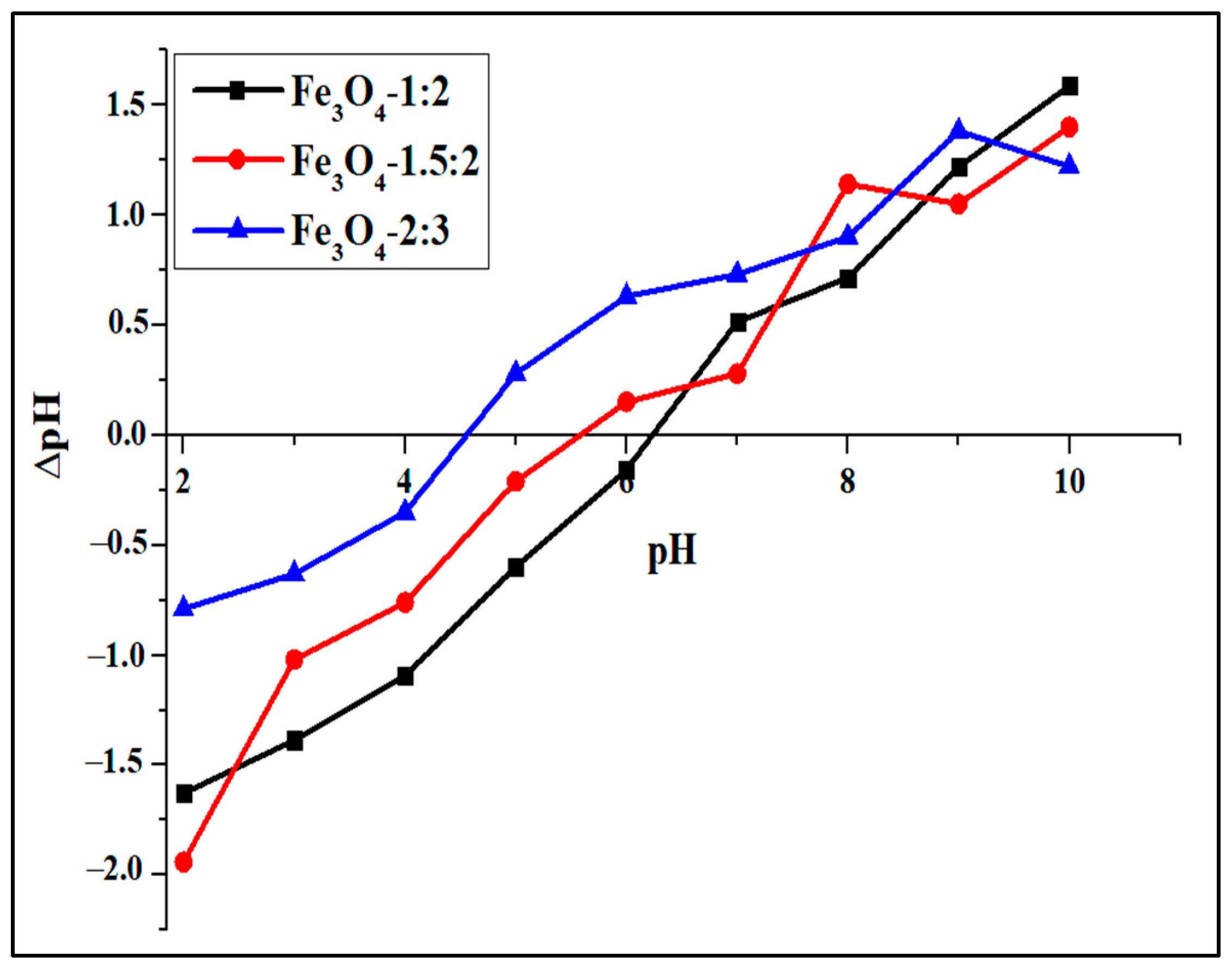
2.3. Surface Properties
3. Adsorption Studies of Magnetite Nanoparticles
4. Photocatalytic Studies of Magnetite Nanoparticles
4.1. Effect of Scavengers on the Degradation of Organic Dyes by Fe3O4 Nanoparticles
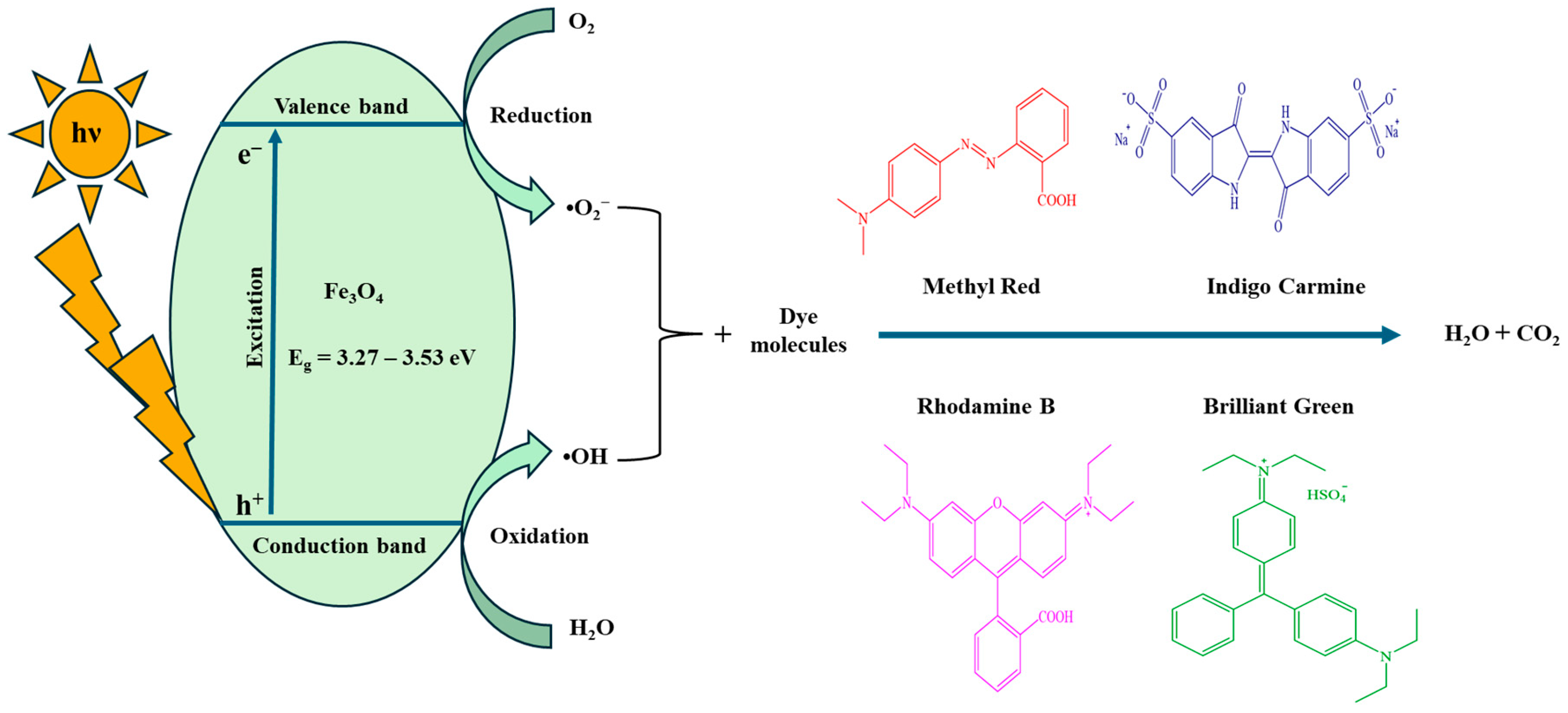
4.2. Proposed Mechanism
4.3. Effect of pH
4.4. Recyclability of Iron Oxide Nanoparticles
5. Materials and Methods
5.1. Materials
5.2. Preparation of Iron Oxide Nanoparticles
5.3. Physical Characterization
5.4. Adsorption Studies
5.5. Photocatalysis Experiment
5.6. Effect of pH
5.7. Effect of Scavengers
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Alsantali, R.; Raja, Q.; Alzahrani, A.; Sadiq, A.; Naeem, N.; Mughal, E.; Al-Rooqi, M.; El Guesmi, N.; Moussa, Z.; Ahmed, S. Miscellaneous azo dyes: A comprehensive review on recent advancements in biological and industrial Applications. Dyes Pigm. 2021, 199, 110050. [Google Scholar] [CrossRef]
- John, A.; Yang, H.-H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Alsukaibi, A.K.D. Various approaches for the detoxification of toxic dyes in wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Hasan, M.N. Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J. Mol. Liq. 2021, 328, 115468. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yang, J.E.; Park, J.E.; Jeong, S.-W.; Choi, S.-J.; Choi, Y.J.; Jeon, J. Rapid and efficient removal of anionic dye in water using a chitosan-coated iron oxide-immobilized polyvinylidene fluoride membrane. ACS Omega 2022, 7, 8759–8766. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, N. Recent progress in photocatalytic removal of environmental pollution hazards in water using nanostructured materials. Separations 2022, 9, 264. [Google Scholar] [CrossRef]
- Van Thuan, D.; Ngo, H.L.; Thi, H.P.; Chu, T.T.H. Photodegradation of hazardous organic pollutants using titanium oxides-based photocatalytic: A review. Environ. Res. 2023, 229, 116000. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Kumar, S.; Verma, A.; Kumar, A.; Ludwig, T.; Frank, M.; Mathur, S.; Prakash, R.; Sinha, I. Development of magnetically recyclable visible light photocatalysts for hydrogen peroxide production. Mater. Sci. Semicond. Process. 2020, 112, 105024. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Kazmi, J.; Mohamed, M.A.; Berhanuddin, D.D. Molarity effects of Fe and NaOH on synthesis and characterisation of magnetite (Fe3O4) nanoparticles for potential application in magnetic hyperthermia therapy. Magnetochemistry 2022, 8, 161. [Google Scholar] [CrossRef]
- Granath, T.; Mandel, K.; Löbmann, P. The Significant influence of the pH value on citrate coordination upon modification of superparamagnetic iron oxide nanoparticles. Part. Syst. Charact. 2022, 39, 2100279. [Google Scholar] [CrossRef]
- Alibeigi, S.; Vaezi, M. Phase transformation of iron oxide nanoparticles by varying the molar ratio of Fe2+:Fe3+. Chem. Eng. Technol. 2008, 31, 1591–1596. [Google Scholar] [CrossRef]
- Dehghanpour, H.R. pH, molar ratio of ferrous to ferric ions and surfactant presence effects on physical properties of iron oxide nanoparticles generated by co-precipitation method. J. Coord. Chem. 2020, 73, 3452–3464. [Google Scholar] [CrossRef]
