Host Cells Upregulate Phosphate Transporter PIT1 to Inhibit Ehrlichia chaffeensis Intracellular Growth
Abstract
:1. Introduction
2. Results
2.1. Host Cells Upregulate the PIT1 Expression upon E. chaffeensis Infection
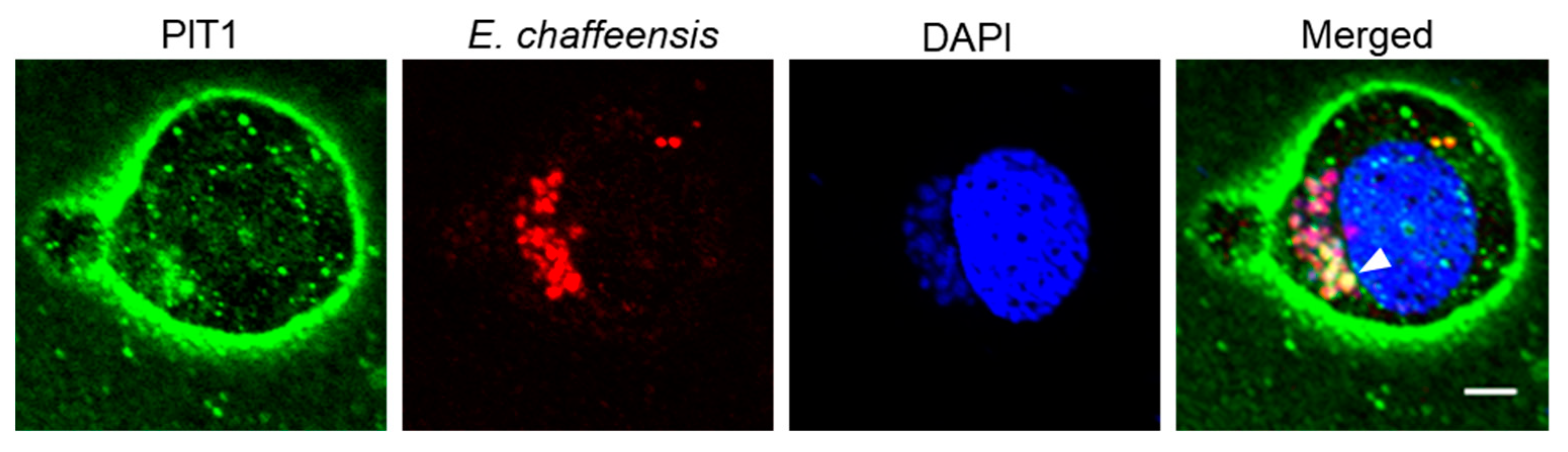
2.2. PIT1 Is Localized on the ECV Membrane in Infected THP-1 Cells
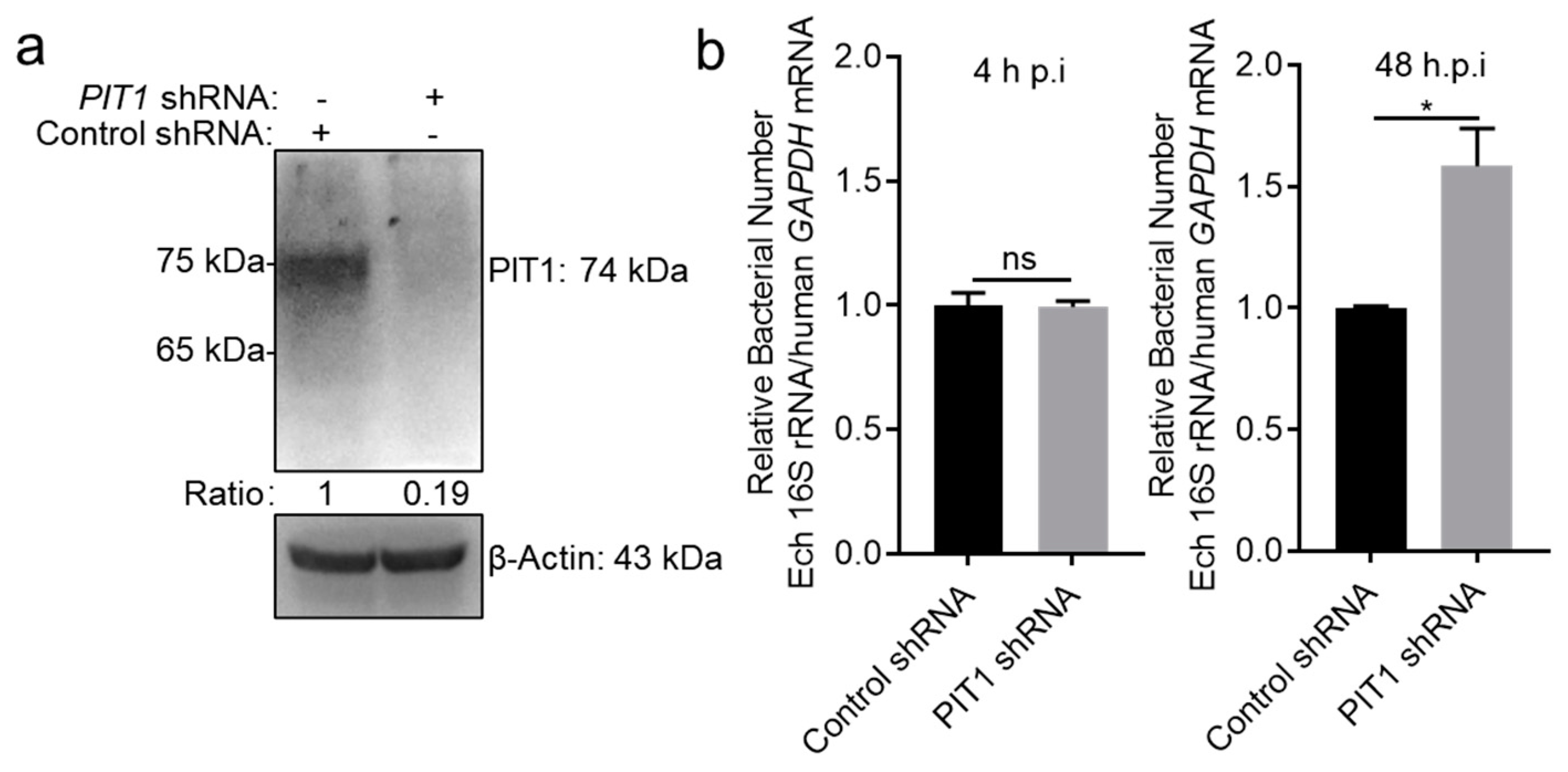
2.3. PIT1 Inhibits E. chaffeensis Intracellular Growth in Host Cells
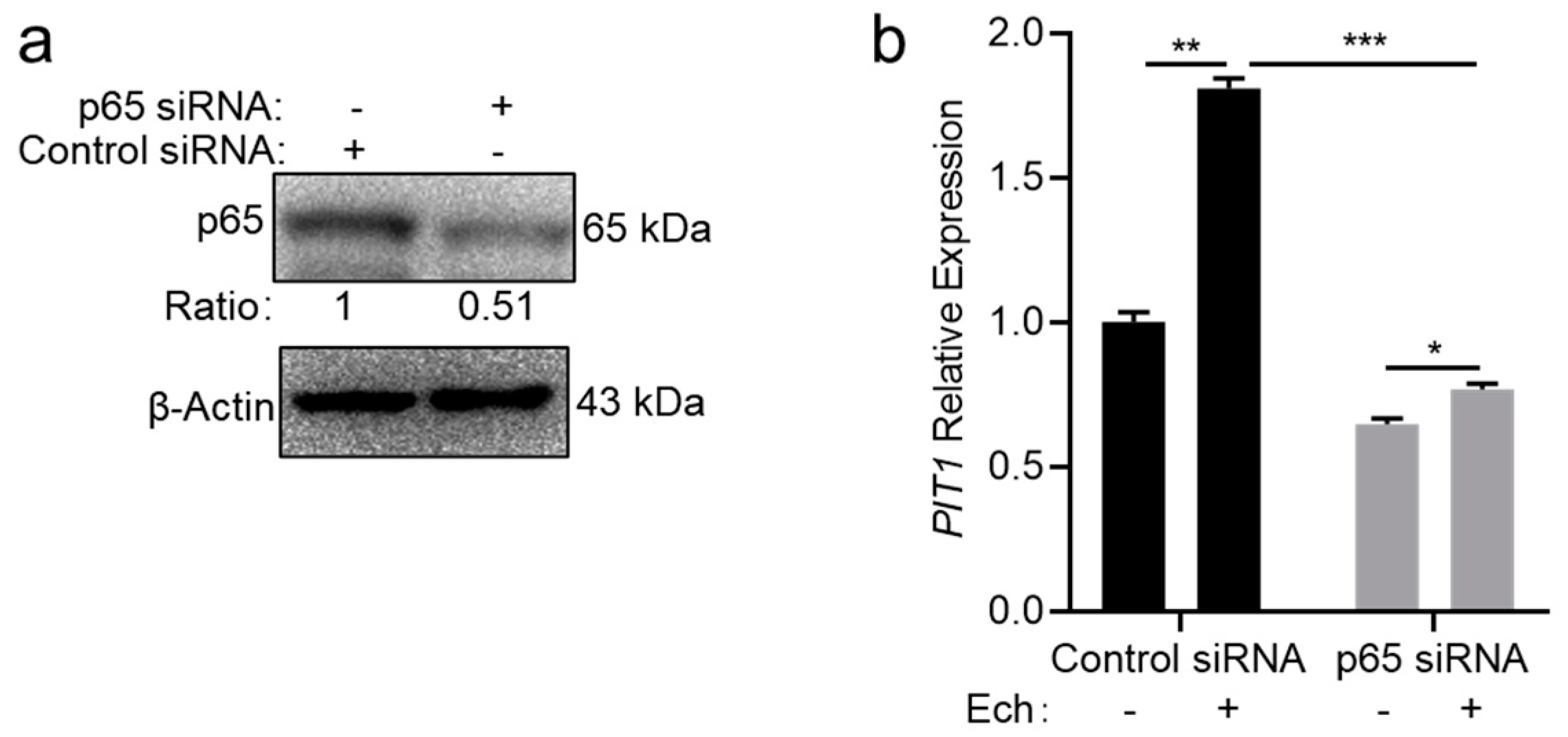
2.4. Host Cells Upregulate the PIT1 Expression in an NF-κB-Dependent Manner
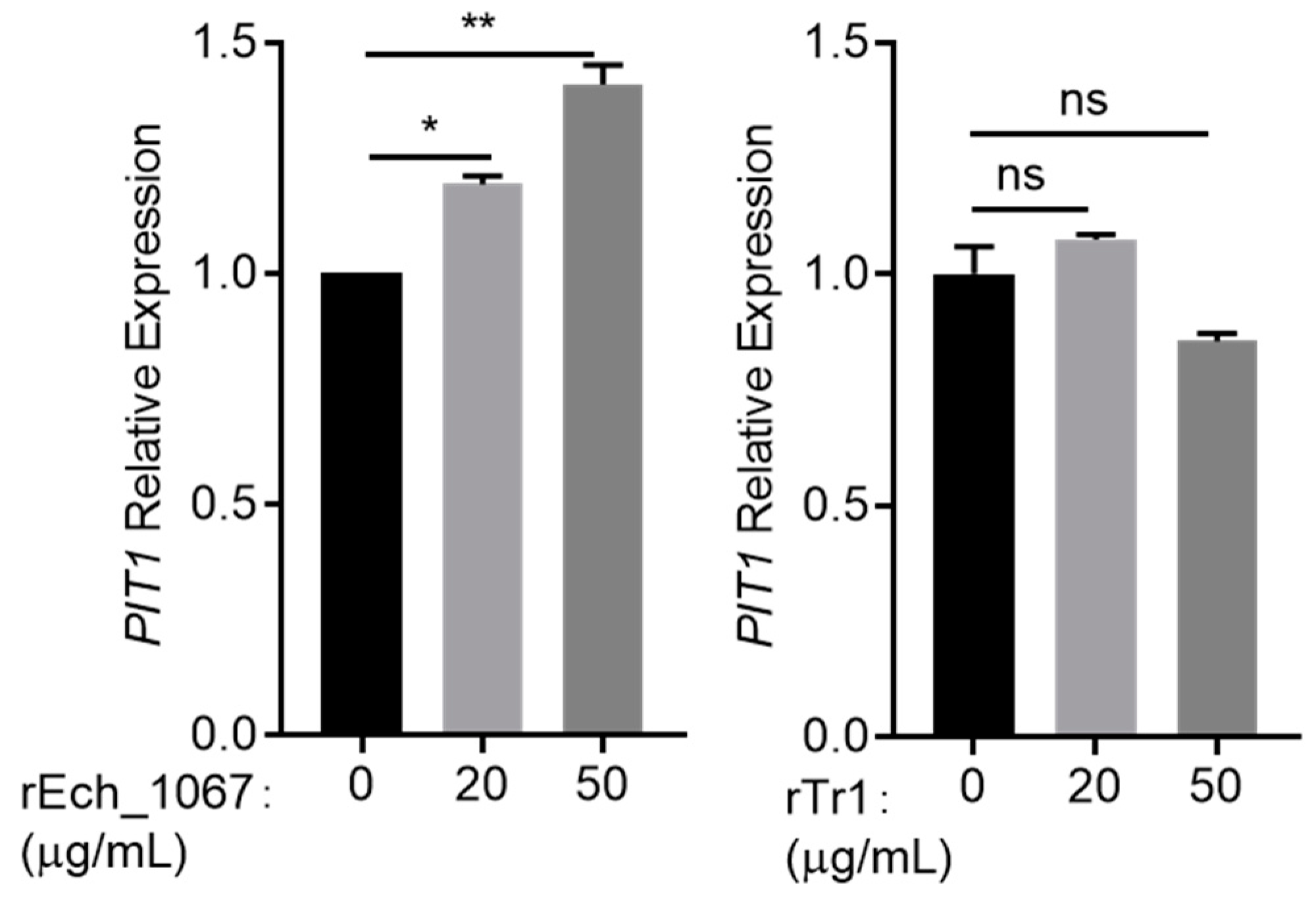
2.5. Host Cells Recognize E. chaffeensis Ech_1067 to Upregulate the PIT1 Expression
3. Discussion
4. Materials and Methods
4.1. Bacterial and Cell Culture, Plasmids, and Primers
4.2. Isolation of Host Cell-Free E. chaffeensis
4.3. Immunofluorescence Microscopy
4.4. Expression and Purification of Recombinant Proteins
4.5. Endotoxin Removal and Assay
4.6. Transfection and Stimulation of THP-1 Cells
4.7. Quantitative RT-PCR
4.8. Western Blotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rikihisa, Y. Molecular pathogenesis of Ehrlichia chaffeensis infection. Annu. Rev. Microbiol. 2015, 69, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.E.; Sims, K.G.; Olson, J.G.; Childs, J.E.; Piesman, J.F.; Happ, C.M.; Maupin, G.O.; Johnson, B.J. Amblyomma americanum: A potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 