Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of S. aureus-Induced Periprosthetic Joint Infection
Abstract
1. Introduction
2. Results
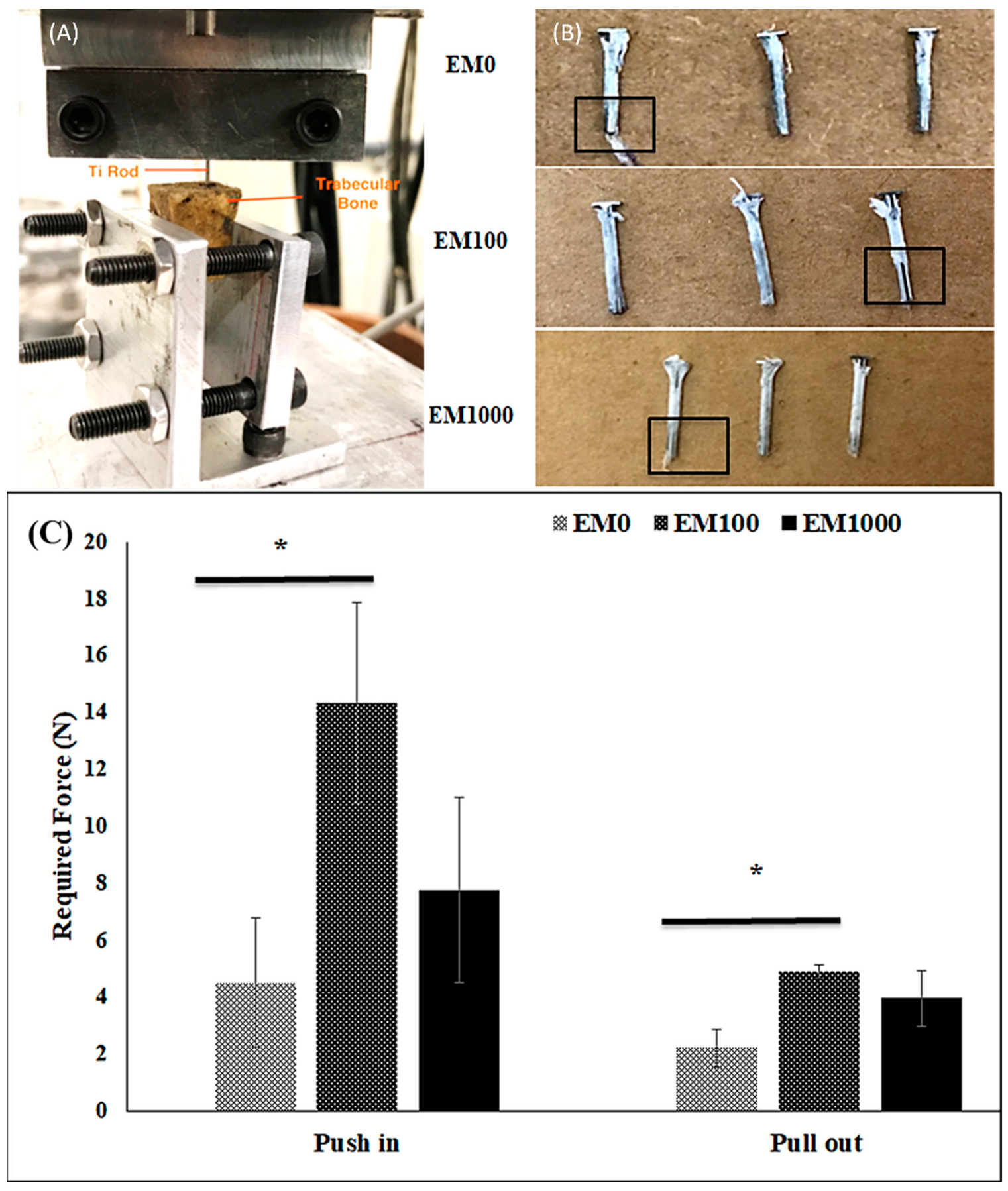
2.1. Bonding Strength of EM-NF Coating on the Surface of Ti Pins
2.2. Animal Study
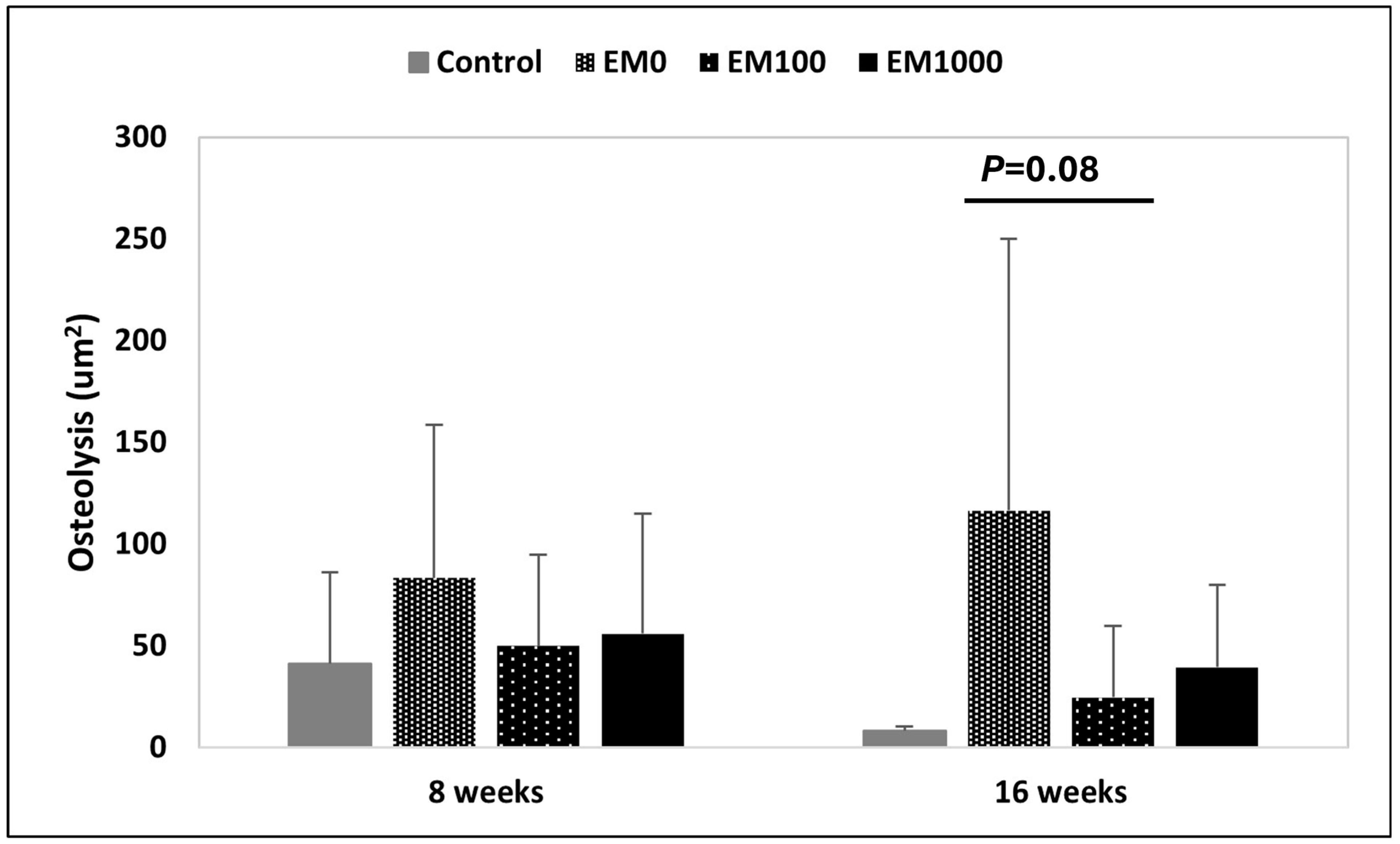
2.3. μCT Evaluation of Osteolysis
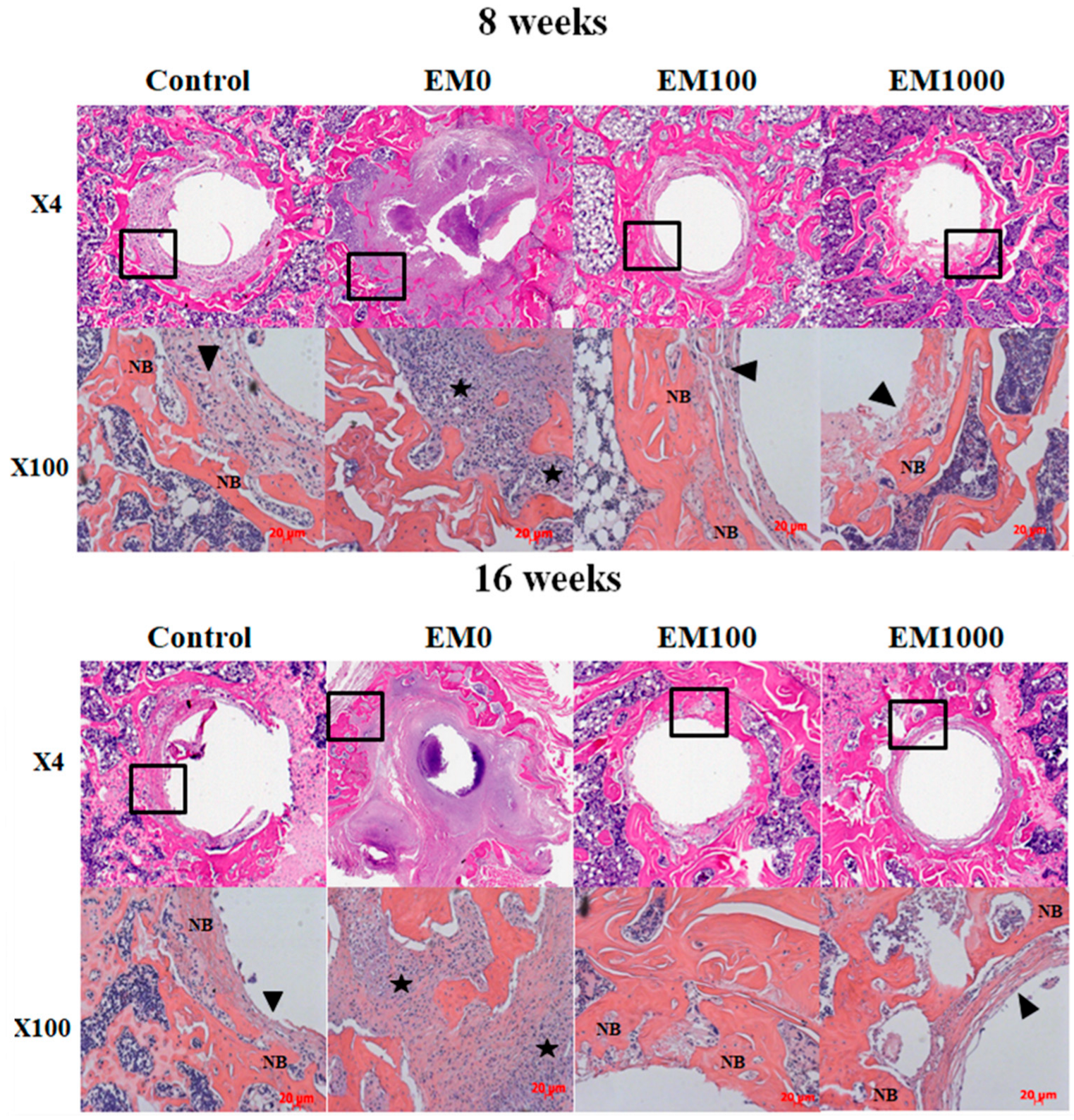
2.4. Histological Analysis of Decalcified Paraffin Tissue Sections
2.5. Hard-Tissue Section Histology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of EM- Doped Coaxial PCL/PLGA (1:1)-PVA NFs
4.3. Bonding Strength of Ti Pins with EM-NF Coating
4.4. Rat Model of S. aureus-Infected Tibia Implantation
4.5. Evaluation of Periprosthetic Osteolysis by Micro-Computed Tomography (μCT)
4.6. Histological Analysis of Decalcified Paraffin Specimens
4.7. Histomorphometry of Hard-Tissue Sections
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Duggal, A.; Barsoum, W.; Schmitt, S.K. Patients with prosthetic joint infection on IV antibiotics are at high risk for readmission. Clin. Orthop. Relat. Res. 2009, 467, 1727–1731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, N.B.; Hersh, B.L.; Kreger, A.; Sayeed, A.; Bullock, A.G.; Rothenberger, S.D.; Klatt, B.; Hamlin, B.; Urish, K.L. Benefits and Adverse Events Associated with Extended Antibiotic Use in Total Knee Arthroplasty Periprosthetic Joint Infection. Clin. Infect. Dis. 2020, 70, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.J.; Jones, R.S. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J. Bone Joint Surg. Br. 2006, 88, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Sudo, A.; Hasegawa, M.; Fukuda, A.; Uchida, A. Treatment of infected hip arthroplasty with antibiotic-impregnated calcium hydroxyapatite. J. Arthroplast. 