MicroRNA-181a Targets GNAI2 and Affects the Proliferation and Induction Ability of Dermal Papilla Cells: The Potential Involvement of the Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Results
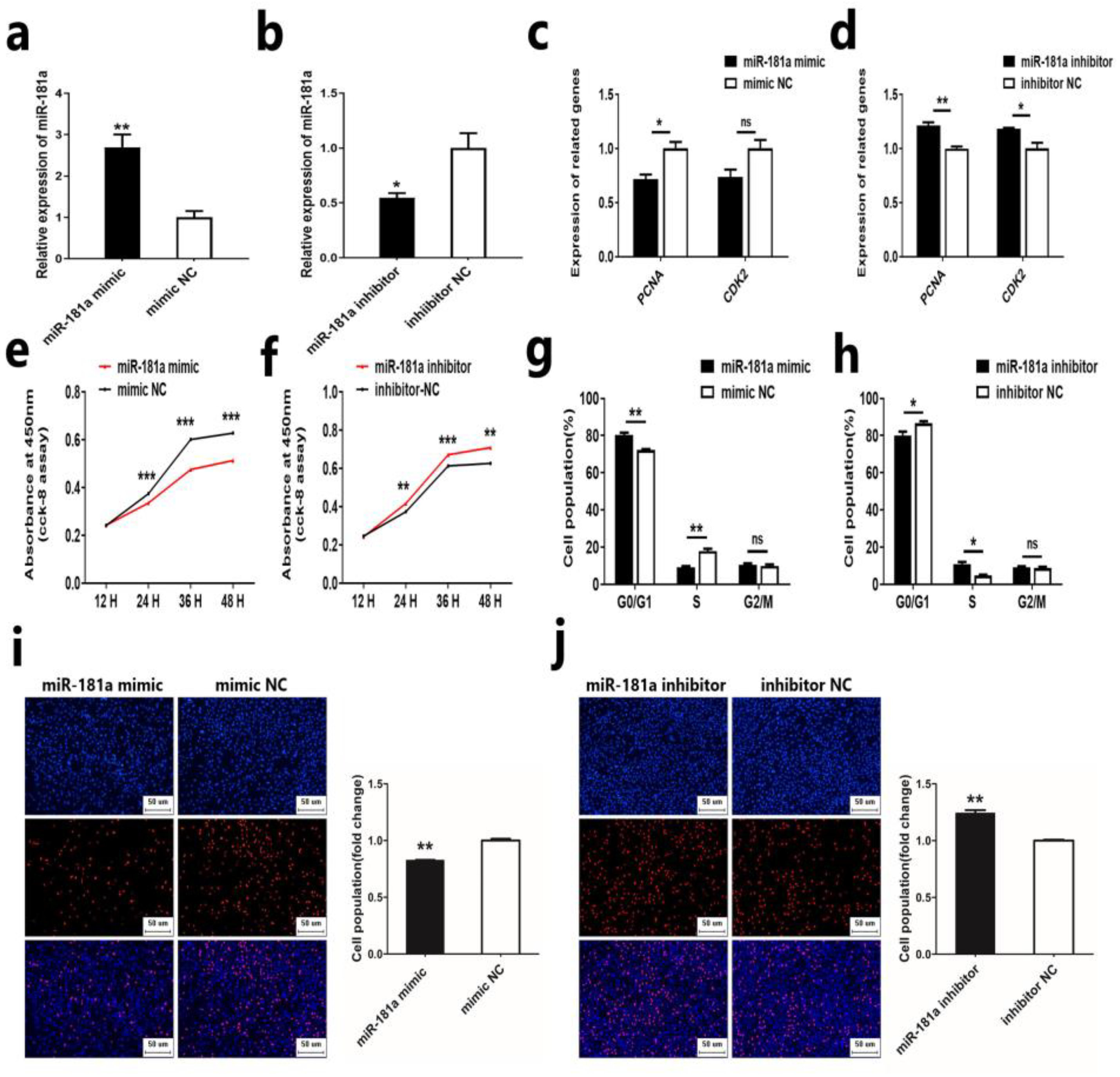
2.1. MiR-181a Inhibits the Proliferation of Ovine DPCs
2.2. MiR-181a Inhibits the Induction Ability of Ovine DPCs
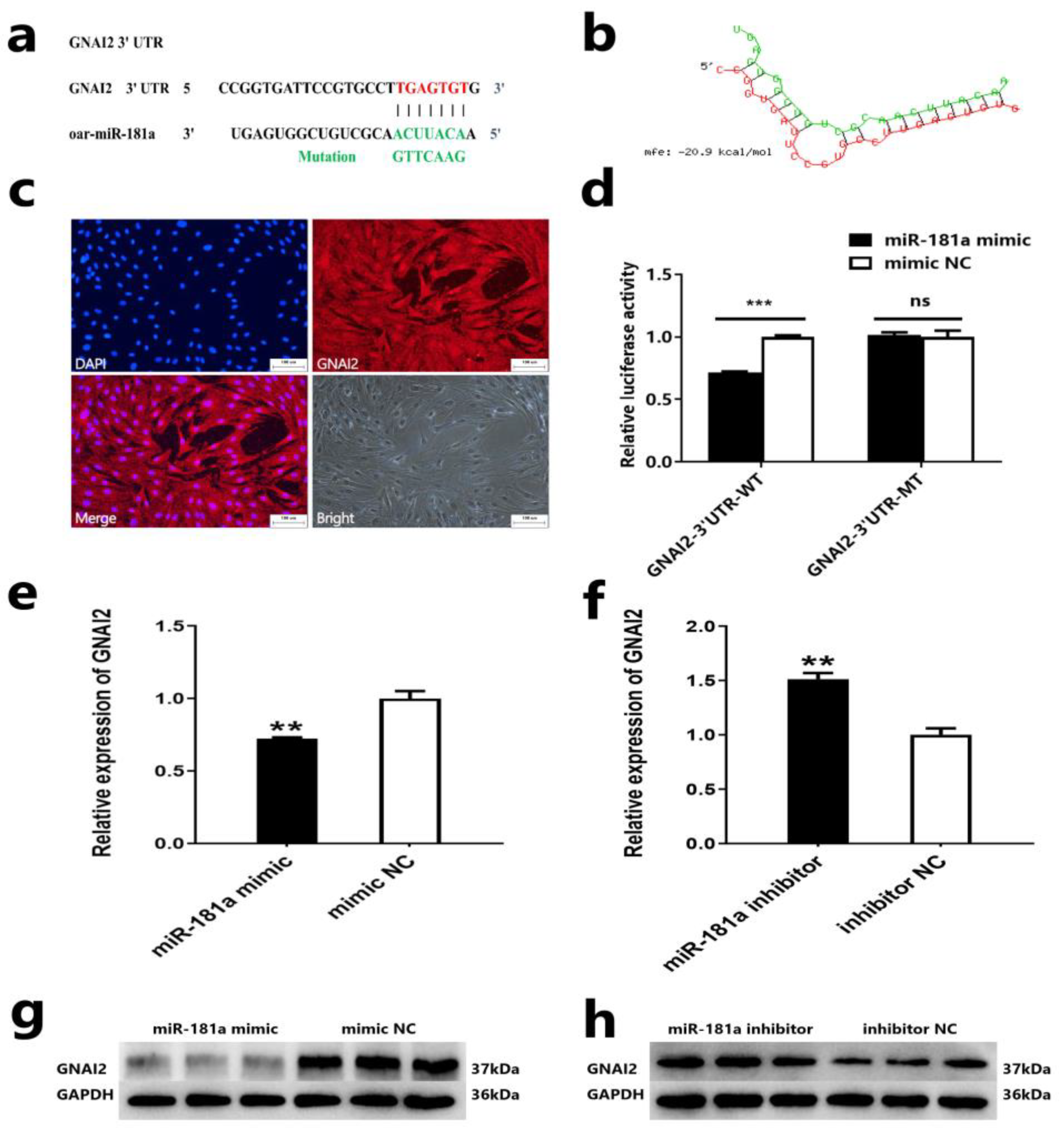
2.3. GNAI2 Is a Target Gene of miR-181a
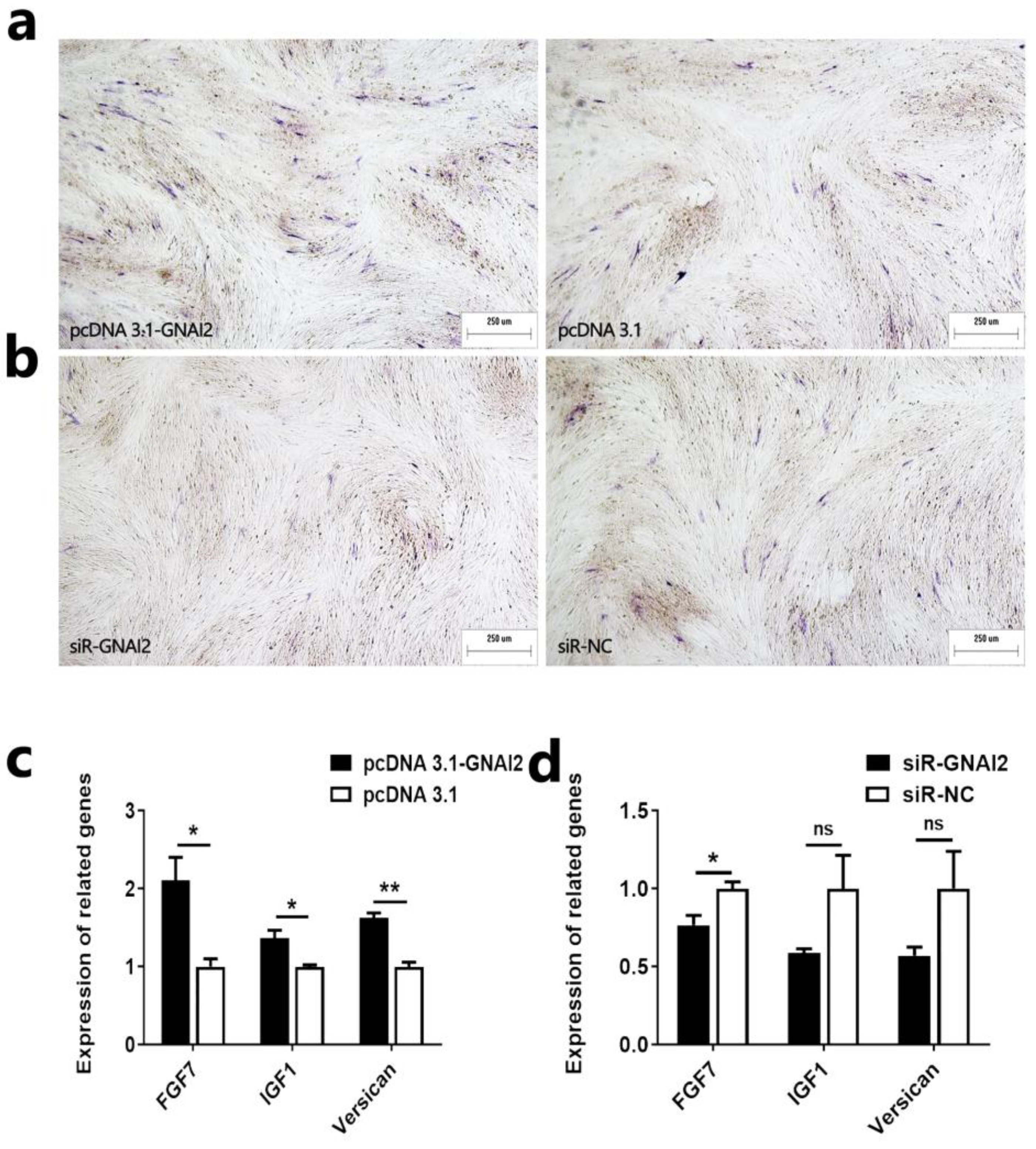
2.4. GNAI2 Promotes the Proliferation of Ovine DPCs
2.5. GNAI2 Promotes the Induction Ability of Ovine DPCs
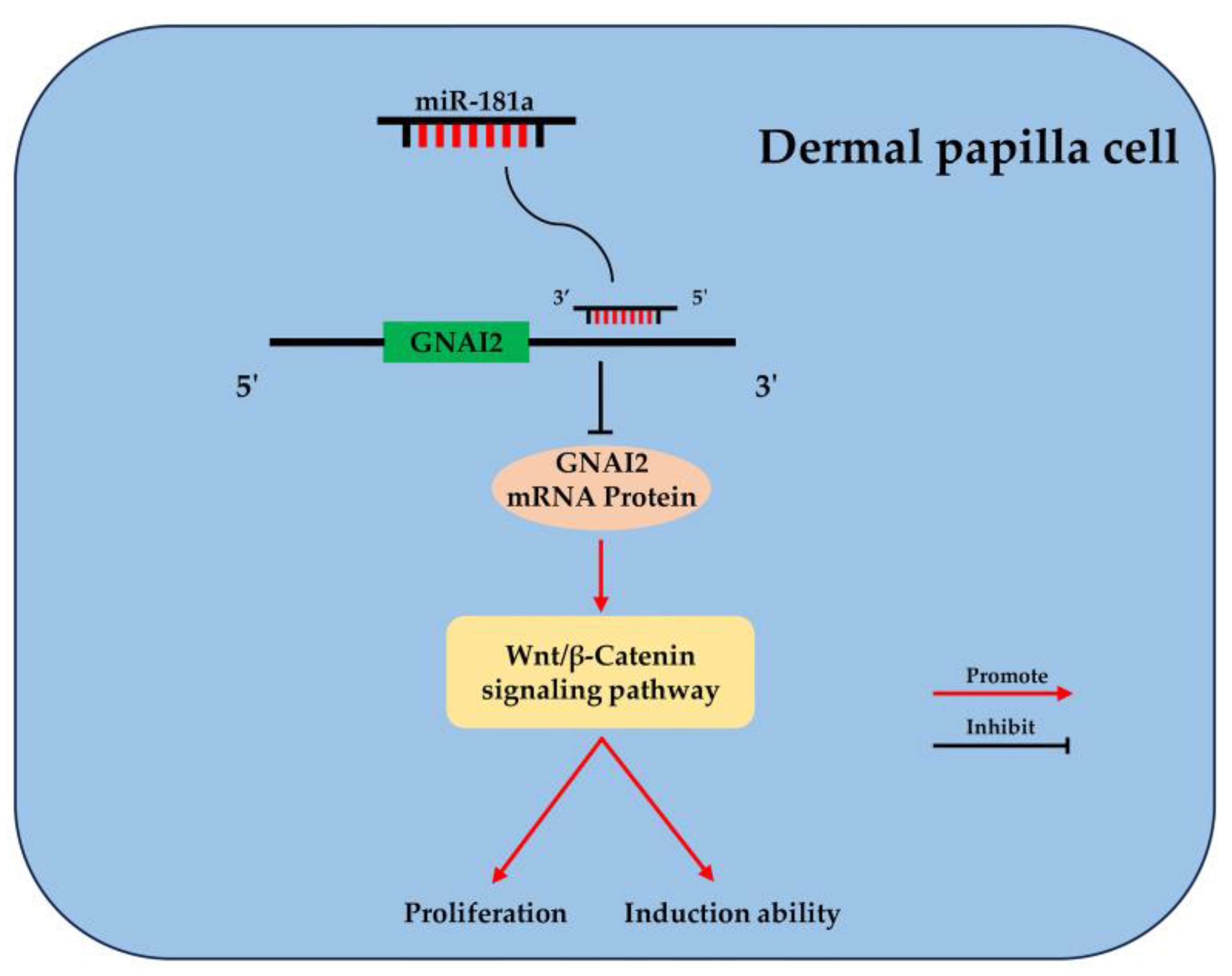
2.6. GNAI2 Is Involved in the Wnt/β-Catenin Signaling Pathway of Ovine DPCs
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Cell Isolation, Culture, and Transfection
4.3. Total RNA Extraction, cDNA Synthesis, Primer Design, and qRT-PCR
4.4. Plasmid Construction and RNA Oligonucleotides
4.5. CCK-8 Assay
4.6. EdU Assay
4.7. Cell Cycle Assay
4.8. Alkaline Phosphatase Activity Assay
4.9. Immunofluorescence Assay
4.10. Dual-Luciferase Assay
4.11. TOP/FOP-Flash Wnt Report Assays
4.12. Total Protein Extraction, and Western Blot Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, X.Y.; Gao, W.; Jin, C.Y.; Wang, L.H.; Wang, Y.; Chen, W.H.; Zou, S.X.; Huang, S.N.; Li, Z.F.; Wang, J.Y. Preliminary study on microR-148a and microR-10a in dermal papilla cells of Hu sheep. BMC Genet. 2019, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.E. Improvement of wool production through genetic engineering. Trends Biotechnol. 1990, 8, 6–11. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Zheng, X.; Liu, Z.; Yang, G.; Hu, X.; Mou, C. Comparative investigation of coarse and fine wool sheep skin indicates the early regulators for skin and wool diversity. Gene 2020, 758, 144968. [Google Scholar] [CrossRef] [PubMed]