1993, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ewing, S.A.; Dawson, J.E.; Kocan, A.A.; Barker, R.W.; Warner, C.K.; Panciera, R.J.; Fox, J.C.; Kocan, K.M.; Blouin, E.F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 1995, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, J.M.; Davidson, W.R.; Stallknecht, D.E.; Dawson, J.E.; Howerth, E.W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 1997, 35, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A prototypical emerging pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Dumler, J.S. Emergence of the ehrlichioses as human health problems. Emerg. Infect. Dis. 1996, 2, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: Subversive manipulators of host cells. Nat. Rev. Microbiol. 2010, 8, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Barnewall, R.E.; Rikihisa, Y.; Lee, E.H. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect. Immun. 1997, 65, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.; Barnewall, R.E.; Rikihisa, Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 1999, 67, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Clemente, T.M.; Angara, R.K.; Gilk, S.D. Establishing the intracellular niche of obligate intracellular vacuolar pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1206037. [Google Scholar] [CrossRef]
- Rikihisa, Y. The “Biological Weapons” of Ehrlichia chaffeensis: Novel Molecules and Mechanisms to Subjugate Host Cells. Front. Cell. Infect. Microbiol. 2021, 11, 830180. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, M.; Qin, S.; Duan, N.; Li, X.; Zhou, Y.; Wang, M.; Jin, Y.; Wu, W.; Cheng, Z. Ehrlichia chaffeensis Etf-3 Induces Host RAB15 Upregulation for Bacterial Intracellular Growth. Int. J. Mol. Sci. 2024, 25, 2551. [Google Scholar] [CrossRef]
- Michigami, T. Extracellular phosphate as a signaling molecule. Contrib. Nephrol. 2013, 180, 14–24. [Google Scholar]
- Wagner, C.A. The basics of phosphate metabolism. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2024, 39, 190–201. [Google Scholar] [CrossRef]
- Wagner, C.A.; Hernando, N.; Forster, I.C.; Biber, J. The SLC34 family of sodium-dependent phosphate transporters. Pflug. Arch. Eur. J. Physiol. 2014, 466, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, Y.; Yan, J.; Kang, C.; Yao, T.; Sun, H.; Cheng, Z. Phosphate (Pi) Transporter PIT1 Induces Pi Starvation in Salmonella-Containing Vacuole in HeLa Cells. Int. J. Mol. Sci. 2023, 24, 17216. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cheng, Z.; Zhang, S.; Li, S.; Li, X.; Li, X.; Zhang, X.; Li, X.; Feng, Y.; Cui, H.; et al. Bladder epithelial cell phosphate transporter inhibition protects mice against uropathogenic Escherichia coli infection. Cell Rep. 2022, 39, 110698. [Google Scholar] [CrossRef]
- Wakeel, A.; Zhu, B.; Yu, X.J.; McBride, J.W. New insights into molecular Ehrlichia chaffeensis-host interactions. Microbes Infect. 2010, 12, 337–345. [Google Scholar] [CrossRef]