2008, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Tone, S.; Naito, Y.; Wakabayashi, H.; Sudo, A. Use of antibiotic-impregnated hydroxyapatite for infection following total knee arthroplasty. Mod. Rheumatol. 2021, 31, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Leprêtre, S.; Chai, F.; Hornez, J.-C.; Vermet, G.; Neut, C.; Descamps, M.; Hildebrand, H.F.; Martel, B. Prolonged local antibiotics delivery from hydroxyapatite functionalised with cyclodextrin polymers. Biomaterials 2009, 30, 6086–6093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar]
- Rafiq, I.; Gambhir, A.K.; Wroblewski, B.M.; Kay, P.R. The microbiology of infected hip arthroplasty. Int. Orthop. 2006, 30, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Ruzaimi, M.Y.; Shahril, Y.; Masbah, O.; Salasawati, H. Antimicrobial properties of erythromycin and colistin impregnated bone cement. An. in vitro analysis. Med. J. Malaysia. 2006, 61 (Suppl. SA), 21–26. [Google Scholar]
- Ren, W.; Li, X.H.; Chen, B.D.; Wooley, P.H. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NF-kappaB activity. J. Orthop. Res. 2004, 22, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wu, B.; Peng, X.; Mayton, L.; Yu, D.; Ren, J.; Chen, B.D.; Wooley, P.H. Erythromycin inhibits wear debris-induced inflammatory osteolysis in a murine model. J. Orthop. Res. 2006, 24, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Henke, M.O.; Rubin, B.K. Macrolide antibiotics as immunomodulatory medications: Proposed mechanisms of action. Pharmacol. Ther. 2008, 117, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages-Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. A 2013, 101, 3033–3045. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, W.; Li, X.; Markel, D.C.; Ren, W. Mitigative effect of erythromycin on PMMA challenged preosteoblastic MC3T3-E1 cells. Sci. World J. 2014, 2014, 107196. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Song, W.; Yu, X.; Wang, S.; Blasier, R.; Markel, D.C.; Mao, G.; Shi, T. Cyclodextrin-erythromycin complexes as a drug delivery device for orthopedic application. Int. J. Nanomed. 2011, 6, 3173–3186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, B.M.; Handorf, A.M.; Ionescu, L.C.; Li, W.J.; Mauck, R.L. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert. Rev. Med. Devices. 2009, 6, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Gluck, J.M.; Rahgozar, P.; Ingle, N.P.; Rofail, F.; Petrosian, A.; Cline, M.G.; Jordan, M.C.; Roos, K.P.; MacLellan, W.R.; Shemin, R.J.; et al. Hybrid coaxial electrospun nanofibrous scaffolds with limited immunological response created for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209. [Google Scholar] [CrossRef] [PubMed]
- Goosen, J.H.; Kums, A.J.; Kollen, B.J.; Verheyen, C.C. Porous-coated femoral components with or without hydroxyapatite in primary uncemented total hip arthroplasty: A systematic review of randomized controlled trials. Arch. Orthop. Trauma. Surg. 2009, 129, 1165–1169. [Google Scholar] [CrossRef]
- Kohgo, T.; Yamada, Y.; Ito, K.; Yajima, A.; Yoshimi, R.; Okabe, K.; Baba, S.; Ueda, M. Bone regeneration with self-assembling peptide nanofiber scaffolds in tissue engineering for osseointegration of dental implants. Int. J. Periodontics Restor. Dent. 2011, 31, e9–e16. [Google Scholar]
- Ifkovits, J.L.; Sundararaghavan, H.G.; Burdick, J.A. Electrospinning fibrous polymer scaffolds for tissue engineering and cell culture. J. Vis. Exp. 2009, 1589–1593. [Google Scholar] [CrossRef]
