Difamilast, a Topical Phosphodiesterase 4 Inhibitor, Produces Soluble ST2 via the AHR–NRF2 Axis in Human Keratinocytes
Abstract
1. Introduction
2. Results
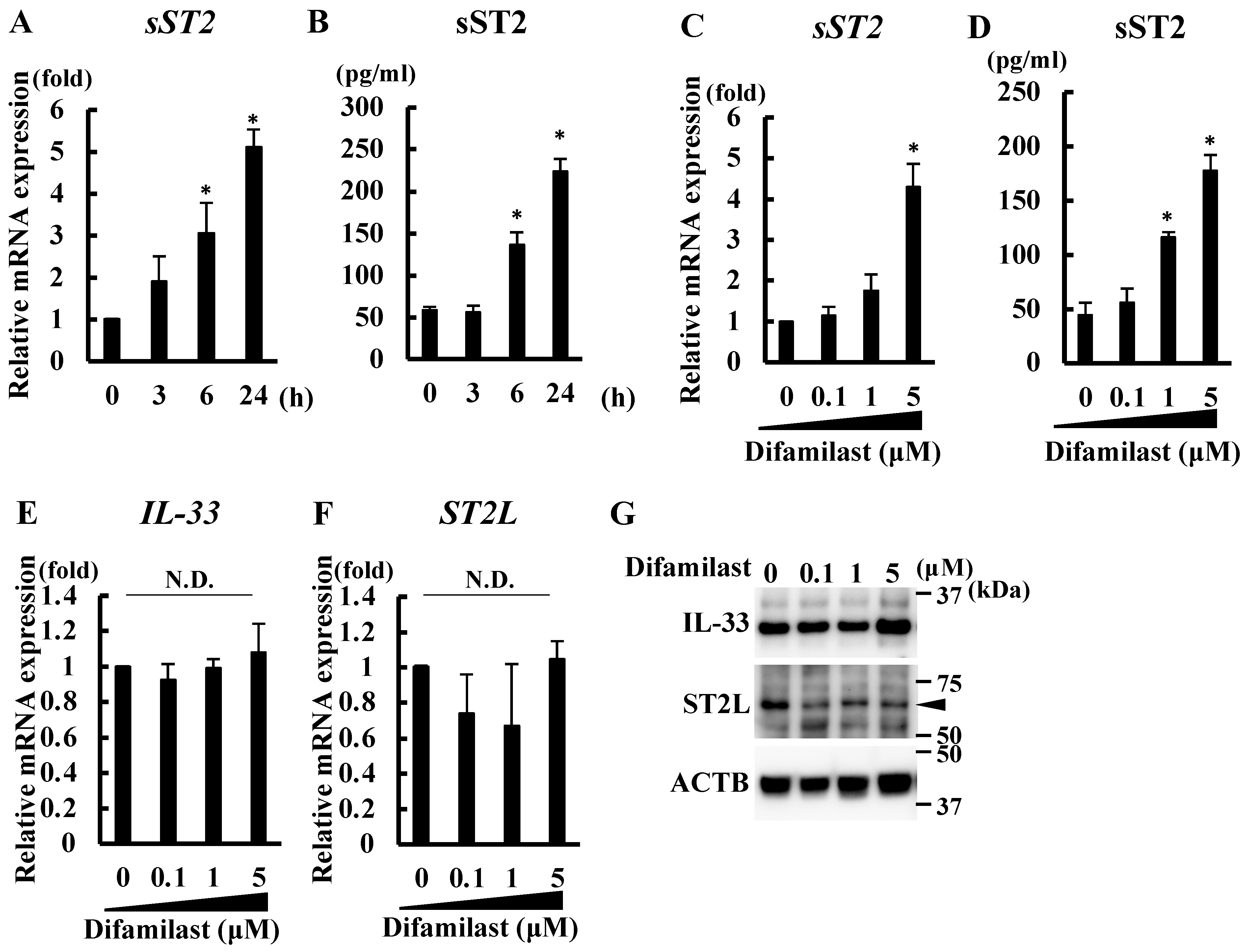
2.1. Difamilast Treatment Produced sST2 in NHEKs
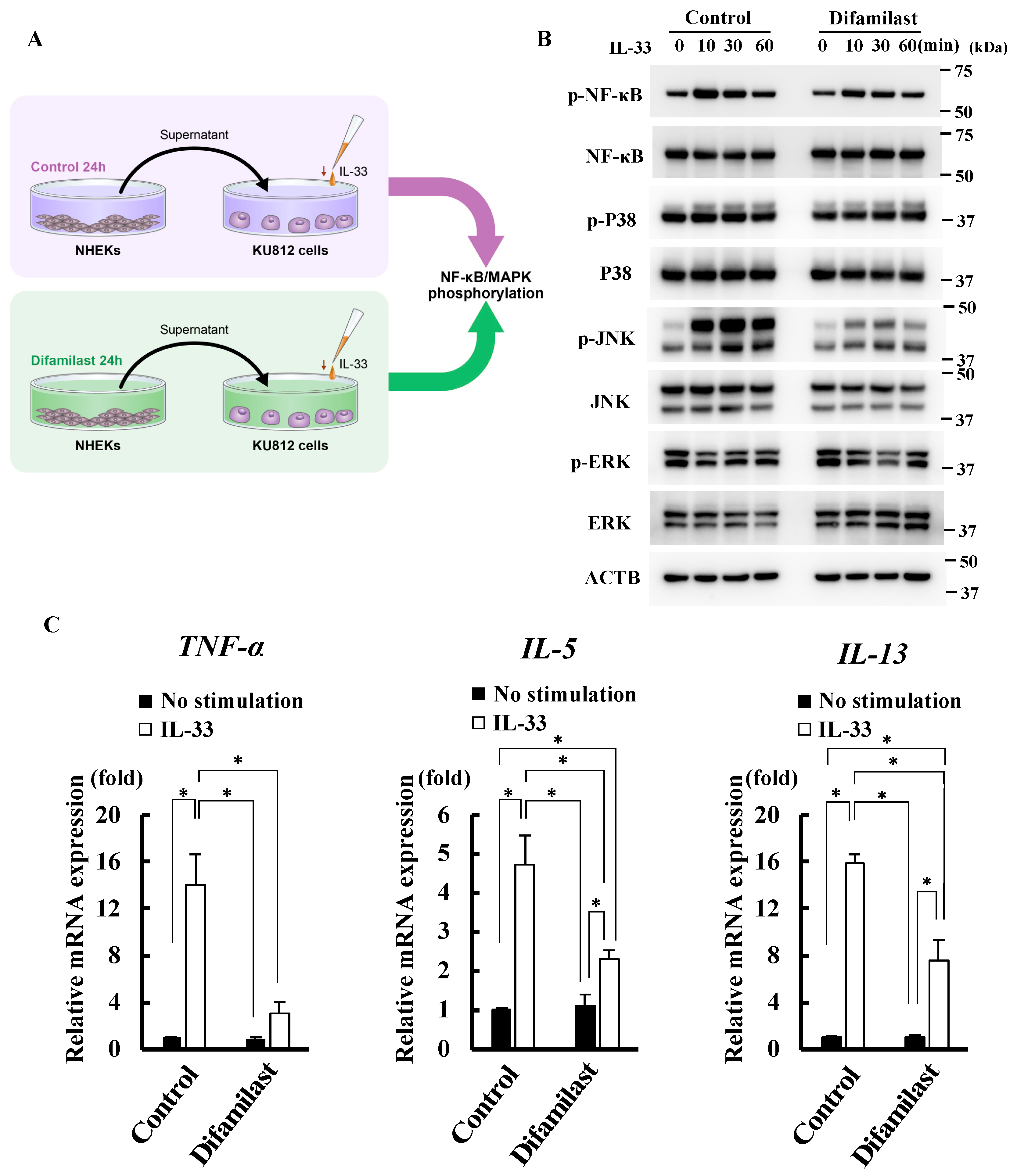
2.2. Difamilast Treatment Inhibited IL-33 Activity via sST2 Production by NHEKs
2.3. Difamilast Treatment Inhibited IL-33 Activity via sST2 Production by NHEKs
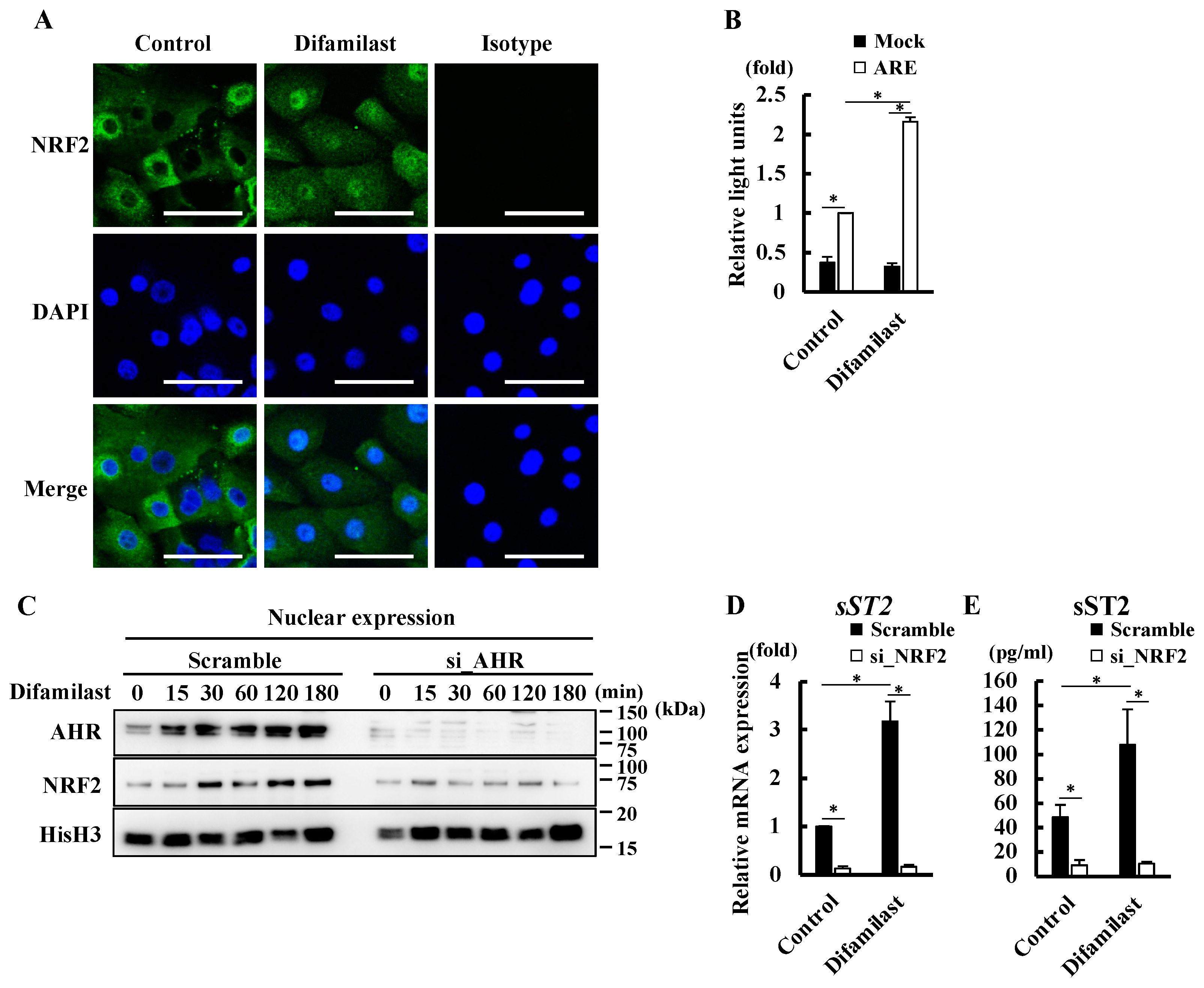
2.4. Difamilast Treatment Produced sST2 via the AHR–NRF2 Axis in NHEKs
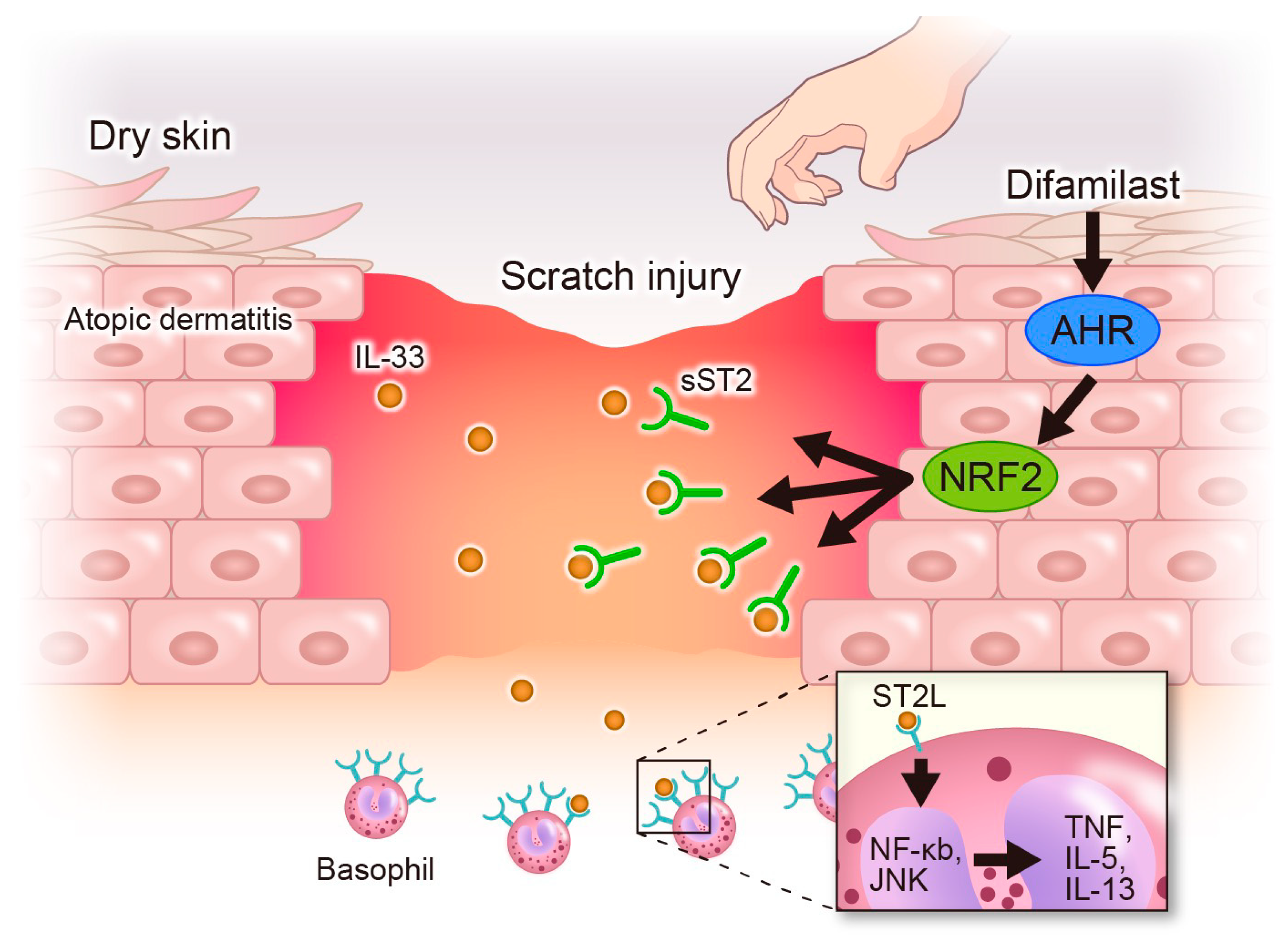
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Stimulation of KU812 Cells
4.6. Small Interfering RNA (siRNA) Transfection
4.7. Protein Extraction
4.8. Western Blotting
4.9. Luciferase Assay for Antioxidant Response Element (ARE) Activity
4.10. Analysis of Reactive Oxygen Species (ROS) Production
4.11. Immunohistochemical Analysis
4.12. Co-IP Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation; barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Samynathan, A.; Fishbein, A.B.; Abbott, S.M.; Booster, G.D.; Zee, P.C.; Sheldon, S.H.; Yosipovitch, G.; Silverberg, J.I. Assessment and management of sleep disturbances in atopic dermatitis: A review. Dermatitis 2024, 35, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Takemoto, S.; Houzawa, H.; Nakayama, M. Desire for alternative treatment options in patients with atopic dermatitis in Japan: Results of a web-based cross-sectional study (AD-JOIN Study). Dermatol. Ther. 