- Kumar, D.M.; Lin, M.; Xiong, Q.; Webber, M.J.; Kural, C.; Rikihisa, Y. EtpE Binding to DNase X Induces Ehrlichial Entry via CD147 and hnRNP-K Recruitment, Followed by Mobilization of N-WASP and Actin. mBio 2015, 6, e01541-15. [Google Scholar] [CrossRef]
- Kumar, D.M.; Yamaguchi, M.; Miura, K.; Lin, M.; Los, M.; Coy, J.F.; Rikihisa, Y. Ehrlichia chaffeensis Uses Its Surface Protein EtpE to Bind GPI-Anchored Protein DNase X and Trigger Entry into Mammalian Cells. PLoS Pathog. 2013, 9, e1003666. [Google Scholar]
- Koumakis, E.; Millet-Botti, J.; Benna, J.E.; Leroy, C.; Boitez, V.; Codogno, P.; Friedlander, G.; Forand, A. Novel function of PiT1/SLC20A1 in LPS-related inflammation and wound healing. Sci. Rep. 2019, 9, 1808. [Google Scholar] [CrossRef]
- Dunning Hotopp, J.C.; Lin, M.; Madupu, R.; Crabtree, J.; Angiuoli, S.V.; Eisen, J.A.; Seshadri, R.; Ren, Q.; Wu, M.; Utterback, T.R.; et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006, 2, e21. [Google Scholar]
- Lin, M.; Rikihisa, Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 2003, 71, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Cheng, Z.; Matsuo, J.; Rikihisa, Y. Penicillin-binding protein of Ehrlichia chaffeensis: Cytokine induction through MyD88-dependent pathway. J. Infect. Dis. 2012, 206, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Arciola, C.R.; Rindi, S.; Di Poto, A.; Missineo, A.; Montanaro, L.; Speziale, P. Toll-like receptors (TLRs) in innate immune defense against Staphylococcus aureus. Int. J. Artif. Organs 2011, 34, 799–810. [Google Scholar] [CrossRef]