- Nissimov, J.N.; Das Chaudhuri, A.B. Hair curvature: A natural dialectic and review. Biol. Rev. 2014, 89, 723–766. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124 Pt 8, 1179–1182. [Google Scholar] [CrossRef]
- Rogers, G.E. Biology of the wool follicle: An excursion into a unique tissue interaction system waiting to be rediscovered. Exp. Dermatol. 2006, 15, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lian, L.; Zhang, D.; Qu, L.; Yang, N. gga-miR-26a targets NEK6 and suppresses Marek’s disease lymphoma cell proliferation. Poult. Sci. 2014, 93, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yuan, L.; Cao, W.; Ye, X.; Ma, X.; Qin, C.; Li, B.; Yu, F.; Fu, X. Regulation of secondary hair follicle cycle in cashmere goats by miR-877-3p targeting IGFBP5 gene. J. Anim. Sci. 2023, 101, skad314. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.-U.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Zhang, X.; Dai, Y.; Yang, N.; Bao, Z.; Chen, Y.; Wu, X. Exosomal miRNA-181a-5p from the cells of the hair follicle dermal papilla promotes the hair follicle growth and development via the Wnt/beta-catenin signaling pathway. Int. J. Biol. Macromol. 2022, 207, 110–120. [Google Scholar] [CrossRef]
- Ma, T.; Li, J.; Li, J.; Wu, S.; Xiangba; Jiang, H.; Zhang, Q. Expression of miRNA-203 and its target gene in hair follicle cycle development of Cashmere goat. Cell Cycle 2021, 20, 204–210. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wei, C.; Huang, X.; Zhang, G.; Mao, J.; Li, X.; Yang, C.; Zhang, W.; Tian, K.; Liu, G. MiR-23b and miR-133 Cotarget TGFβ2/NOTCH1 in Sheep Dermal Fibroblasts, Affecting Hair Follicle Development. Cells 2024, 13, 557. [Google Scholar] [CrossRef]
