Vasorelaxant and Hypotensive Effects of Galla chinensis in Rats
Abstract
:1. Introduction
2. Results
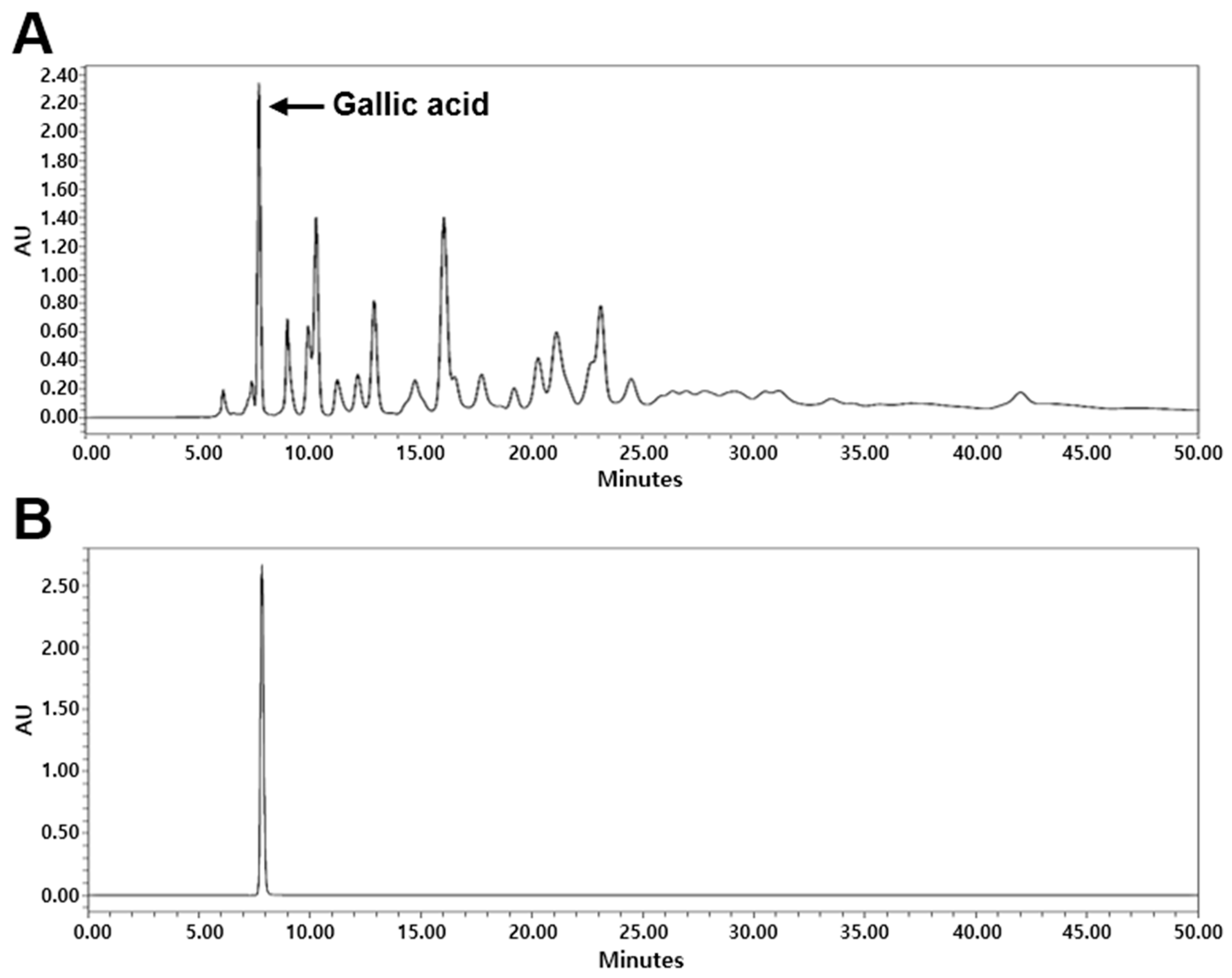
2.1. High-Performance Liquid Chromatography (HPLC) Analysis of G. chinensis
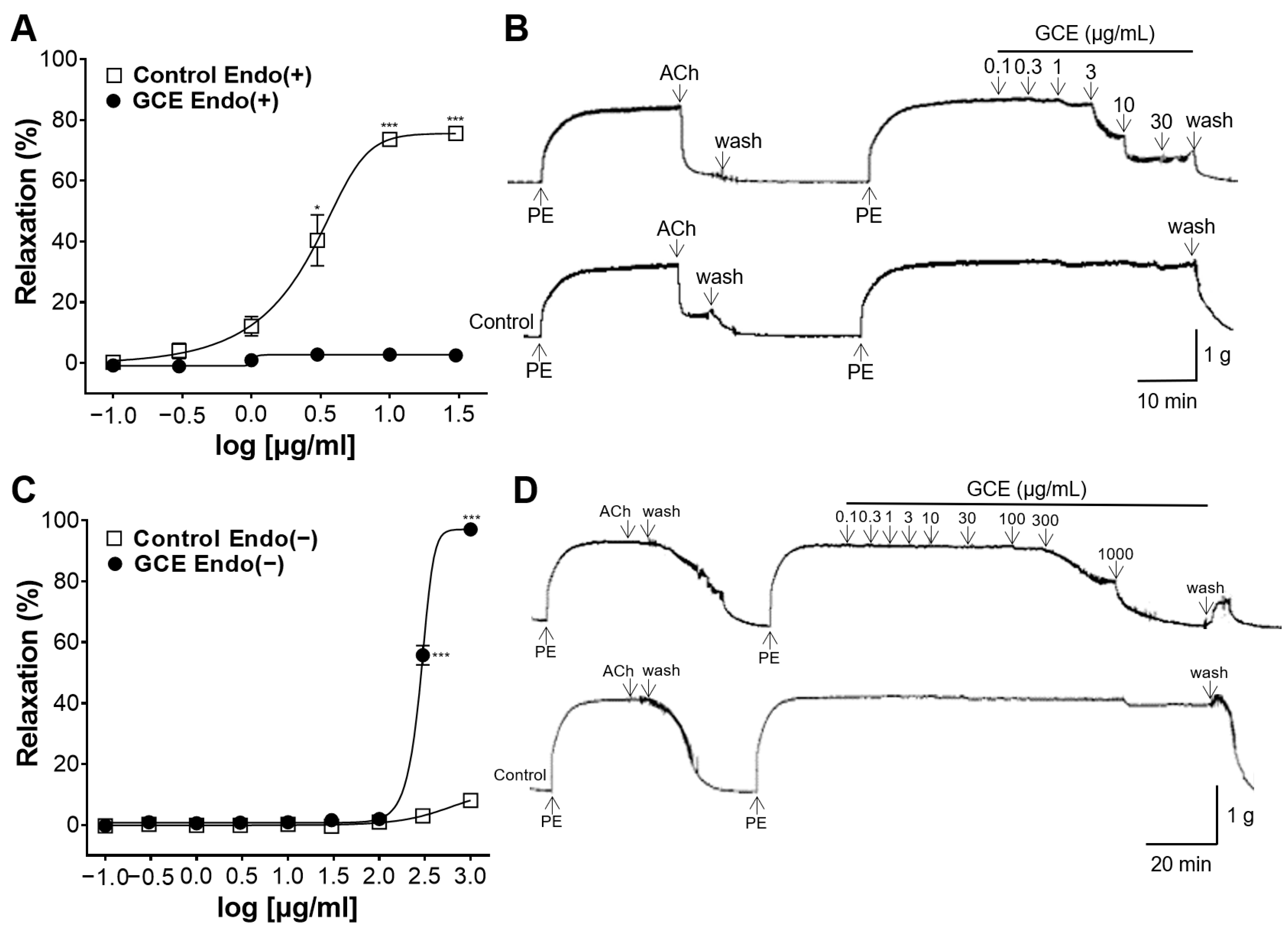