- Šutka, A.; Bitina, S.; Smits, K.; Sutka, A.; Ignatane, L.; Mairov, M.; Käämbre, T.; Timusk, M.; Laipniece, L.; Lazdovica, K. Rapid, high-yield aqueous synthesis of ultrafine magnetite nanoparticles from Fe(III) precursor at room temperature. J. Mater. Sci. 2023, 59, 447–457. [Google Scholar] [CrossRef]
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M.; Soleymani Goloujeh, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; N’Deh, K.P.U.; Kim, G.-J.; Choi, J.; Kim, J.; Kim, E.-K.; An, J. Fe2+:Fe3+ molar ratio influences the immunomodulatory properties of maghemite (γ-Fe2O3) nanoparticles in an atopic dermatitis model. ACS Appl. Bio Mater. 2021, 4, 1252–1267. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The future of semiconductors nanoparticles: Synthesis, properties and applications. Mater. Sci. Eng. 2021, 272, 115363. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K.; Kuvarega, A.T. Fabrication of magnetic recoverable Fe3O4/TiO2 heterostructure for photocatalytic degradation of rhodamine B dye. Inorg. Chem. Commun. 2022, 145, 109966. [Google Scholar] [CrossRef]
- Khan, I.; Zada, N.; Khan, I.; Sadiq, M.; Saeed, K. Enhancement of photocatalytic potential and recoverability of Fe3O4 nanoparticles by decorating over monoclinic zirconia. J. Environ. Health Sci. Eng. 2020, 18, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, M.; Balaji; Sumathi, S. Photocatalytic, antimicrobial and antifungal activity of biogenic iron oxide nanoparticles synthesised using aegle marmelos Extracts. J. Inorg. Organomet. Polym. 2021, 31, 1738–1744. [Google Scholar] [CrossRef]
- Ravelo-Nieto, E.; Ovalle-Serrano, S.A.; Gutiérrez-Pineda, E.A.; Blanco-Tirado, C.; Combariza, M.Y. Textile wastewater depuration using a green cellulose based Fe3O4 bionanocomposite. J. Environ. Chem. Eng. 2023, 11, 109516. [Google Scholar] [CrossRef]
- Accogli, A.; Bertoli, L.; Panzeri, G.; Gibertini, E.; Pesce, R.; Bussetti, G.; Magagnin, L. Electrochemical characterization of magnetite (Fe3O4) nanoaggregates in acidic and alkaline solutions. ACS Omega 2021, 6, 26880–26887. [Google Scholar] [CrossRef] [PubMed]
- Sosun; Ali, A.; Mannan, A.; Ali Shah, U.; Zia, M. Removal of of toxic metal ions (Ni2+ and Cd2+) from wastewater by using TOPO decorated iron oxide nanoparticles. Appl. Water Sci. 2022, 12, 86. [Google Scholar] [CrossRef]
- Ahmouda, K.; Boudiaf, M.; Barani, D.; Benhaoua, B. The effect of mediating plant extract on the photocatalytic activity of different eco-friendly synthesized magnetite nanoparticles against cresol red photodegradation. J. Photochem. Photobiol. 2024, 450, 115442. [Google Scholar] [CrossRef]
- Spivakov, A.; Lin, C.-R.; Chang, Y.-C.; Wang, C.-C.; Sarychev, D. Magnetic and magneto-optical oroperties of iron oxides nanoparticles synthesized under atmospheric pressure. Nanomaterials 2020, 10, 1888. [Google Scholar] [CrossRef] [PubMed]
- Syahida, A.N.; Sutanto, H.; Alkian, I.; Irianti, F.D.D.; Wibowo, A.A.; Priyono, P. Synthesized and characterization nanosized synthesis Fe3O4 powder from natural iron sand. J. Phys. Conf. Ser. 2021, 1943, 012013. [Google Scholar] [CrossRef]