- Lv, X.; Chen, W.; Wang, S.; Cao, X.; Yuan, Z.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. Integrated Hair Follicle Profiles of microRNAs and mRNAs to Reveal the Pattern Formation of Hu Sheep Lambskin. Genes 2022, 13, 342. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Huch, M. Definitions for adult stem cells debated. Nature 2018, 563, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Bastepe, M. GNAS Spectrum of Disorders. Curr. Osteoporos. Rep. 2015, 13, 146–158. [Google Scholar] [CrossRef]
- Hu, S.S.; Dai, Y.Y.; Bai, S.C.; Zhao, B.H.; Wu, X.S.; Chen, Y. GNAI2 Promotes Proliferation and Decreases Apoptosis in Rabbit Melanocytes. Genes 2021, 12, 1130. [Google Scholar] [CrossRef]
- Fu, X.D.; Li, Y.M.; Alvero, A.; Li, J.N.; Wu, Q.H.; Xiao, Q.; Peng, Y.L.; Hu, Y.B.; Li, X.; Yan, W.G. MicroRNA-222-3p/GNAI2/AKT axis inhibits epithelial ovarian cancer cell growth and associates with good overall survival. Oncotarget 2016, 7, 80633–80654. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.F.; Cao, X.K.; Ge, L.; He, M.L.; Zhang, W.B.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Quan, K. The identification and validation of target genes of IGFBP3 protein in sheep skeletal muscle cells. Anim. Biotechnol. 2023, 34, 4580–4587. [Google Scholar] [CrossRef]
