TRPA1, TRPV1, and Caffeine: Pain and Analgesia
Abstract
:1. Introduction
2. Methodology
3. TRP Channel Overview
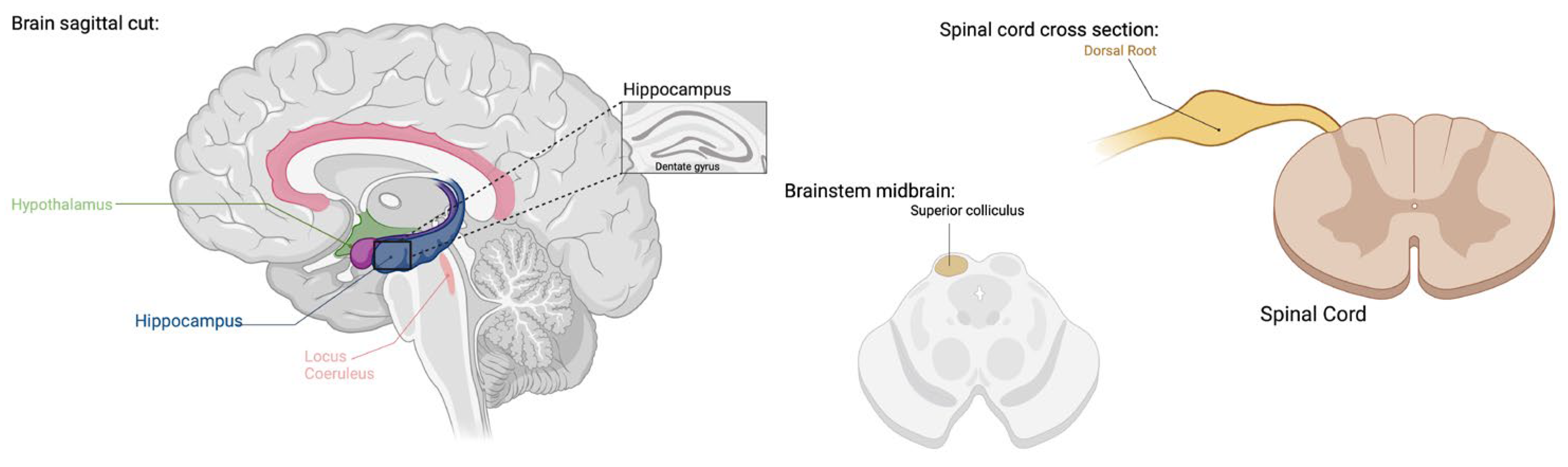
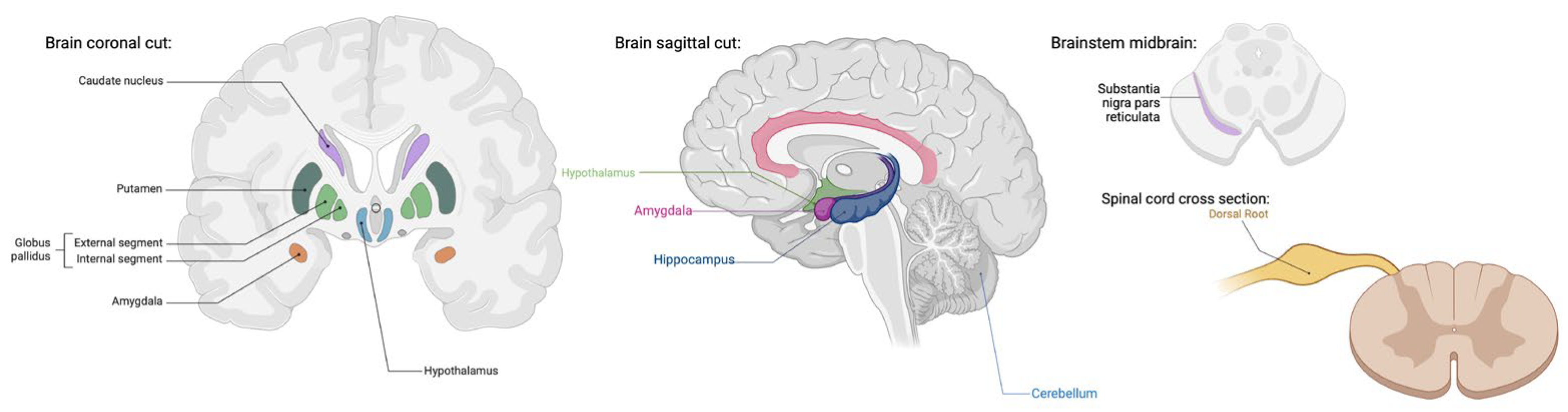
3.1. TRPV1 and TRPA1: Central Nervous System (CNS) Distribution
3.2. TRPV1 and TRPA1: Structure and Function in Pain
4. Caffeine in Analgesia
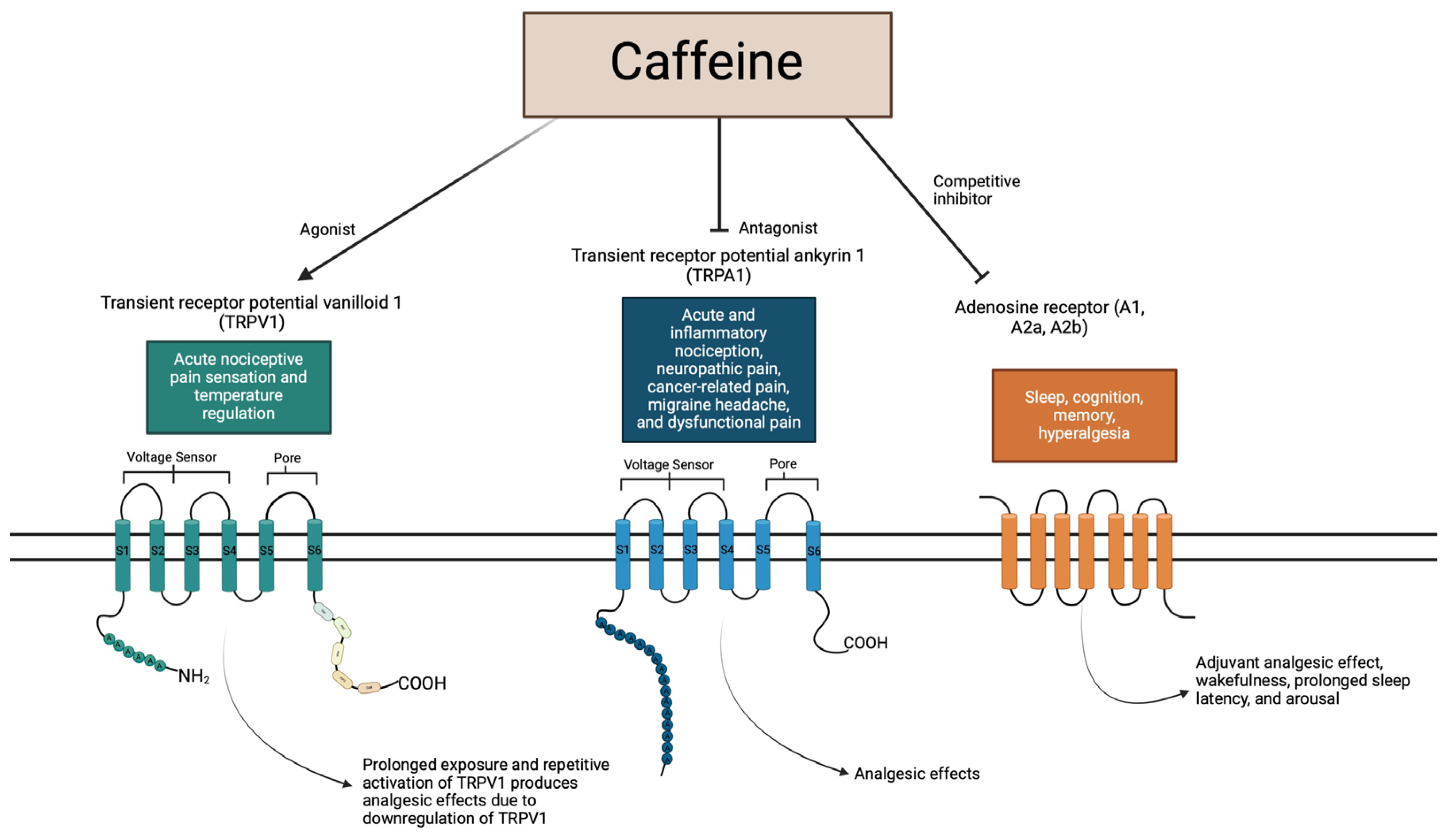
4.1. Mechanism of Action
4.2. Caffeine: Adjuvant Analgesia
4.3. Caffeine: Surgical Uses
5. TRP and Caffeine
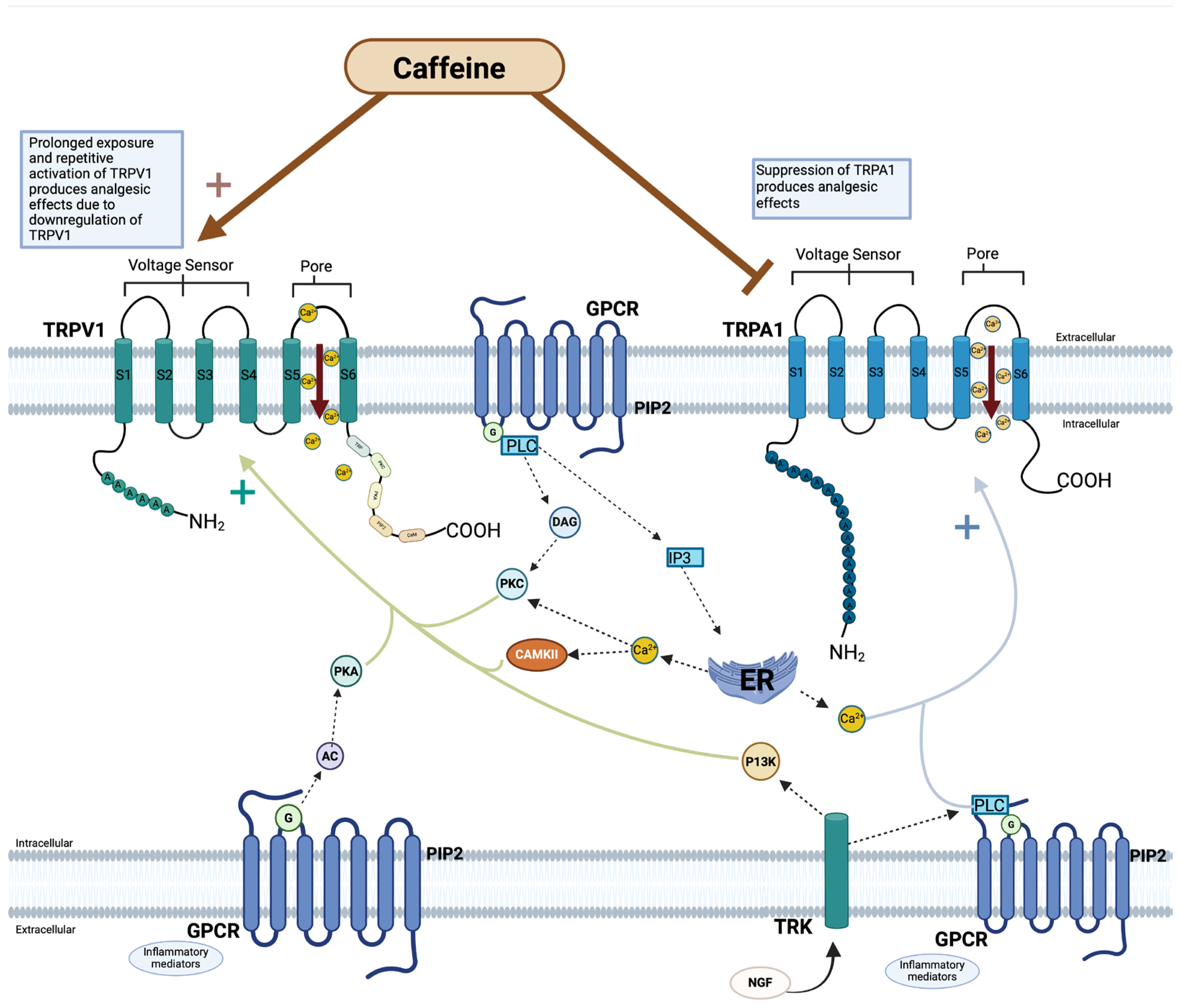
5.1. TRPV1 and Caffeine
5.2. TRPA1 and Caffeine
6. Discussion
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Cao, E. Structural mechanisms of transient receptor potential ion channels. J. Gen. Physiol. 2020, 152, e201811998. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, U.A.; Gaudet, R. Structural biology of TRP channels. Handb. Exp. Pharmacol. 2014, 223, 963–990. [Google Scholar] [PubMed]
- Duitama, M.; Moreno, Y.; Santander, S.P.; Casas, Z.; Sutachan, J.J.; Torres, Y.P.; Albarracín, S.L. TRP Channels as Molecular Targets to Relieve Cancer Pain. Biomolecules 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Yang, J. Structural biology of TRP channels. Adv. Exp. Med. Biol. 2011, 704, 1–23. [Google Scholar] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. Trp (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Duggan, A.; Kumar, G.; García-Añoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B. Transient receptor potential (TRP) channels in The brain: The good and the ugly. Eur. Rev. 2012, 20, 343–355. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.S.; Lee, S.Y.; Hwang, S.W.; Koo, J.; Cho, H.; Oh, U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004, 279, 7048–7054. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.A.; Ahern, G.P. Voltage is a partial activator of rat thermosensitive TRP channels. J. Physiol. 2007, 585, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [PubMed]
- Wu, H.H.; Meng, T.T.; Chen, J.M.; Meng, F.L.; Wang, S.Y.; Liu, R.H.; Chen, J.N.; Ning, B.; Li, Y.; Su, G.H. Asenapine maleate inhibits angiotensin II-induced proliferation and activation of cardiac fibroblasts via the ROS/TGFβ1/MAPK signaling pathway. Biochem. Biophys. Res. Commun. 2021, 553, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capó-Aponte, J.E.; Pleyer, U.; et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Li, M.; Zhou, X. Transient Receptor Potential Melastatin 8 (TRPM8)-Based Mechanisms Underlie Both the Cold Temperature-Induced Inflammatory Reactions and the Synergistic Effect of Cigarette Smoke in Human Bronchial Epithelial (16HBE) Cells. Front. Physiol. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kim, C.Y.; Zheng, C.; Jin, S.W.; Kim, J.Y.; Lee, S.Y.; Kim, M.Y.; Han, E.H.; Hwang, Y.P.; Jeong, H.G. Rutaecarpine Increases Nitric Oxide Synthesis via eNOS Phosphorylation by TRPV1-Dependent CaMKII and CaMKKβ/AMPK Signaling Pathway in Human Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 9407. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.; Li, C.X.; Feng, J.; Cicchetti, M.; Yue, L. TRP Channels in Stroke. Neurosci. Bull. 2023; published online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.S.; Bernardes, L.B.; Trevisan, G. TRP channels in cancer pain. Eur. J. Pharmacol. 2021, 904, 174185. [Google Scholar] [CrossRef] [PubMed]
- Shirolkar, P.; Mishra, S.K. Role of TRP ion channels in pruritus. Neurosci. Lett. 2022, 768, 136379. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gu, L.; Chen, M.; Zheng, Y.; Xiong, X.; Zhu, S. Novel Targets for Stroke Therapy: Special Focus on TRPC Channels and TRPC6. Front. Aging Neurosci. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jones, B.; Korchev, Y.; Bloom, S.R.; Pacchetti, B.; Anand, P.; Sodergren, M.H. CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons. J. Pain. Res. 2020, 13, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.-J.; Guo, W.; Zheng, D.-H.; Zhang, C.-Q.; Li, S.; Liu, S.-Y.; Yin, Q.; Yang, H.; Shu, H.-F. Increased Expression of TRPV1 in the Cortex and Hippocampus from Patients with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2013, 49, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Steinlein, O.K. Calcium Signaling and Epilepsy. Cell Tissue Res. 2014, 357, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals 2016, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Shalygin, A.; Gudermann, T.; Chubanov, V.; Dietrich, A. TRPM2 channels are essential for regulation of cytokine production in lung interstitial macrophages. J. Cell Physiol. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Körtési, T.; Vécsei, L. TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine. Int. J. Mol. Sci. 2022, 24, 700. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.W.; Ward, N.J.; Calkins, D.J. TRPV1: A stress response protein in the central nervous system. Am. J. Neurodegener. Dis. 2012, 1, 1–14. [Google Scholar] [PubMed]
- Roberts, J.C.; Davis, J.B.; Benham, C.D. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004, 995, 176–183. [Google Scholar] [CrossRef]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, X.L.; Kong, W.L.; Zeng, M.L.; Shao, L.; Jiang, G.T.; Cheng, J.J.; Kong, S.; He, X.H.; Lin, W.H.; et al. TRPV1 translocated to astrocytic membrane to promote migration and inflammatory infiltration thus promotes epilepsy after hypoxic ischemia in immature brain. J. Neuroinflammation 2019, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Tecuapetla, C.; Cuellar-Herrera, M.; Luna-Munguia, H. Insights into Potential Targets for Therapeutic Intervention in Epilepsy. Int. J. Mol. Sci. 2020, 21, 8573. [Google Scholar] [CrossRef]