- Gaur, R.; Shahabuddin, S.; Ahmad, I.; Sridewi, N. Role of alkylamines in tuning the morphology and optical properties of SnS2 nanoparticles synthesized by via facile thermal decomposition approach. Nanomaterials 2022, 12, 3950. [Google Scholar] [CrossRef]
- Chuburu, F.; Malystkyi, V.; Moreau, J.; Callewaert, M.; Henoumont, C.; Cadiou, C.; Feuillie, C.; Laurent, S.; Molinari, M. Synthesis and characterization of conjugated hyaluronic acids. Application to stability studies of chitosan-hyaluronic acid nanogels based on fluorescence resonance energy transfer. Gels 2022, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Matalobos, J.; Bermejo-Barrera, P.; Aboal-Somoza, M.; Fondo, M.; García-Deibe, A.M.; Corredoira-Vázquez, J.; Alves-Iglesias, Y. Semiconductor quantum dots as target analytes: Properties, surface chemistry and detection. Nanomaterials 2022, 12, 2501. [Google Scholar] [CrossRef] [PubMed]
- Abu-Noqta, O.A.; Aziz, A.A.; Usman, A.I. Colloidal stability of iron oxide nanoparticles coated with different capping agents. Mater. Today Proc. 2019, 17, 1072–1077. [Google Scholar] [CrossRef]
- Sakthi Sri, S.P.; Taj, J.; George, M. Facile synthesis of magnetite nanocubes using deep eutectic solvent: An insight to anticancer and photo-Fenton efficacy. Surf. Interf. 2020, 20, 100609. [Google Scholar] [CrossRef]
- Jain, R. Effect of gadolinium doping on the structural, magnetic, electrical, optical, and elastic properties of magnetite nanoparticles. Mater. Sci. Eng. 2023, 296, 116631. [Google Scholar] [CrossRef]
- León-Flores, J.; Pérez-Mazariego, J.L.; Marquina, M.; Gómez, R.; Escamilla, R.; Tehuacanero-Cuapa, S.; Reyes-Damián, C.; Arenas-Alatorre, J. Controlled formation of hematite—Magnetite nanoparticles by a biosynthesis method and its photocatalytic removal potential against methyl orange dye. J. Cluster Sci. 2023, 34, 2381–2395. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Preparation, phase analysis and electrochemistry of magnetite (Fe3O4) and maghemite (γ-Fe2O3) nanoparticles. Int. J. Electrochem. Sci. 2022, 17, 22124. [Google Scholar] [CrossRef]
- Shamaila, S.; Bano, T.; Sajjad, A.K.L. Efficient visible light magnetic modified iron oxide photocatalysts. Ceram. Int. 2017, 43, 14672–14677. [Google Scholar] [CrossRef]
- Bayer, T.; Wei, R.; Kappler, A.; Byrne, J.M. Cu(II) and Cd(II) removal efficiency of microbially redox-activated magnetite nanoparticles. ACS Earth Space Chem. 2023, 7, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.M.; Feuser, P.E.; Cercená, R.; Peterson, M.; Dal-Bó, A.G. Obtention of magnetite nanoparticles via the hydrothermal method and effect of synthesis parameters. J. Magn. Magn. Mater. 2023, 580, 170925. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr(VI) and Cu(II) in mixed solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, N.; Saxena, R. Highly efficient and rapid removal of a toxic dye: Adsorption kinetics, isotherm, and mechanism studies on functionalized multiwalled carbon nanotubes. Surf. Interf. 2020, 21, 100639. [Google Scholar] [CrossRef]