- Xiang, B.; Li, Y.M.; Li, J.P.; Li, J.Y.; Jiang, H.Z.; Zhang, Q.L. MiR-19 3b regulated the formation of coat colors by targeting WNT10A and GNAI2 in Cashmere goats. Anim. Biotechnol. 2023, 34, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.L.; Hu, T.X.; Yang, H.C.; Zhang, L.; Song, Q.; Xiang, F.; Yang, X.C.; Li, Y.H. Twist1 Contributes to the Maintenance of Some Biological Properties of Dermal Papilla Cells by Forming a Complex With Tcf4 and β-Catenin. Front. Cell Dev. Biol. 2020, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Li, Y.C.; Wang, Y.Y.; Wu, J.J.; Yang, G.H.; Yang, T.; Gao, Y.; Lu, Y.G. Versican gene: Regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J. Dermatol. Sci. 2012, 68, 157–163. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Lv, X.Y.; Cao, X.K.; Yuan, Z.H.; Getachew, T.; Li, Y.T.; Wang, S.H.; Sun, W. SOX18 Promotes the Proliferation of Dermal Papilla Cells via the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 16672. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, H.; Panteleyev, A.A.; de Berker, D.; Tobin, D.J.; Christiano, A.M. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation 2004, 72, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Adam, R.C.; Ge, Y.; Hua, Z.L.; Fuchs, E. Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 2017, 169, 483–496 e413. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Grisanti, L.; Zemla, R.; Rezza, A.; Barros, R.; Sennett, R.; Mazloom, A.R.; Chung, C.Y.; Cai, X.; Cai, C.L. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev. Cell 2012, 23, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef]
- Laron, E.A.; Aamar, E.; Enshell-Seijffers, D. The Mesenchymal Niche of the Hair Follicle Induces Regeneration by Releasing Primed Progenitors from Inhibitory Effects of Quiescent Stem Cells. Cell Rep. 2018, 24, 909–921.e3. [Google Scholar] [CrossRef]