- Anand, U.; Otto, W.R.; Casula, M.A.; Day, N.C.; Davis, J.B.; Bountra, C.; Birch, R.; Anand, P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci. Lett. 2006, 399, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Menigoz, A.; Boudes, M. The Expression Pattern of TRPV1 in Brain. J. Neurosci. 2011, 31, 13025–13027. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Boczán, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Edes, I.; Csiba, L.; Blumberg, P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Gold-Binder, M.; Ciotu, C.I.; Witek, M.; Ninidze, N.; Kress, H.G.; Fischer, M.J. A Human TRPA1-Specific Pain Model. J. Neurosci. 2019, 39, 3845–3855. [Google Scholar] [CrossRef] [PubMed]
- Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Otto, W.R.; Facer, P.; Zebda, N.; Selmer, I.; Gunthorpe, M.J.; Chessell, I.P.; Sinisi, M.; Birch, R.; Anand, P. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci. Lett. 2008, 438, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Hwang, S.W. Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception. Biomol. Ther. 2018, 26, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert. Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- McEntire, D.M.; Kirkpatrick, D.R.; Dueck, N.P.; Kerfeld, M.J.; Smith, T.A.; Nelson, T.J.; Reisbig, M.D.; Agrawal, D.K. Pain transduction: A pharmacologic perspective. Expert. Rev. Clin. Pharmacol. 2016, 9, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Ringkamp, M.; Dougherty, P.M.; Raja, S.N. Anatomy and Physiology of the Pain Signaling Process. In Essentials of Pain Medicine, 4th ed.; Benzon, H.T., Raja, S.N., Liu, S.S., Fishman, S.M., Cohen, S.P., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 3–10. [Google Scholar]
- Zheng, W.; Wen, H. Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2019, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Numazaki, M.; Tominaga, T.; Takeuchi, K.; Murayama, N.; Toyooka, H.; Tominaga, M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. USA 2003, 100, 8002–8006. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, Y.; Liu, L.; Ren, S.; Tian, Y.; Yang, F. Molecular mechanism underlying modulation of TRPV1 heat activation by polyols. J. Biol. Chem. 2021, 297, 100806. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond neuronal heat sensing: Diversity of TRPV1 heat-capsaicin receptor-channel functions. Front. Cell Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.; Rossaneis, A.; Pinho-Ribeiro, F.; Verri, W. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Gawalska, A.; Kołaczkowski, M.; Bucki, A. Structural Modeling of TRPA1 Ion Channel-Determination of the Binding Site for Antagonists. Molecules 2022, 27, 3077. [Google Scholar] [CrossRef] [PubMed]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. Anktm1, a Trp-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Sinica, V.; Moparthi, V.K.; Kreir, M.; Vignane, T.; Filipovic, M.R.; Vlachova, V.; Zygmunt, P.M. The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain. Nat. Commun. 2022, 13, 6113. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Cui, L.W.; Liu, Z.; Gao, Y.M.; Wang, S.; Li, H.; Liu, H.X.; Yu, L.J. Effects of TRPA1 activation and inhibition on TRPA1 and CGRP expression in dorsal root ganglion neurons. Neural Regen. Res. 2019, 14, 140–148. [Google Scholar] [PubMed]