- Wang, J.; Shah, A.; Yu, X. The influence of fiber thickness, wall thickness and gap distance on the spiral nanofibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2011, 31, 50–56. [Google Scholar] [CrossRef]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; E Kast, R.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 2017, 12, 045008. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mazeh, H.; Guardia, A.; Song, W.; Begeman, P.; Markel, D.C.; Ren, W. Sustained release of strontium (Sr2+) from polycaprolactone/poly (D,L-lactide-co-glycolide)-polyvinyl alcohol coaxial nanofibers enhances osteoblastic differentiation. J. Biomater. Appl. 2019, 34, 533–545. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Liu, Q.; Wang, D.; Xie, T.; Guo, T.; Duan, K.; Weng, J. Room-temperature attachment of PLGA microspheres to titanium surfaces for implant-based drug release. Appl. Surf. Sci. 2014, 309, 112–118. [Google Scholar] [CrossRef]
- Ren, W.; Yu, X.; Chen, L.; Shi, T.; Bou-Akl, T.; Markel, D.C. Osteoblastic differentiation and bactericidal activity are enhanced by erythromycin released from PCL/PLGA-PVA coaxial nanofibers. J. Biomater. Appl. 2022, 37, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Hwang, M.G.; Lim, J. In vitro bioactivity of titanium implants coated with bicomponent hybrid biodegradable polymers. J. Sol-Gel Sci. Technol. 2012, 64, 756–764. [Google Scholar] [CrossRef]
- Pastorino, D.; Canal, C.; Ginebra, M.P. Drug delivery from injectable calcium phosphate foams by tailoring the macroporosity-drug interaction. Acta Biomater. 2015, 12, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2021, 7, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Hinarejos, P.; Guirro, P.; Leal, J.; Montserrat, F.; Pelfort, X.; Sorli, M.; Horcajada, J.; Puig, L. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: A prospective randomized study in 3000 knees. J. Bone Jt. Surg. 2013, 95, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Nien, Y.H.; Lin, S.W.; Hsu, Y.N. Preparation and characterization of acrylic bone cement with high drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 974–978. [Google Scholar] [CrossRef]