- Saxena, N.; Mok, K.W.; Rendl, M. An updated classification of hair follicle morphogenesis. Exp. Dermatol. 2019, 28, 332–344. [Google Scholar] [CrossRef]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Deschene, E.R.; Zito, G.; Gonzalez, D.G.; Saotome, I.; Haberman, A.M.; Greco, V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487, 496-U1600. [Google Scholar] [CrossRef]
- Mardaryev, A.N.; Ahmed, M.I.; Vlahov, N.V.; Fessing, M.Y.; Gill, J.H.; Sharov, A.A.; Botchkareva, N.V. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. FASEB J. 2010, 24, 3869–3881. [Google Scholar] [CrossRef]
- Yi, R.; O’Carroll, D.; Pasolli, H.A.; Zhang, Z.H.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Bai, C.W.; Lu, L.; Zhang, J.N.; Zhou, C.Y.; Ni, Y.C.; Li, K.R.; Yao, J.; Zhou, X.Z.; Lan, C.G.; Cao, C. G protein subunit alpha i2’s pivotal role in angiogenesis. Theranostics 2024, 14, 2190–2209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Dai, Y.; Liu, X.Q.; Wang, C.; Wang, A.X.; Chen, Z.J.; Heidbreder, C.E.; Kolokythas, A.; Zhou, X.F. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum. Genet. 2011, 129, 189–197. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Zhu, N.X.; Huang, K.; Liu, Y.; Zhang, H.; Lin, E.; Zeng, Y.; Li, H.H.; Xu, Y.M.; Cai, B.Z.; Yuan, Y.P. miR-195-5p Regulates Hair Follicle Inductivity of Dermal Papilla Cells by Suppressing Wnt/β-Catenin Activation. Biomed. Res. Int. 2018, 2018, 4924356. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′–3′) | Annealing Temperature (°C) |
|---|---|---|
| miR-181a | F: CGAACATTCAACGCTGTCG | 58 |
| R: AGTGCAGGGTCCGAGGTATT | ||
| Stem-loop primer | AACATTCAACGCTGTCGGTGAGTGTCGTATCCAG | 60 |