- Vélez-Ortega, A.C.; Stepanyan, R.; Edelmann, S.E.; Torres-Gallego, S.; Park, C.; Marinkova, D.A.; Nowacki, J.S.; Sinha, G.P.; Frolenkov, G.I. TRPA1 activation in non-sensory supporting cells contributes to regulation of cochlear sensitivity after acoustic trauma. Nat. Commun. 2023, 14, 3871. [Google Scholar] [CrossRef] [PubMed]
- Deering-Rice, C.E.; Shapiro, D.; Romero, E.G.; Stockmann, C.; Bevans, T.S.; Phan, Q.M.; Stone, B.L.; Fassl, B.; Nkoy, F.; Uchida, D.A.; et al. Activation of Transient Receptor Potential Ankyrin-1 by Insoluble Particulate Material and Association with Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 53, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Habgood, M.; Seiferth, D.; Zaki, A.-M.; Alibay, I.; Biggin, P.C. Atomistic mechanisms of human TRPA1 activation by electrophile irritants through molecular dynamics simulation and mutual information analysis. Sci. Rep. 2022, 12, 4929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.; Ro, J.Y.; Chung, M.K. Warmth suppresses and desensitizes damage-sensing ion channel TRPA1. Mol. Pain. 2012, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kuwahara, K.; Kozai, D.; Sakaguchi, R.; Mori, Y. TRP Channels: Their Function and Potentiality as Drug Targets. In Innovative Medicine; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015; pp. 195–218. [Google Scholar]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J. Methylxanthines and pain. Methylxanthines 2010, 200, 311–329. [Google Scholar]
- Baratloo, A.; Rouhipour, A.; Forouzanfar, M.M.; Safari, S.; Amiri, M.; Negida, A. The Role of Caffeine in Pain Management: A Brief Literature Review. Anesth. Pain. Med. 2016, 6, e33193. [Google Scholar] [CrossRef] [PubMed]
- Do, H.N.; Akhter, S.; Miao, Y. Pathways and Mechanism of Caffeine Binding to Human Adenosine A2A Receptor. Front. Mol. Biosci. 2021, 8, 673170. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cordomí, A.; Llinas del Torrent, C.; Lillo, A.; Serrano-Marín, J.; Navarro, G.; Pardo, L. Structure and function of adenosine receptor heteromers. Cell Mol. Life Sci. 2021, 78, 3957–3968. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Sebastião, A.M. Caffeine and adenosine. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Deboer, T.; Landolt, H. Adenosine, caffeine, and sleep–wake regulation: State of the science and perspectives. J. Sleep. Res. 2022, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Porkka-Heiskanen, T.; Kalinchuk, A.V. Adenosine, energy metabolism and sleep homeostasis. Sleep. Med. Rev. 2011, 15, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zylka, M.J. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 2011, 17, 188–196. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Borea, P.A.; Varani, K. Targeting Adenosine Receptors: A Potential Pharmacological Avenue for Acute and Chronic Pain. Int. J. Mol. Sci. 2020, 21, 8710. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, Y.O.; Levine, J.D. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience 1990, 38, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Abo-Salem, O.M.; Hayallah, A.M.; Bilkei-Gorzo, A.; Filipek, B.; Zimmer, A.; Müller, C.E. Antinociceptive Effects of Novel A2B Adenosine Receptor Antagonists. J. Pharmacol. Exp. Ther. 2004, 308, 358–366. [Google Scholar] [CrossRef]
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014, 12, Cd009281. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Veiga-Herreros, P.; Sánchez-Oliver, A.J.; Montoya, J.J.; Ramos-Álvarez, J.J.; Miguel-Tobal, F.; Lago-Rodríguez, Á.; Jodra, P. Acute Effects of Caffeine Intake on Psychological Responses and High-Intensity Exercise Performance. Int. J. Environ. Res. Public Health 2021, 18, 584. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Lambert, T.; Kaye, R.J.; Gaignard, S.M.; Ragusa, J.; Wheat, S.; Moll, V.; Cornett, E.M.; Urman, R.D.; Kaye, A.D. Adjuvants in clinical regional anesthesia practice: A comprehensive review. Best. Pract. Res. Clin. Anaesthesiol. 2019, 33, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Weiser, T.; Richter, E. Efficacy and Safety of a Fixed-Dose Combination of Ibuprofen and Caffeine in the Management of Moderate to Severe Dental Pain after Third Molar Extraction. Eur. J. Pain 2017, 22, 28–38. [Google Scholar] [CrossRef]
- Laska, E.M.; Sunshine, A.; Mueller, F.; Elvers, W.B.; Siegel, C.; Rubin, A. Caffeine as an Analgesic Adjuvant. JAMA 1984, 251, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Diener, H.C.; Robbins, M.S.; Garas, S.Y.; Patel, K. Caffeine in the management of patients with headache. J. Headache Pain 2017, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, L.; Haroutiunian, S. Caffeine as Adjuvant Analgeticum for Treating Acute Pain. Ugeskr. Laeger 2013, 175, 2486–2488. [Google Scholar] [PubMed]
- Fong, R.; Wang, L. Caffeine Accelerates Emergence from Isoflurane Anesthesia in Humans. Anesthesiology 2018, 129, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. The TRP Superfamily of Cation Channels. Sci. STKE 2005, 272, re3. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.L.; Tordoff, M.G. The Taste of Caffeine. J. Caffeine Res. 2017, 7, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.H.; Mokkapatti, R.; Levitan, E.S. Effects of caffeine on intracellular calcium, calcium current and calcium-dependent potassium current in anterior pituitary GH3 cells. Pflug. Arch. 1994, 426, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Karhapää, L.; Törnquist, K. Effects of caffeine on the influx of extracellular calcium in GH4C1 pituitary cells. J. Cell Physiol. 1997, 171, 52–60. [Google Scholar] [CrossRef]