2.2. Vascular Relaxation Induced by GCE on the Endothelium-Intact or -Removed Aorta
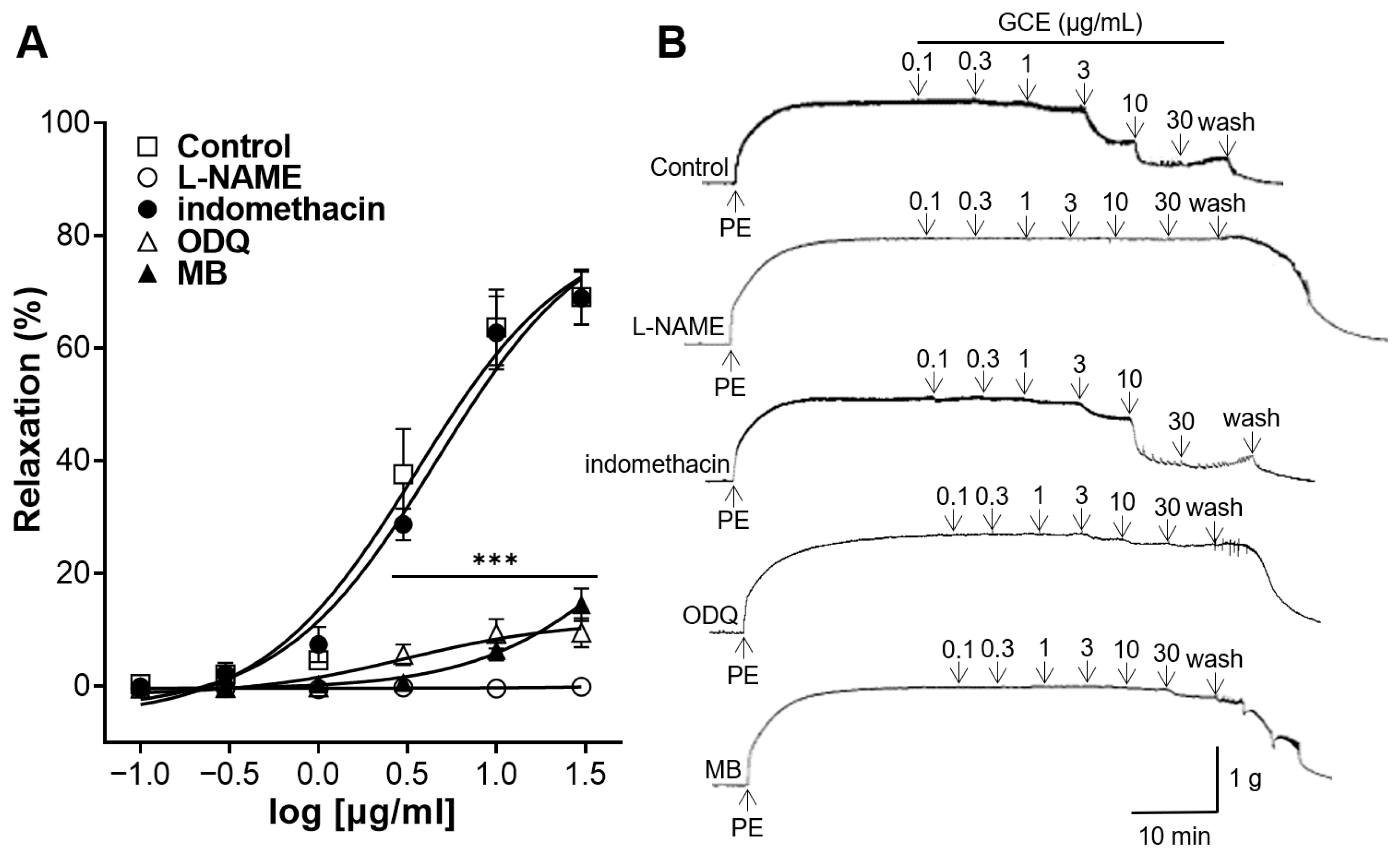
2.3. Vascular Relaxation Induced by GCE on Aortas Incubated with Nitric Oxide (NO) Synthase Inhibitor, Cyclooxygenase (COX) Inhibitor, Soluble Guanylate Cyclase (sGC) Inhibitor or cGMP Inhibitor
2.4. Inhibitory Effect of GCE on Vasoconstriction Induced by Ca2+
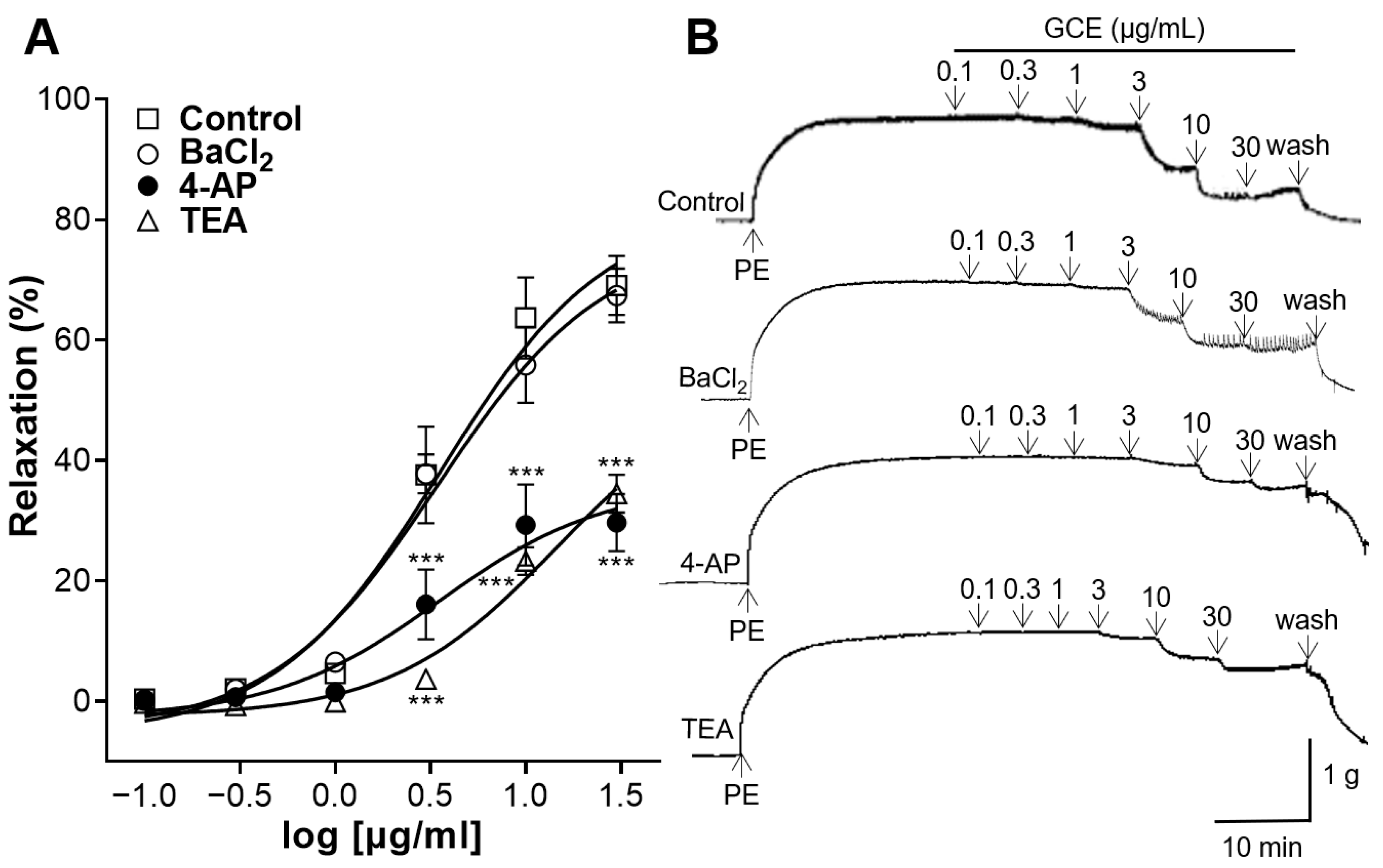
2.5. Vascular Relaxation Induced by GCE on Aortas Pre-Treated with K+ Channel Blockers