- Duan, N.; Ma, X.; Cui, H.; Wang, Z.; Chai, Z.; Yan, J.; Li, X.; Feng, Y.; Cao, Y.; Jin, Y.; et al. Insights into the mechanism regulating the differential expression of the P28-OMP outer membrane proteins in obligatory intracellular pathogen Ehrlichia chaffeensis. Emerg. Microbes Infect. 2021, 10, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 2013, 34, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Rikihisa, Y. Surface-exposed proteins of Ehrlichia chaffeensis. Infect. Immun. 2007, 75, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.L.; Yu, X.; Walker, D.H. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: Preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 2000, 28, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Byerly, C.D.; Patterson, L.L.; McBride, J.W. Ehrlichia TRP effectors: Moonlighting, mimicry and infection. Pathog. Dis. 2021, 79, ftab026. [Google Scholar] [CrossRef]
- Patterson, L.L.; Byerly, C.D.; Solomon, R.; Pittner, N.; Bui, D.C.; Patel, J.; McBride, J.W. Ehrlichia Notch signaling induction promotes XIAP stability and inhibits apoptosis. Infect. Immun. 2023, 91, e0000223. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.L.; Velayutham, T.S.; Byerly, C.D.; Bui, D.C.; Patel, J.; Veljkovic, V.; Paessler, S.; McBride, J.W. Ehrlichia SLiM ligand mimetic activates Notch signaling in human monocytes. mBio 2022, 