- Hagenacker, T.; Büsselberg, D. Modulation of intracellular calcium influences capsaicin-induced currents of TRPV-1and voltage-activated channel currents in Nociceptive neurones. J. Peripher. Nerv. Syst. 2007, 12, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Masuho, I.; Tateyama, M.; Saitoh, O. Characterization of bitter taste responses of intestinal STC-1 cells. Chem. Senses 2005, 30, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 2008, 105, 17373–17388. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 73–118. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Chen, Y.; Wang, C. β-arrestin-1: Bridging GPCRs to active TRP channels. Channels 2017, 11, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Simon, S. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, 3rd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bourinet, E.; Altier, C.; Hildebrand, M.E.; Trang, T.; Salter, M.W.; Zamponi, G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94, 81–140. [Google Scholar] [CrossRef] [PubMed]
- Janscó, G.; Kiraly, E.; Janscó-Gábor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar]
- Rosenberger, D.C.; Binzen, U.; Treede, R.-D.; Greffrath, W. The capsaicin receptor TRPV1 is the first line defense protecting from acute non damaging heat: A translational approach. J. Transl. Med. 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Salvador, L.; Andrés-Borderia, A.; Ferrer-Montiel, A.; Planells-Cases, R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 2012, 287, 19462–19471. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Ciotu, C.I.; Szallasi, A. The mysteries of capsaicin-sensitive afferents. Front. Physiol. 2020, 220, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Q.; Ma, S.; Wang, D.H. Selective ablation of TRPV1 by intrathecal injection of resiniferatoxin in rats increases renal sympathoexcitatory responses and salt sensitivity. Hypertens. Res. 2018, 41, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.P.; Gover, T.D.; Moreira, T.H.; Lopes, V.G.; Weinreich, D. The identification of a caffeine-induced Ca2+ influx pathway in rat primary sensory neurons. Mol. Cell Biochem. 2009, 327, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Cleland, K.; Chang, J.; Bukiya, A.N.; Dopico, A.M. Extra-endothelial TRPV1 channels participate in alcohol and caffeine actions on cerebral artery diameter. Alcohol 2018, 73, 45–55. [Google Scholar]
- Chen, J.; Hackos, D.H. TRPA1 as a drug target—Promise and challenges. Naunyn Schmiedebergs Arch. 2015, 388, 451–463. [Google Scholar] [CrossRef]