- Tang, P.; Low, D.E.; Atkinson, S.; Pike, K.; Ashi-Sulaiman, A.; Simor, A.; Richardson, S.; Willey, B.M. Investigation of Staphylococcus aureus isolates identified as erythromycin intermediate by the Vitek-1 System: Comparison with results obtained with the Vitek-2 and Phoenix systems. J. Clin. Microbiol. 2003, 41, 4823–4825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, W.; Yu, X.; Markel, D.C.; Shi, T.; Ren, W. Coaxial PCL/PVA electrospun nanofibers: Osseointegration enhancer and controlled drug release device. Biofabrication 2013, 5, 035006. [Google Scholar] [CrossRef] [PubMed]
- Monzón, M.; García-Álvare, F.; Laclériga, A.; Gracia, E.; Leiva, J.; Oteiza, C.; Amorena, B. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J. Orthop. Res. 2001, 19, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Back, D.A.; Pauly, S.; Rommel, L.; Haas, N.P.; Schmidmaier, G.; Wildemann, B.; Greiner, S.H. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet. Disord. 2012, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Guo, C.; Dong, W.; He, F.M.; Zhao, S.F.; Yang, G.L. Effect of thin nano-hydroxyapatite coating on implant osseointegration in ovariectomized rats. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 113, e48–e53. [Google Scholar] [CrossRef] [PubMed]
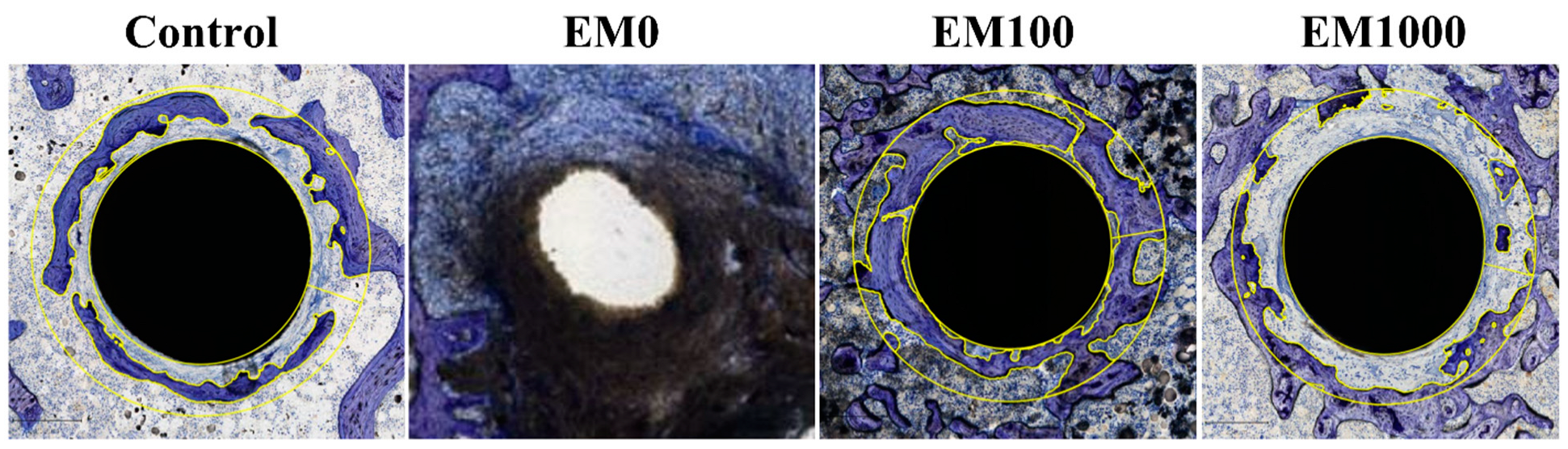

| Group | BIC (%) | BAFO | Bone-to-implant contact (BIC): The BIC was calculated as the percentage of implant surface in direct contact with the bone over the entire length of the implant within the section examined. Bone area fraction occupancy (BAFO): The BAFO was the area occupied by the mineralized bone matrix within 200 um of the implant within the section. Measurement was made by outlining the bone surface area from the total field within a 200 um radius of the implant and was expressed as percentage (implant area was removed). |