| TGCGAATACCTCGGACCCTGCACTGGATACGAC | ||
| U6 | F: CTCGCTTCGGCAGCACA | 60 |
| R: AACGCTTCACGAATTTGCGT |
| Gene | Primer Sequence (5′–3′) | Product Size (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|
| GNAI2 | F: GAGTACCAGCTCAATGACTCTGCC | 150 | 60 | NM_001162566.1 |
| R: TAGGTCTTTGAAGGTGAAGTGCGT | ||||
| PCNA | F: CGAGGGCTTCGACACTTAC | 97 | 60 | XM_004014340.5 |
| R: GTCTTCATTGCCAGCACATT | ||||
| CDK2 | F: AGAAGTGGCTGCATCACAAG | 92 | 60 | NM_001142509.1 |
| R: TCTCAGAATCTCCAGGGAATAG | ||||
| IGF1 | F: TGTGCTTGCTCGCCTTCA | 216 | 60 | XM_027965760.2 |
| R: AGTACATCTCCAGCCTCCTCA | ||||
| FGF7 | F: CTGCCAAGTTTGCTCTAC | 286 | 60 | NM_001009235.2 |
| R: CAGCCACTGTCCTGATTT | ||||
| Versican | F: TACAAAGGGAGGGTGTCGGT | 226 | 60 | XM_004009067.5 |
| R: AAGCCTTCTGTGCCATCTCA | ||||
| CTNNB1 | F: GAGGACAAGCCACAGGATTAT | 101 | 60 | NM_001308590.1 |
| R: CCAAGATCAGCGGTCTCATT | ||||
| TCF4 | F: AACCCTTTCGCCCACCAA | 299 | 60 | XM_012103768.4 |
| R: CAGGCTGATTCATCCCAC | ||||
| LEF1 | F: CAGGTGGTGTTGGACAGATAA | 179 | 60 | XM_042251146.1 |
| R: ATGAGGGATGCCAGTTGTG | ||||
| c-MYC | F: CCCTACCCGCTCAACGACA | 295 | 60 | NM_001009426.1 |
| R: GGCTGTGAGGAGGTTTGC | ||||
| cyclinD1 | F: CCGAGGAGAACAAGCAGATC | 91 | 60 | XM_027959928.2 |
| R: GAGGGTGGGTTGGAAATG | ||||
| GAPDH | F: TCTCAAGGGCATTCTAGGCTAC | 151 | 60 | NM_001190390.1 |
| R: GCCGAATTCATTGTCGTACCAG |
| Primer Name | Primer Sequence (5′–3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| OE-GNAI2 | F: CTAGCGTTTAAACTTAAGCTT ATGCAGAGATCGCCGCTCG | 1068 | 62 |
| R: CCACACTGGACTAGTGGATCCCTATCCAGAGATGCAGGCGCTG | |||
| GNAI2-3′UTR-WT | F: AAAAGATCCTTTATT AAGCTT ACTGCAAACCTAGAAAACTTTTTAGAAA | 242 | 60 |