2022, 12, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 2013, 70, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Yumine, A.; Takemura, M.; Kido-Nakahara, M.; Ito, T.; Yamamura, K.; Nakahara, T. PDE4 inhibition by difamilast regulates filaggrin and loricrin expression via keratinocyte proline-rich protein in human keratinocytes. J. Dermatol. Sci. 2023, 110, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P.H.; Adams, M.; Horan, G.; Truzzi, F.; Marconi, A.; Pincelli, C. Apremilast normalizes gene expression of inflammatory mediators in human keratinocytes and reduces antigen-induced atopic dermatitis in mice. Drugs R D 2019, 19, 329–338. [Google Scholar] [CrossRef]
- Takahashi, K.; Miyake, K.; Ito, J.; Shimamura, H.; Suenaga, T.; Karasuyama, H.; Ohashi, K. Topical application of a PDE4 inhibitor ameliorates atopic dermatitis through inhibition of basophil IL-4 production. J. Investig. Dermatol. 2024, 144, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Imamura, T.; Yokota, D.; Tsubouchi, H. Difamilast ointment in Japanese adult and pediatric patients with atopic dermatitis: A phase III, long-term, open-label study. Dermatol. Ther. 2022, 12, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Oshio, T.; Komine, M.; Tsuda, H.; Tominaga, S.-I.; Saito, H.; Nakae, S.; Ohtsuki, M. Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J. Dermatol. Sci. 2017, 85, 106–114. [Google Scholar] [CrossRef]