- Medhat, A.; El-Maghrabi, H.H.; Abdelghany, A.; Abdel Menem, N.M.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently activated carbons from corn cob for methylene blue adsorption. Appl. Surf. Sci. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E. Photocatalytic degradation of single and binary mixture of brilliant green and rhodamine B dyes by zinc sulfide quantum dots. Molecules 2021, 26, 7686. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Tao, F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.H.A.; Siciliano, P.H.C.; Alves, O.C.; Cesar, D.V.; Henriques, C.A.; Gaspar, A.B. Synthesis of a magnetic Fe3O4/RGO composite for the rapid photo-fenton discoloration of indigo carmine dye. Top. Catal. 2020, 63, 1017–1029. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Saini, C.; Ambati, R.; Gogate, P.R. Synthesis of ultrasound assisted nanostuctured photocatalyst (NiO supported over CeO2) and its application for photocatalytic as well as sonocatalytic dye degradation. Catal. Today 2018, 300, 50–57. [Google Scholar] [CrossRef]
- Soltani-nezhad, F.; Saljooqi, A.; Mostafavi, A.; Shamspur, T. Synthesis of Fe3O4/CdS–ZnS nanostructure and its application for photocatalytic degradation of chlorpyrifos pesticide and brilliant green dye from aqueous solutions. Ecotoxicol. Environ. Saf. 2020, 189, 109886. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, J.R.; Botsa, S.M. SnO2/Fe2O3/Ag Nanocomposite via hydrothermal approach: A novel highly efficient photodegradation of eosin yellow and brilliant green dyes under visible light irradiation. Chem. Afr. 2019, 2, 635–644. [Google Scholar] [CrossRef]
- AbouSeada, N.; Ahmed, M.A.; Elmahgary, M.G. Synthesis and characterization of novel magnetic nanoparticles for photocatalytic degradation of indigo carmine dye. Mater. Sci. Energy Technol. 2022, 5, 116–124. [Google Scholar] [CrossRef]
- Dayana, P.N.; Abel, M.J.; Inbaraj, P.F.H.; Sivaranjani, S.; Thiruneelakandan, R.; Prince, J.J. Zirconium doped copper ferrite (CuFe2O4) nanoparticles for the enhancement of visible light-responsive photocatalytic degradation of rose bengal and indigo carmine dyes. J. Cluster Sci. 2022, 33, 1739–1749. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. Visible light-induced degradation of indigo carmine over ZnFe2O4/Tannin/ZnO: Role of tannin as a modifier and its degradation mechanism. Int. J. Hydrogen Energy 2018, 43, 8779–8793. [Google Scholar] [CrossRef]
- Gupta, N.K.; Ghaffari, Y.; Bae, J.; Kim, K.S. Synthesis of coral-like α-Fe2O3 nanoparticles for dye degradation at neutral pH. J. Mol. Liq. 2020, 301, 112473. [Google Scholar] [CrossRef]
- Pegu, B.; Konwer, S. Facile green synthesis of reduced graphene oxide/iron nanocomposites using tea leaf extract as efficient photocatalyst for methyl red degradation. Graphene 2D Mater. 2023, 8, 121–133. [Google Scholar] [CrossRef]
- Bilgic, A. Fabrication of monoBODIPY-functionalized Fe3O4@SiO2@TiO2 nanoparticles for the photocatalytic degradation of rhodamine B under UV irradiation and the detection and removal of Cu(II) ions in aqueous solutions. J. Alloys Compd. 2022, 899, 163360. [Google Scholar] [CrossRef]