13, e0007622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yu, K.; Qi, L.; Liu, Y.; Cheng, S.; Wu, M.; Wang, Z.; Fu, J.; Liu, X. A proteomic view of Salmonella Typhimurium in response to phosphate limitation. Proteomes 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Lin, M.; Huang, W.; Teymournejad, O.; Johnson, J.M.; Hays, F.A.; Liang, Z.; Li, G.; Rikihisa, Y. Ehrlichia type IV secretion system effector Etf-2 binds to active RAB5 and delays endosome maturation. Proc. Natl. Acad. Sci. USA 2018, 115, E8977–E8986. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yang, N.; Li, X.; Duan, N.; Qin, S.; Wang, M.; Zhou, Y.; Jin, Y.; Wu, W.; Jin, S.; et al. Host Cells Upregulate Phosphate Transporter PIT1 to Inhibit Ehrlichia chaffeensis Intracellular Growth. Int. J. Mol. Sci. 2024, 25, 7895. https://doi.org/10.3390/ijms25147895
Li M, Yang N, Li X, Duan N, Qin S, Wang M, Zhou Y, Jin Y, Wu W, Jin S, et al. Host Cells Upregulate Phosphate Transporter PIT1 to Inhibit Ehrlichia chaffeensis Intracellular Growth. International Journal of Molecular Sciences. 2024; 25(14):7895. https://doi.org/10.3390/ijms25147895
Chicago/Turabian StyleLi, Meifang, Nan Yang, Xiaoxiao Li, Nan Duan, Shanhua Qin, Mengyao Wang, Yuhong Zhou, Yongxin Jin, Weihui Wu, Shouguang Jin, and et al. 2024. "Host Cells Upregulate Phosphate Transporter PIT1 to Inhibit Ehrlichia chaffeensis Intracellular Growth" International Journal of Molecular Sciences 25, no. 14: 7895. https://doi.org/10.3390/ijms25147895