- Luo, C.H.; Lai, A.C.Y.; Tsai, C.C.; Chen, W.Y.; Chang, Y.S.; Chung, E.J.C.; Chang, Y.J. Staphylococcus aureus exacerbates dermal IL-33-ILC2 axis activation through evoking RIPK3/MLKL-mediated necroptosis of dry skin. JCI Insight 2024, 9, e166821. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Duffen, J.L.; Nocka, K.H.; Kasaian, M.T. IL-13 controls IL-33 activity through modulation of ST2. J. Immunol. 2021, 207, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, H.; Hayakawa, M.; Tominaga, S.I. Soluble ST2 suppresses the effect of interleukin-33 on lung type 2 innate lymphoid cells. Biochem. Biophys. Rep. 2016, 5, 401–407. [Google Scholar] [CrossRef][Green Version]
- Hayakawa, H.; Hayakawa, M.; Kume, A.; Tominaga, S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 2007, 282, 26369–26380. [Google Scholar] [CrossRef]
- Lee, H.Y.; Rhee, C.K.; Kang, J.Y.; Byun, J.H.; Choi, J.Y.; Kim, S.J.; Kim, Y.K.; Kwon, S.S.; Lee, S.Y. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp. Lung Res. 2014, 40, 66–76. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Tsuji, G.; Yamamura, K.; Kawamura, K.; Kido-Nakahara, M.; Ito, T.; Nakahara, T. Regulatory mechanism of the IL-33-IL-37 axis via aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2023, 24, 14633. [Google Scholar] [CrossRef]
- Trier, A.M.; Mack, M.R.; Fredman, A.; Tamari, M.; Heul, A.M.V.; Zhao, Y.; Guo, C.J.; Avraham, O.; Ford, Z.K.; Oetjen, L.K.; et al. IL-33 signaling in sensory neurons promotes dry skin itch. J. Allergy Clin. Immunol. 2022, 149, 1473–1480.e6. [Google Scholar] [CrossRef]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef]