- Maqbool, S.; Ahmed, A.; Mukhtar, A.; Jamshaid, M.; Rehman, A.U.; Anjum, S. Efficient photocatalytic degradation of Rhodamine B dye using solar light-driven La-Mn co-doped Fe2O3 nanoparticles. Environ. Sci. Pollut. Res. 2023, 30, 7121–7137. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.C.L.; Mortari, S.R.; Vizzotto, B.S.; Chuy, G.; dos Santos, C.; Brum, L.F.W.; da Silva, W.L. Iron oxide nanocatalyst with titanium and silver nanoparticles: Synthesis, characterization and photocatalytic activity on the degradation of rhodamine B dye. Sci. Rep. 2020, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Farrokhnia, A.; Garcıa-Lopez, E.I.; Zargar Shoushtari, M. Superparamagnetic recoverable flowerlike Fe3O4@Bi2O3 core–shell with g-C3N4 sheet nanocomposite: Synthesis, characterization, mechanism and kinetic study of photo-catalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1022–1033. [Google Scholar] [CrossRef]
- Kaushik, J.; Saluja, T.; Tisha; Nisha; Baig, A.; Sonal; Dubey, P.; Sonkar, S.K. Photoactive Fe3O4@Fe2O3 synthesized from industrial iron oxide dust for Fenton-free degradation of multiple organic dyes. Ind. Eng. Chem. Res. 2023, 62, 10487–10497. [Google Scholar] [CrossRef]
- Purkait, P.K.; Majumder, S.; Roy, S.; Maitra, S.; Chandra Das, G.; Chaudhuri, M.G. Enhanced heterogeneous photocatalytic degradation of florasulam in aqueous media using green synthesized TiO2 nanoparticle under UV light irradiation. Inorg. Chem. Commun. 2023, 155, 111017. [Google Scholar] [CrossRef]
- El Sharkawy, H.M.; Shawky, A.M.; Elshypany, R.; Selim, H. Efficient photocatalytic degradation of organic pollutants over TiO2 nanoparticles modified with nitrogen and MoS2 under visible light irradiation. Sci. Rep. 2023, 13, 8845. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Mishra, S.; Sahu, T.K.; Verma, P.; Kumar, P.; Samanta, S.K. Microwave-assisted catalytic degradation of brilliant green by spinel zinc ferrite sheets. ACS Omega 2019, 4, 10411–10418. [Google Scholar] [CrossRef] [PubMed]
- Ritika; Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K.; Khan, M.A.; Algarni, H. Enhanced solar light-mediated photocatalytic degradation of brilliant green dye in aqueous phase using BiPO4 nanospindles and MoS2/BiPO4 nanorods. J. Mater. Sci. Mater. Electron. 2019, 30, 20741–20750. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Liu, J.-J.; Hu, Y.-X.; Kong, L.-J.; Jiang, D.; Xu, X.-R. Comparative study of rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Sep. Purif. Technol. 2017, 184, 374–383. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, H.; Ma, J.; Kong, Y.; Komarneni, S. Efficient degradation of rhodamine B by magnetically separable ZnS–ZnFe2O4 composite with the synergistic effect from persulfate. Chemosphere 2019, 237, 124547. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Abd El-Lateef, H.M.; Taha, K.K.; Modwi, A. Mesoporous TiO2@g-C3N4 composite: Construction, characterization, and boosting indigo carmine dye destruction. Diam. Relat. Mater. 2021, 118, 108491. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented indigo carmine dye photodegradation via mesoporous MgO@g-C3N4 nanocomposite. J. Photochem. Photobiol. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; El-Shall, M.S.; Awad, F.S. Facile synthesis of amorphous zirconium phosphate graphitic carbon nitride composite and its high performance for photocatalytic degradation of indigo carmine dye in water. J. Mater. Res. Technol. 2022, 20, 1456–1469. [Google Scholar] [CrossRef]