| Negative control | 3.43 | 0.39 | |
| EM0 | 0 | 0 | |
| EM100 | 35.08 | 0.63 | |
| EM1000 | 0 | 0.3 |
| Description | 8 Weeks | 16 Weeks |
|---|---|---|
| NF coating, no infection, negative control | 4 | 4 |
| EM0-NF coating, positive control | 8 | 8 |
| EM100-NF coating | 8 | 8 |
| EM1000-NF coating | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markel, D.C.; Powell, D.; Wu, B.; Pawlitz, P.; Bou-Akl, T.; Chen, L.; Shi, T.; Ren, W. Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of S. aureus-Induced Periprosthetic Joint Infection. Int. J. Mol. Sci. 2024, 25, 7926. https://doi.org/10.3390/ijms25147926
Markel DC, Powell D, Wu B, Pawlitz P, Bou-Akl T, Chen L, Shi T, Ren W. Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of S. aureus-Induced Periprosthetic Joint Infection. International Journal of Molecular Sciences. 2024; 25(14):7926. https://doi.org/10.3390/ijms25147926
Chicago/Turabian StyleMarkel, David C., Dexter Powell, Bin Wu, Paula Pawlitz, Therese Bou-Akl, Liang Chen, Tong Shi, and Weiping Ren. 2024. "Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of S. aureus-Induced Periprosthetic Joint Infection" International Journal of Molecular Sciences 25, no. 14: 7926. https://doi.org/10.3390/ijms25147926
APA StyleMarkel, D. C., Powell, D., Wu, B., Pawlitz, P., Bou-Akl, T., Chen, L., Shi, T., & Ren, W. (2024). Therapeutic Efficacy of an Erythromycin-Loaded Coaxial Nanofiber Coating in a Rat Model of S. aureus-Induced Periprosthetic Joint Infection. International Journal of Molecular Sciences, 25(14), 7926. https://doi.org/10.3390/ijms25147926