- Bianchi, B.R.; Zhang, X.F.; Reilly, R.M.; Kym, P.R.; Yao, B.B.; Chen, J. Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J. Pharmacol. Exp. Ther. 2012, 341, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.F.; Kort, M.E.; Huth, J.R.; Sun, C.; Miesbauer, L.J.; Cassar, S.C.; Neelands, T.; Scott, V.E.; Moreland, R.B.; et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008, 28, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Ishii, H.; Yamamoto, T.; Nakajo, K.; Kubo, Y. The met268pro mutation of mouse TRPA1 changes the effect of caffeine from activation to suppression. Biophys. J. 2010, 99, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, A. The adenosine story goes ionic: Cav2.1-type ca2+ channels identified as effectors of adenosine’s somnogenic actions. Sleep 2013, 36, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.O.; Miteva, A.S.; Gaidukov, A.E.; Balezina, O.P. The role of adenosine receptors and L-type calcium channels in the regulation of the mediator secretion in mouse motor synapses. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2015, 9, 318–328. [Google Scholar] [CrossRef]
- Tarifa, C.; Jiménez-Sábado, V.; Franco, R.; Montiel, J.; Guerra, J.; Ciruela, F.; Hove-Madsen, L. Expression and impact of adenosine A3 receptors on calcium homeostasis in human right atrium. Int. J. Mol. Sci. 2023, 24, 4404. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.W.; Torres, R.P.; Bezerra, T.F. Beneficial And Adverse Effects Of Caffeine Consumption On Human Body: A Comprehensive Review. Int. J. Sci. 2021, 16, 132. [Google Scholar]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wiffen, P.J.; Moore, A.R. Single Dose Oral Ibuprofen plus Caffeine for Acute Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2015, 7, CD011509. [Google Scholar]
- de Freitas, M.C.; Cholewa, J.M.; Gobbo, L.A.; de Oliveira, J.V.N.S.; Lira, F.S.; Rossi, F.E. Acute capsaicin supplementation improves 1,500-m running time-trial performance and rate of perceived exertion in physically active adults. J. Strength. Cond. Res. 2018, 32, 572–577. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.C.; Cholewa, J.M.; Panissa, V.L.G.; Toloi, G.G.; Netto, H.C.; de Freitas, C.Z.; Freire, R.V.; Lira, F.S.; Rossi, F.E. Acute capsaicin supplementation improved resistance exercise performance performed after a high-intensity intermittent running in resistance-trained men. J. Strength. Cond. Res. 2022, 36, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.B.; Gomes, P.L.C.; Silva, R.A.; Fonseca, I.; Fonseca, M.; Cruz, V.M.; Drummond, M.D. Acute caffeine and capsaicin supplementation and performance in resistance training. Motriz 2022, 28, e1021010121. [Google Scholar] [CrossRef]