- Castex-Rizzi, N.; Galliano, M.F.; Aries, M.F.; Hernandez-Pigeon, H.; Vaissiere, C.; Delga, H.; Caruana, A.; Carrasco, C.; Lévêque, M.; Duplan, H.; et al. In vitro approaches to pharmacological screening in the field of atopic dermatitis. Br. J. Dermatol. 2014, 170 (Suppl. S1), 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pecaric-Petkovic, T.; Didichenko, S.A.; Kaempfer, S.; Spiegl, N.; Dahinden, C.A. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 2009, 113, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Tare, N.; Li, H.; Morschauser, A.; Cote-Sierra, J.; Ju, G.; Renzetti, L.; Lin, T.A. KU812 cells provide a novel in vitro model of the human IL-33/ST2L axis: Functional responses and identification of signaling pathways. Exp. Cell Res. 2010, 316, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Suzukawa, M.; Iikura, M.; Koketsu, R.; Nagase, H.; Tamura, C.; Komiya, A.; Nakae, S.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 2008, 181, 5981–5989. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Xie, Y.; Nie, S.; Chen, B.; Wu, Z. Roflumilast enhances the melanogenesis and attenuates oxidative stress-triggered damage in melanocytes. J. Dermatol. Sci. 2023, 110, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Oesch-Bartlomowicz, B.; Huelster, A.; Wiss, O.; Antoniou-Lipfert, P.; Dietrich, C.; Arand, M.; Weiss, C.; Bockamp, E.; Oesch, F. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: Divergent signaling pathways. Proc. Natl. Acad. Sci. USA 2005, 102, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sogawa, K.; Fujii-Kuriyama, Y. Cooperative interaction between AhR.Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J. Biol. Chem. 1996, 271, 12310–12316. [Google Scholar] [CrossRef]
- Manzella, C.; Singhal, M.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep. 2018, 8, 6103. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef]
- Kim, D.-J.; Baek, S.-Y.; Park, M.-K.; Park, K.-S.; Lee, J.H.; Park, S.-H.; Kim, H.-Y.; Kwok, S.-K. Serum level of interleukin-33 and soluble ST2 and their association with disease activity in patients with Behcet’s disease. J. Korean Med. Sci. 2013, 28, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Mildner, M.; Storka, A.; Lichtenauer, M.; Mlitz, V.; Ghannadan, M.; Hoetzenecker, K.; Nickl, S.; Dome, B.; Tschachler, E.; Ankersmit, H.J. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc. Res. 2010, 87, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Serrels, B.; McGivern, N.; Canel, M.; Byron, A.; Johnson, S.C.; McSorley, H.J.; Quinn, N.; Taggart, D.; Von Kreigsheim, A.; Anderton, S.M.; et al. IL-33 and ST2 mediate FAK-dependent antitumor immune evasion through transcriptional networks. Sci. Signal. 2017, 10, eaan8355. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Bretheau, F.; Castellanos-Molina, A.; Bélanger, D.; Kusik, M.; Mailhot, B.; Boisvert, A.; Vallières, N.; Lessard, M.; Gunzer, M.; Liu, X.; et al. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat. Commun. 2022, 13, 5786. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y. The role of KEAP1-NRF2 system in atopic dermatitis and psoriasis. Antioxidants 2022, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.-M.; Vega-Mendoza, D.; Strakosha, M.; Deng, L.; Choi, S.; Miyake, K.; Karasuyama, H.; Chiu, I.M.; Phipatanakul, W.; Geha, R.S. Basophils are important for development of allergic skin inflammation. J. Allergy Clin. Immunol. 2024, 153, 1344–1354.e5. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; Van Drongelen, V.; Mulder, A.; Van Esch, J.; Scott, H.; Van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Greaves, M.W.; Schmelz, M. Itch. Lancet 2003, 9358, 690–694. [Google Scholar] [CrossRef]
- Molfino, N.A.; Gossage, D.; Kolbeck, R.; Parker, J.M.; Geba, G.P. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy 2012, 42, 712–737. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kouzaki, H.; Kikuoka, H.; Kato, T.; Tojima, I.; Shimizu, S.; Shimizu, T. Soluble ST2 suppresses IL-5 production by human basophilic KU812 cells, induced by epithelial cell-derived IL-33. Allergol. Int. 2018, 67S, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, D.; Limberg, M.M.; Gray, N.; Raap, U. Basophils in pruritic skin diseases. Front. Immunol. 2023, 14, 1213138. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.R.; Margulis, A.; Wood, N.; Goldman, S.J.; Kasaian, M.; Chaudhary, D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 2010, 59, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakamoto, K.; Inui, T.; Sada, M.; Chibana, K.; Miyaoka, C.; Yoshida, Y.; Aso, J.; Nunokawa, H.; Honda, K.; et al. Soluble ST2 enhances IL-33-induced neutrophilic and pro-type 2 inflammation in the lungs. Allergy 2022, 77, 3137–3141. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, G.; Yumine, A.; Kawamura, K.; Takemura, M.; Kido-Nakahara, M.; Yamamura, K.; Nakahara, T. Difamilast, a Topical Phosphodiesterase 4 Inhibitor, Produces Soluble ST2 via the AHR–NRF2 Axis in Human Keratinocytes. Int. J. Mol. Sci. 2024, 25, 7910. https://doi.org/10.3390/ijms25147910
Tsuji G, Yumine A, Kawamura K, Takemura M, Kido-Nakahara M, Yamamura K, Nakahara T. Difamilast, a Topical Phosphodiesterase 4 Inhibitor, Produces Soluble ST2 via the AHR–NRF2 Axis in Human Keratinocytes. International Journal of Molecular Sciences. 2024; 25(14):7910. https://doi.org/10.3390/ijms25147910
Chicago/Turabian StyleTsuji, Gaku, Ayako Yumine, Koji Kawamura, Masaki Takemura, Makiko Kido-Nakahara, Kazuhiko Yamamura, and Takeshi Nakahara. 2024. "Difamilast, a Topical Phosphodiesterase 4 Inhibitor, Produces Soluble ST2 via the AHR–NRF2 Axis in Human Keratinocytes" International Journal of Molecular Sciences 25, no. 14: 7910. https://doi.org/10.3390/ijms25147910
APA StyleTsuji, G., Yumine, A., Kawamura, K., Takemura, M., Kido-Nakahara, M., Yamamura, K., & Nakahara, T. (2024). Difamilast, a Topical Phosphodiesterase 4 Inhibitor, Produces Soluble ST2 via the AHR–NRF2 Axis in Human Keratinocytes. International Journal of Molecular Sciences, 25(14), 7910. https://doi.org/10.3390/ijms25147910