- Chaudhary, R.G.; Sonkusare, V.; Bhusari, G.; Mondal, A.; Potbhare, A.; Juneja, H.; Abdala, A.; Sharma, R. Preparation of mesoporous ThO2 nanoparticles: Influence of calcination on morphology and visible-light-driven photocatalytic degradation of indigo carmine and methylene blue. Environ. Res. 2023, 222, 115363. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, V.N.; Chaudhary, R.G.; Bhusari, G.S.; Mondal, A.; Potbhare, A.K.; Mishra, R.K.; Juneja, H.D.; Abdala, A.A. Mesoporous octahedron-shaped tricobalt tetroxide nanoparticles for photocatalytic degradation of toxic dyes. ACS Omega 2020, 5, 7823–7835. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Farooq, A.; Alam, J. Microwave-assisted rapid degradation of methyl red dye using polyfuran/polythiophene and its co-oligomers as catalysts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 302, 123106. [Google Scholar] [CrossRef] [PubMed]
- Ziyaadini, M.; Ghashang, M. Sunlight-induced photocatalytic degradation of indigo carmine using Bi5Ti3FeO15 layered structure. Optik 2021, 228, 166207. [Google Scholar] [CrossRef]
- Bhapkar, A.R.; Geetha, M.; Jaspal, D.; Gheisari, K.; Laad, M.; Cabibihan, J.-J.; Sadasivuni, K.K.; Bhame, S. Aluminium doped ZnO nanostructures for efficient photodegradation of indigo carmine and azo carmine G in solar irradiation. Appl. Nanosci. 2023, 13, 5777–5793. [Google Scholar] [CrossRef]
- Nandisha, P.S.; Sowbhagya, S.; Yallappa, S. Synthesis and characterization of ternary NiO@Bi2MoO6–MoS heterojunction with enhanced photodegradation efficiency towards indigo carmine dye. Solid State Sci. 2023, 139, 107157. [Google Scholar] [CrossRef]
- Rao, C.V.; Giri, A.S.; Goud, V.V.; Golder, A.K. Studies on pH-dependent color variation and decomposition mechanism of brilliant green dye in Fenton reaction. Int. J. Ind. Chem. 2016, 7, 71–80. [Google Scholar] [CrossRef]
- Abadi, R.W.; Setiawan, C.M.; Santoso, S.P.; Bundjaja, V.; Angkawijaya, A.E.; Jiang, Y.-F.; Wijaya, C.J.; Ismadji, S.; Retnoningtyas, E.S.; Soetaredjo, F.E.; et al. Polystyrene-templated hollow mesoporous magnetite as a bifunctional adsorbent for the removal of rhodamine B via simultaneous adsorption and degradation. J. Environ. Chem. Eng. 2022, 10, 108194. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Gupta, H. Activated banana peel carbon: A potential adsorbent for rhodamine B decontamination from aqueous system. Appl. Water Sci. 2020, 10, 185. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Sahroni, I.; Wijayana, A.; Faraswati, M.; Dwi Putri, A.; Oh, W.-C.; Doong, R.-A. Magnetically-separable photocatalyst of magnetic biochar from snake fruit peel for rhodamine B photooxidation. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100669. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, S.; Yang, J. Preparation of Ag2O/g-C3N4/Fe3O4 composites and the application in the photocatalytic degradation of rhodamine B under visible light. J. Alloys Compd. 2017, 708, 1141–1149. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Effective removal of indigo carmine dye from wastewaters by adsorption onto mesoporous magnesium ferrite nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100550. [Google Scholar] [CrossRef]
- Yazdi, M.G.; Ivanic, M.; Mohamed, A.; Uheida, A. Surface modified composite nanofibers for the removal of indigo carmine dye from polluted water. RSC Adv. 2018, 8, 24588–24598. [Google Scholar] [CrossRef] [PubMed]
- Hadjltaief, H.B.; Bairq, Z.A.S.; Shi, C.; Benzina, M. Evaluation of sono-assisted solar/Fenton process for indigo carmine degradation over magnetic ZnO-Fe3O4 supported Tunisian kaolinite clay. Surf. Interf. 2021, 26, 101395. [Google Scholar] [CrossRef]
- Anjum, F.; Asiri, A.M.; Khan, M.A.; Khan, M.I.; Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Alamry, K.A.; Alfifi, S.Y.; Chakraborty, S. Photo-degradation, thermodynamic and kinetic study of carcinogenic dyes via zinc oxide/graphene oxide nanocomposites. J. Mater. Res. Technol. 2021, 15, 3171–3191. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Debnath, A.; Saha, B. Fabrication of PANI@Fe–Mn–Zr hybrid material and assessments in sono-assisted adsorption of methyl red dye: Uptake performance and response surface optimization. J. Indian Chem. Soc. 2022, 99, 100635. [Google Scholar] [CrossRef]
- Sharma, S.C.D.; Sun, Q.; Li, J.; Wang, Y.; Suanon, F.; Yang, J.; Yu, C.-P. Decolorization of azo dye methyl red by suspended and co-immobilized bacterial cells with mediators anthraquinone-2,6-disulfonate and Fe3O4 nanoparticles. Int. Biodeterior. Biodegrad. 2016, 112, 88–97. [Google Scholar] [CrossRef]
- Pervez, M.N.; Stylios, G.K.; Liang, Y.; Ouyang, F.; Cai, Y. Low-temperature synthesis of novel polyvinylalcohol (PVA) nanofibrous membranes for catalytic dye degradation. J. Clean. Prod. 2020, 262, 121301. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Xing, B.; Liu, Y.; Zhang, C.; Xia, H. Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J. Clean. Prod. 2022, 348, 131301. [Google Scholar] [CrossRef]
- Ye, L.; Zhou, L.; Lu, Y. Direct continuous synthesis of oleic acid-modified Fe3O4 nanoparticles in a microflow system. Ind. Eng. Chem. Res. 2022, 61, 4320–4328. [Google Scholar] [CrossRef]
- Mirzaei, K.; Jafarpour, E.; Shojaei, A.; Khasraghi, S.S.; Jafarpour, P. An investigation on the influence of highly acidic media on the microstructural stability and dye adsorption performance of UiO-66. Appl. Surf. Sci. 2023, 618, 156531. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K.; Saini, J.K. Synthesis of graphitic carbon nitride/zinc oxide (g-C3N4/ZnO) hybrid nanostructures and investigation of the effect of ZnO on the photodegradation activity of g-C3N4 against the brilliant cresyl blue (BCB) dye under visible light irradiation. Adv. Compos. Hybrid Mater. 2022, 6, 16. [Google Scholar] [CrossRef]
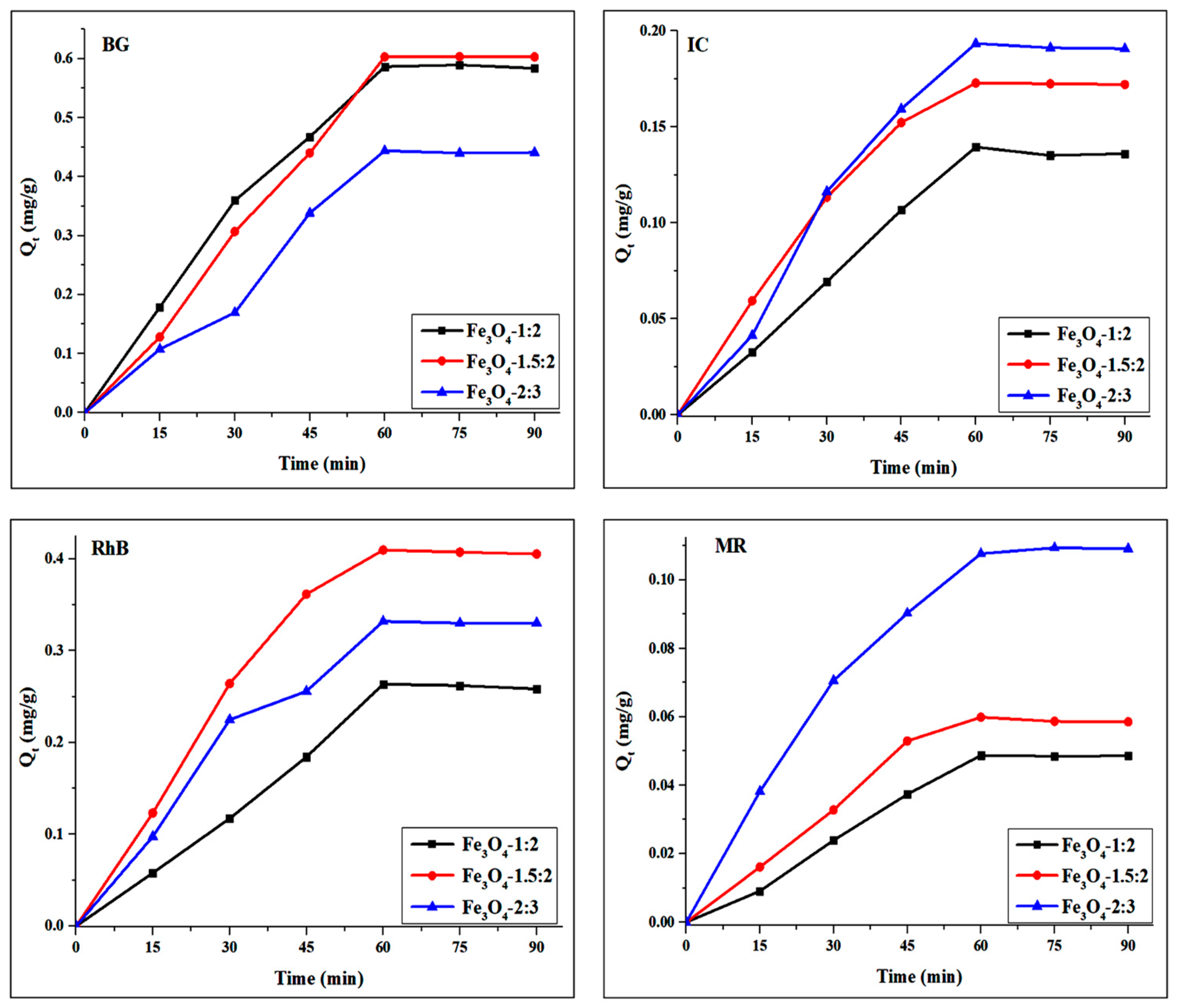