| R: CATAGGCCGGCATAG ACGCGT CCAGGGCCACTGGGGTGG | |||
| GNAI2-3′UTR-MT | F: TGCCTGACTGACGTCTACGTGTTTACACCCATCCC | 6686 | 62 |
| R: TAGACGTCAGTCAGGCACGGAATCACCGGAAA |
| Fragment Name | Sequence (5′–3′) |
|---|---|
| miR-181a mimic | AACAUUCAACGCUGUCGGUGAGU |
| ACUCACCGACAGCGUUGAAUGUU | |
| mimic-NC | UUCUCCGAACGUGUCACGUTT |
| ACGUGACACGUUCGGAGAATT | |
| miR-181a inhibitor | ACUCACCGACAGCGUUGAAUGUU |
| inhibitor-NC | CAGUACUUUUGUGUAGUACAA |
| siRNA-432 | GCGGGAGUACCAGCUCAAUTT |
| AUUGAGCUGGUACUCCCGCTT | |
| siRNA-551 | GCAUCGUGGAGACGCACUUTT |
| AAGUGCGUCUCCACGAUGCTT | |
| siRNA-740 | GCAUCGUGGAGACGCACUUTT |
| AAGUGCGUCUCCACGAUGCTT | |
| siRNA-NC | UUCUCCGAACGUGUCACGUTT |
| ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Lv, X.; Mwacharo, J.M.; Li, Y.; Wang, S.; Sun, W. MicroRNA-181a Targets GNAI2 and Affects the Proliferation and Induction Ability of Dermal Papilla Cells: The Potential Involvement of the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 7950. https://doi.org/10.3390/ijms25147950
He M, Lv X, Mwacharo JM, Li Y, Wang S, Sun W. MicroRNA-181a Targets GNAI2 and Affects the Proliferation and Induction Ability of Dermal Papilla Cells: The Potential Involvement of the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(14):7950. https://doi.org/10.3390/ijms25147950
Chicago/Turabian StyleHe, Mingliang, Xiaoyang Lv, Joram M. Mwacharo, Yutao Li, Shanhe Wang, and Wei Sun. 2024. "MicroRNA-181a Targets GNAI2 and Affects the Proliferation and Induction Ability of Dermal Papilla Cells: The Potential Involvement of the Wnt/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 25, no. 14: 7950. https://doi.org/10.3390/ijms25147950