- Salatto, R.W.; Arevalo, J.A.; Brown, L.E.; Wiersma, L.D.; Coburn, J.W. Caffeine’s effects on an upper-body resistance exercise workout. J. Strength. Cond. Res. 2020, 34, 1643–1648. [Google Scholar] [CrossRef]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflügers Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Güler, A.D.; Caterina, M.J. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 2008, 11, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Iadorola, M. Unilateral Periganglionic Resiniferatoxin for Personalized Pain Treatment. Pain. Med. 2021, 22, 767–768. [Google Scholar] [CrossRef]
- Manitpisitkul, P.; Flores, C.M.; Moyer, J.A.; Romano, G.; Shalayda, K.; Tatikola, K.; Hutchison, J.S.; Mayorga, A.J. A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain. 2018, 18, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Samieirad, S.; Afrasiabi, H.; Tohidi, E.; Qolizade, M.; Shaban, B.; Hashemipour, M.A. Evaluation of caffeine versus codeine for pain and swelling management after implant surgeries: A triple blind clinical trial. J. Cranio-Maxillofac. Surg. 2017, 45, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Björnsson, M.; Svensson, O.; Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 2013, 37, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, P.; Chiche, D.; Brown, W.; Miller, J.; Treister, R.; Leff, R.; Walker, P.; Katz, N. NEO6860, modality-selective TRPV1 antagonist: A randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018, 3, e696. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, C.; Silva, M.A.; Rossato, M.F.; Trevisan, G.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Ferreira, J. Participation of the TRPV1 receptor in the development of acute gout attacks. Rheumatology 2014, 53, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Edelmayer, R.M.; Le, L.N.; Yan, J.; Wei, X.; Nassini, R.; Materazzi, S.; Preti, D.; Appendino, G.; Geppetti, P.; Dodick, D.W.; et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012, 153, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Shatillo, A.; Koroleva, K.; Giniatullina, R.; Naumenko, N.; Slastnikova, A.A.; Aliev, R.R.; Bart, G.; Atalay, M.; Gu, C.; Khazipov, R.; et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 2013, 253, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hämäläinen, M.M.; Saarnilehto, M.; Koivisto, A.; Pertovaara, A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology 2009, 111, 147–154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puthumana, E.A.; Muhamad, L.; Young, L.A.; Chu, X.-P. TRPA1, TRPV1, and Caffeine: Pain and Analgesia. Int. J. Mol. Sci. 2024, 25, 7903. https://doi.org/10.3390/ijms25147903
Puthumana EA, Muhamad L, Young LA, Chu X-P. TRPA1, TRPV1, and Caffeine: Pain and Analgesia. International Journal of Molecular Sciences. 2024; 25(14):7903. https://doi.org/10.3390/ijms25147903
Chicago/Turabian StylePuthumana, Elizabeth A., Luna Muhamad, Lexi A. Young, and Xiang-Ping Chu. 2024. "TRPA1, TRPV1, and Caffeine: Pain and Analgesia" International Journal of Molecular Sciences 25, no. 14: 7903. https://doi.org/10.3390/ijms25147903