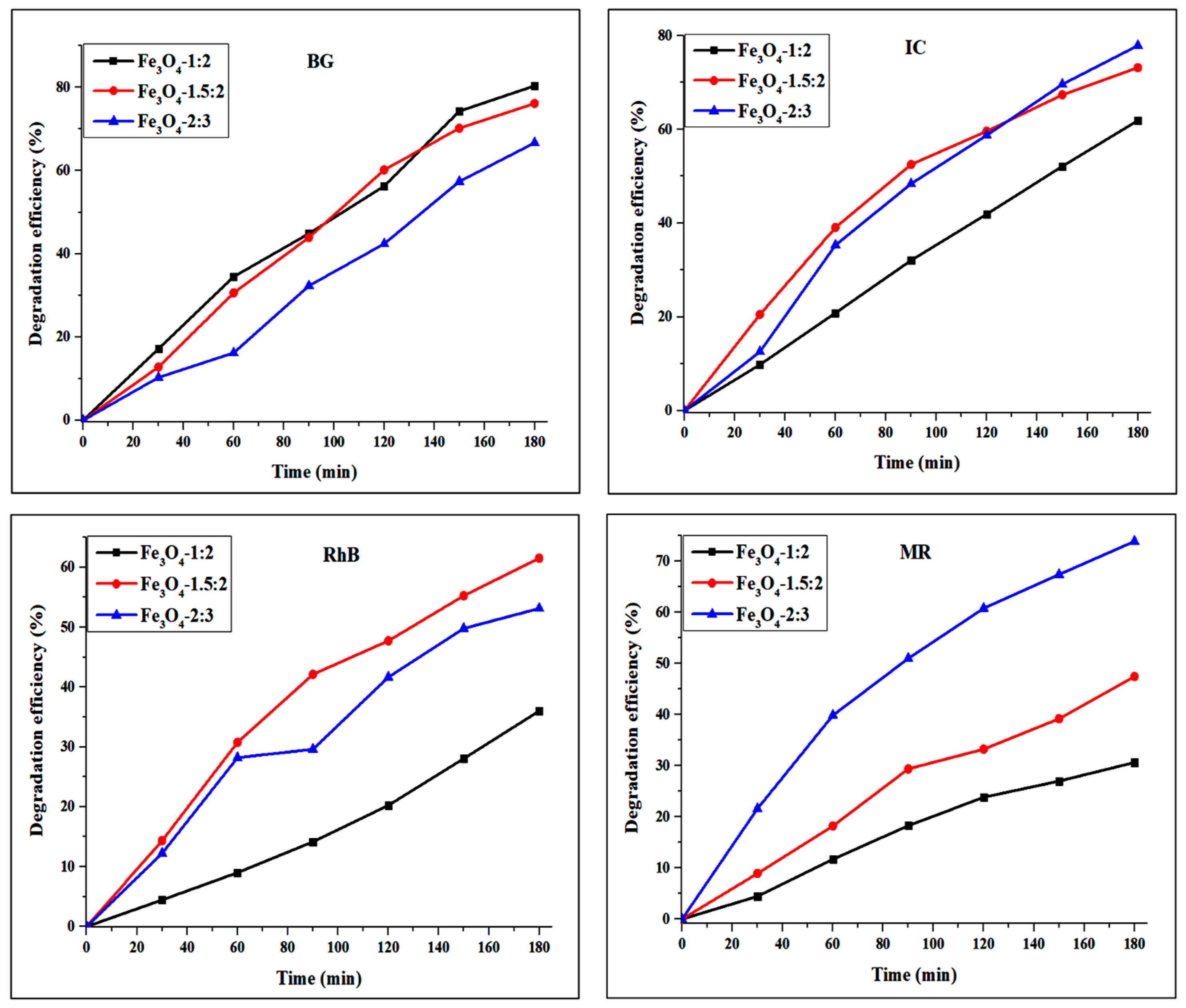
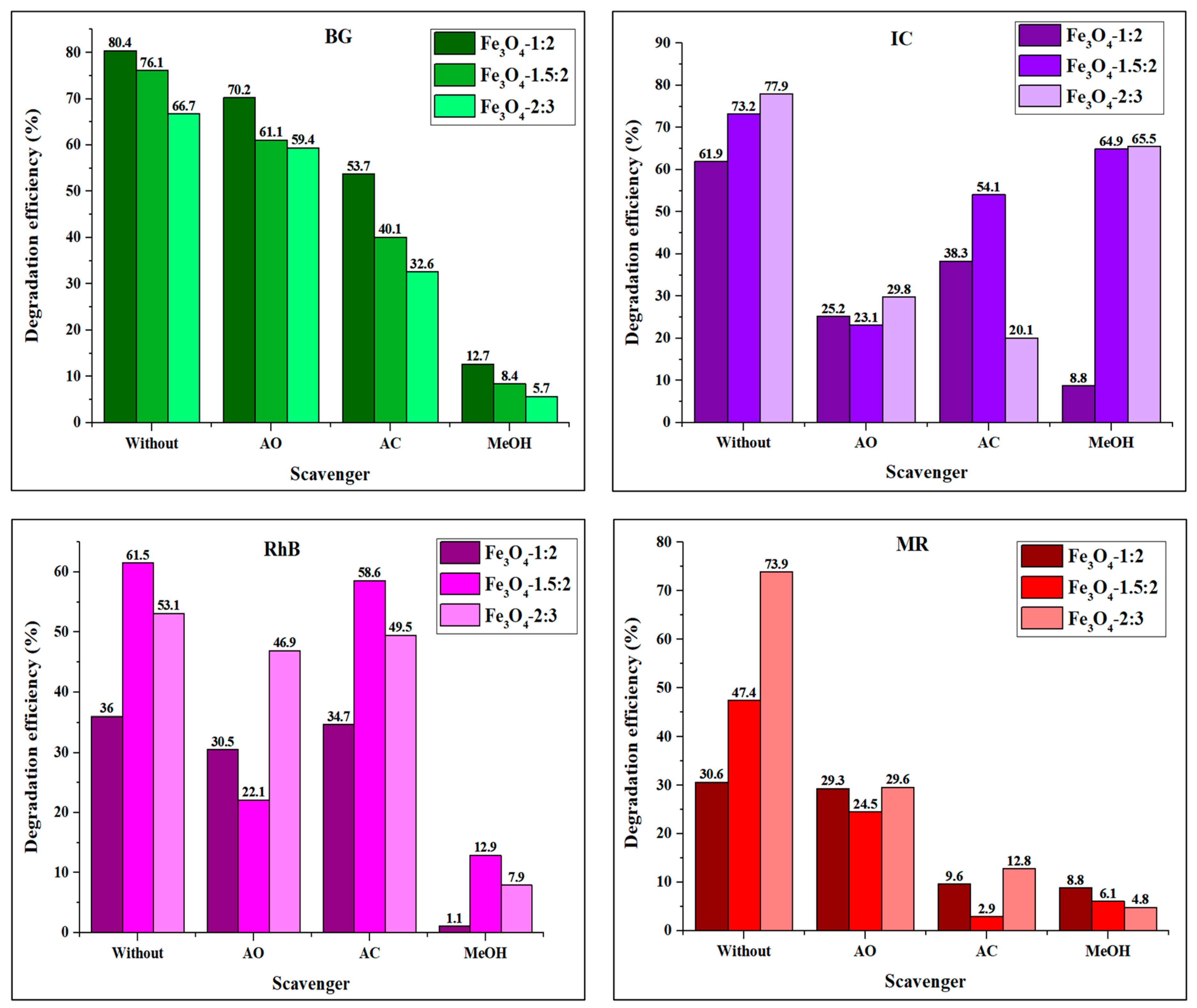
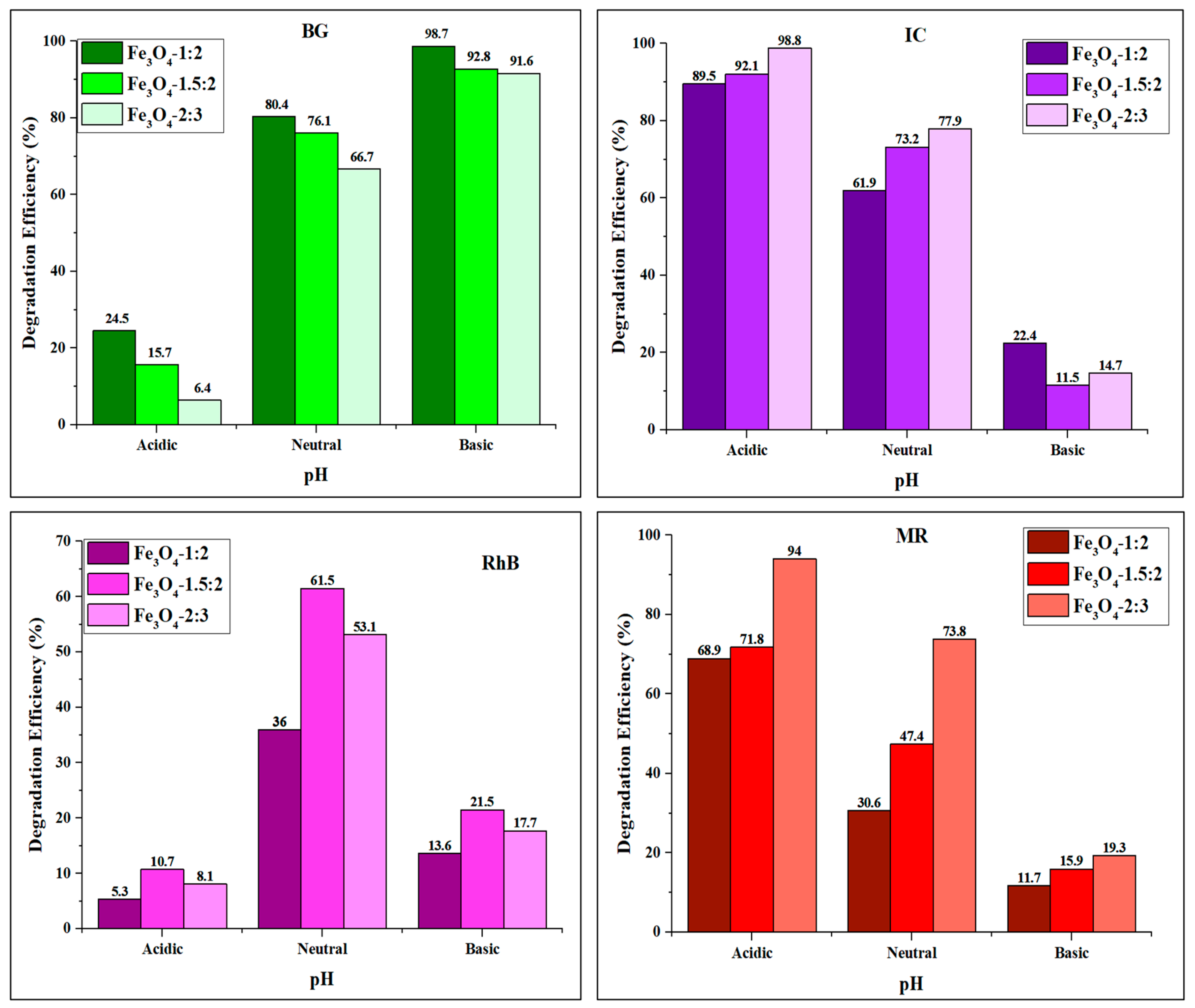
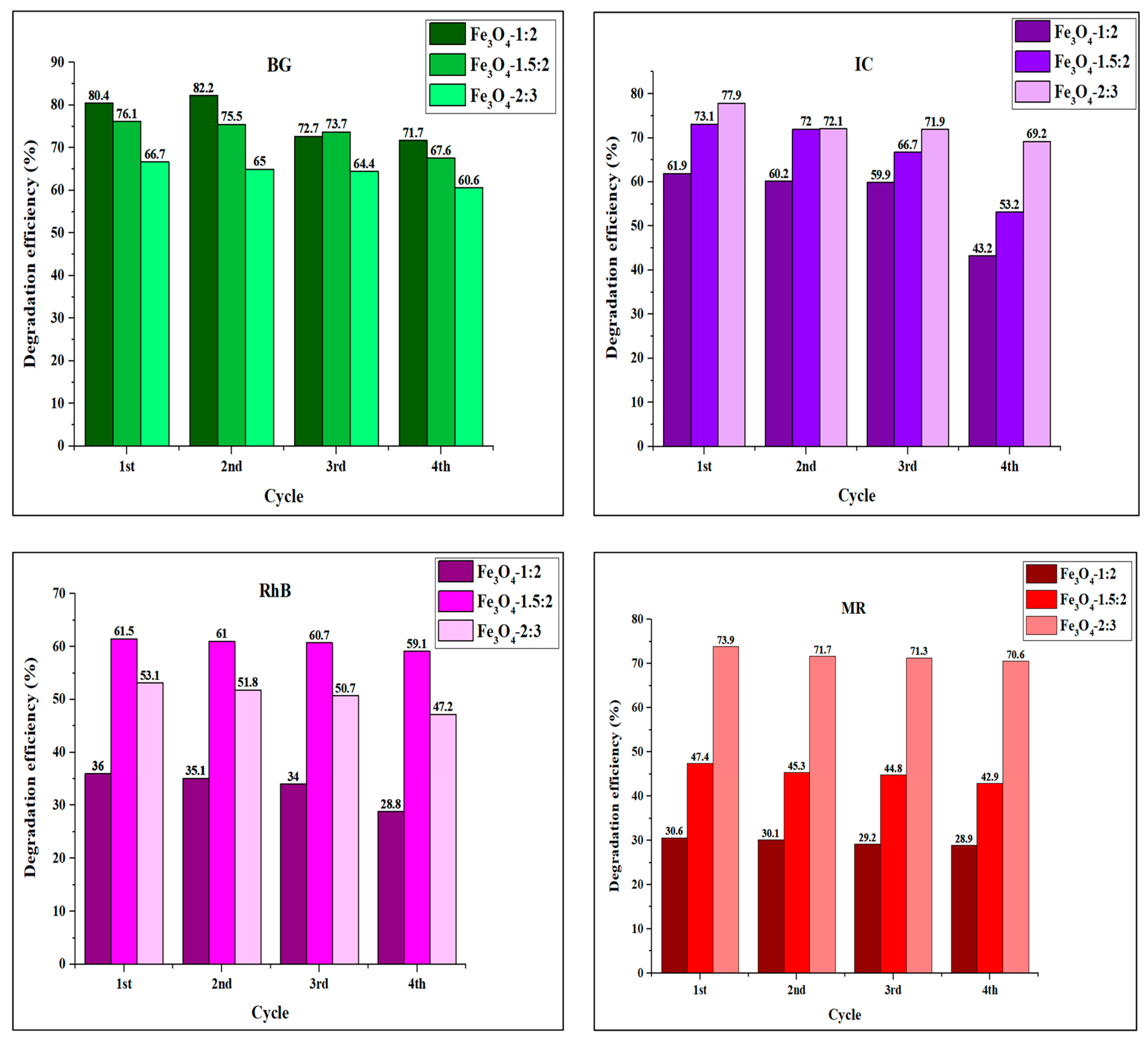
| Dye | Catalyst | Method of Synthesis | Size (nm) | Catalyst Load (mg) | Dye Concentration (ppm) | Degradation Efficiency (%) | Time (min) | Light Source | Reference |
|---|---|---|---|---|---|---|---|---|---|
| BG | NiO/CeO2 | ultrasound | - | 200 | 10 | 81.9 | 240 | 8 W UV light | [48] |
| Fe3O4/ CdS–ZnS | ultrasound | 25–45 | 1.5 | 10 | 92.93 | 360 | 300 W tungsten lamp | [49] | |
| SnO2/Fe2O3 | hydrothermal | 21.41 | 50 | 10 | 74 | 75 | 300 W visible light | [50] | |
| Fe3O4-1:2 | co-precipitation | 5.48 | 5 | 10 | 80.4 | 180 | 70 W visible light | This study | |
| IC | CoFe2O4/ SnO2 | sol-gel/ sonochemical | 13 | 100 | 5 | 55 | 120 | 15 W UV-A lamp | [51] |
| Zr/CuFe2O4 | chemical precipitation | 42 | 100 | 20 | 71 | 120 | 150 W tungsten lamp | [52] | |
| ZnFe2O4 | solvothermal | - | 50 | 16 | 66.3 | 300 | 128 W visible light | [53] | |
| Fe3O4-2:3 | co-precipitation | 6.98 | 5 | 10 | 77.9 | 180 | 70 W visible light | This study | |
| MR | α-Fe2O3 | co-precipitation | 54 | 100 | 10 | 76 | 5 | 32 W UV/H2O2 | [54] |
| Fe3O4/ZrO2 | chemical reduction | 803.54 ± 5.11 | 20 | 70 | 84 | 40 | UV-light | [22] | |
| GO/Fe | in situ | - | 0.03 | 10 | 70 | 30 | 250 W Xenon lamp | [55] | |
| Fe3O4-2:3 | co-precipitation | 6.98 | 5 | 10 | 73.9 | 180 | 70 W visible light | This study | |
| RhB | Fe3O4@SiO2@TiO2 | co-precipitation/Stöber | 8–18 | 50 | 30 | 29.49 | 60 | UV 8/18 | [56] |
| Fe2O3 | co-precipitation | 42–49 | 40 | 50 | 58.21 | 240 | Sunlight | [57] | |
| Fe2O3 | chemical precipitation | 3 | 1410 | 24.25 | 38.03 | 120 | 202 W visible light | [58] | |
| Fe3O4-1.5:2 | co-precipitation | 6.02 | 5 | 10 | 61.5 | 180 | 70 W visible light | This study |
| Catalyst | BG | RhB | IC | MR | ||||
|---|---|---|---|---|---|---|---|---|
| k (min−1) | R2 | k (min−1) | R2 | k (min−1) | R2 | k (min−1) | R2 | |
| Fe3O4-1:2 | 0.0091 | 0.9836 | 0.0024 | 0.9825 | 0.0053 | 0.9925 | 0.0021 | 0.9955 |
| Fe3O4-1.5:2 | 0.0083 | 0.9942 | 0.0053 | 0.9966 | 0.0073 | 0.9977 | 0.0035 | 0.9961 |
| Fe3O4-2:3 | 0.0061 | 0.9797 | 0.0043 | 0.9912 | 0.0084 | 0.9959 | 0.0074 | 0.9987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbuyazi, T.B.; Ajibade, P.A. Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation. Int. J. Mol. Sci. 2024, 25, 7876. https://doi.org/10.3390/ijms25147876
Mbuyazi TB, Ajibade PA. Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation. International Journal of Molecular Sciences. 2024; 25(14):7876. https://doi.org/10.3390/ijms25147876
Chicago/Turabian StyleMbuyazi, Thandi B., and Peter A. Ajibade. 2024. "Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation" International Journal of Molecular Sciences 25, no. 14: 7876. https://doi.org/10.3390/ijms25147876
APA StyleMbuyazi, T. B., & Ajibade, P. A. (2024). Photocatalytic Degradation of Organic Dyes by Magnetite Nanoparticles Prepared by Co-Precipitation. International Journal of Molecular Sciences, 25(14), 7876. https://doi.org/10.3390/ijms25147876