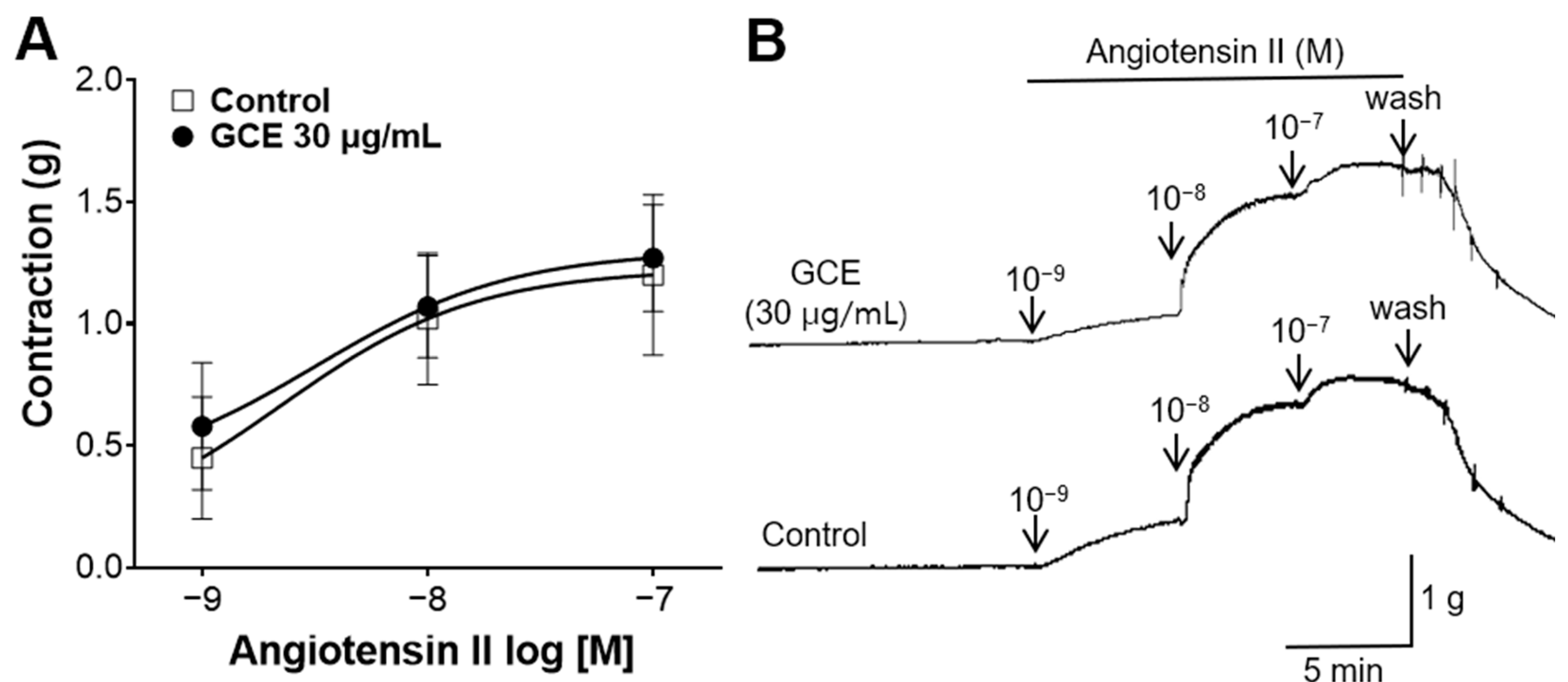
2.6. Inhibitory Effect of GCE on Vasoconstriction Induced by Angiotensin II
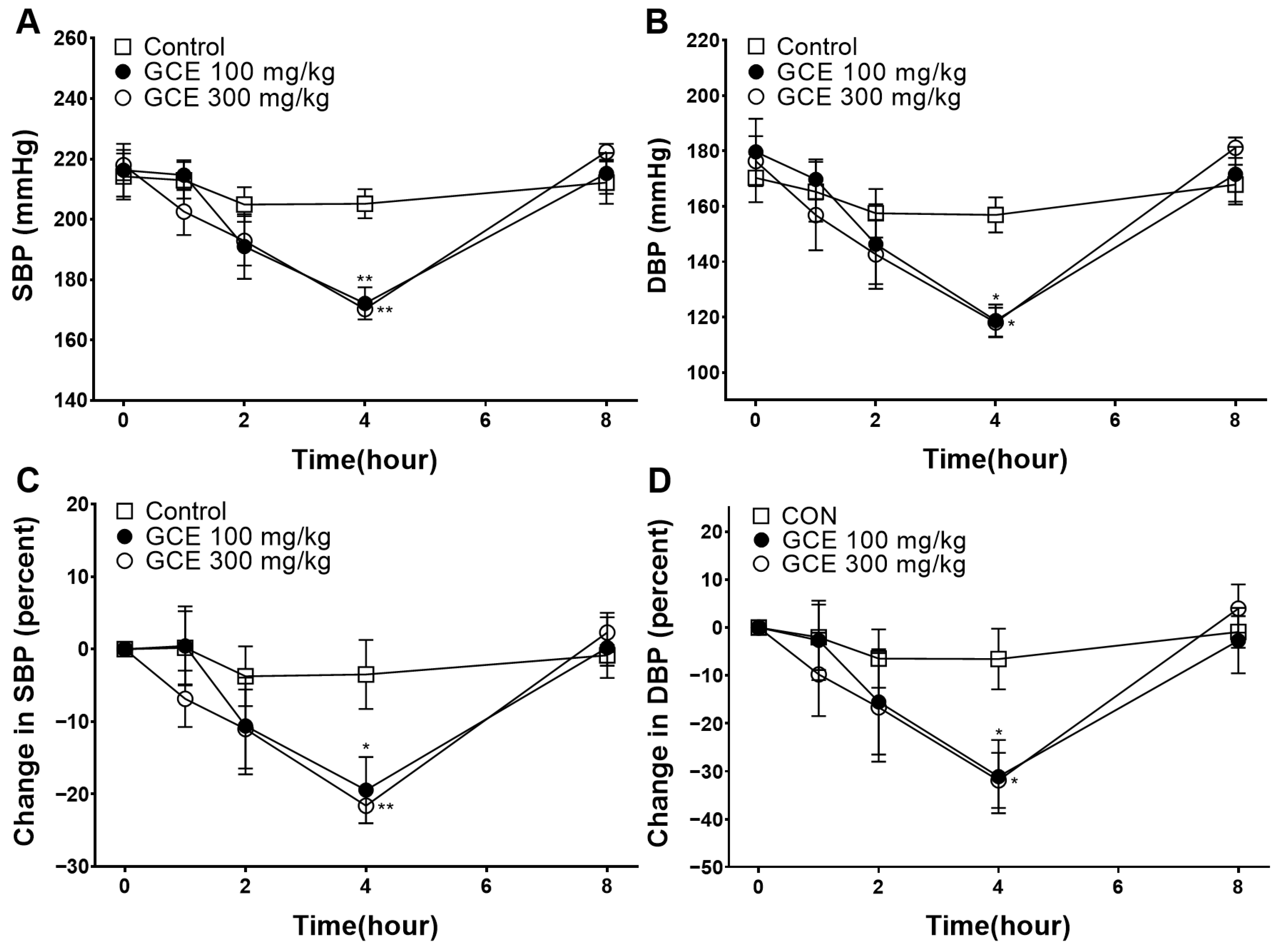
2.7. Hypotensive Effect of GCE
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract
4.2. HPLC Analysis of GCE
4.3. Animals
4.4. Chemicals and Buffer Preparation
4.5. Preparation of Aortic Rings and Measurement of the Tension
4.5.1. General Experimental Procedures
4.5.2. Vascular Relaxation Induced by GCE on the Endothelium-Intact or -Removed Aortic Rings
4.5.3. Vascular Relaxation Induced by GCE on Aortic Rings Pre-Treated with L-NAME, Indomethacin, ODQ, or MB
4.5.4. Inhibitory Effect of GCE on Vasoconstriction Induced by Ca2+
4.5.5. Vascular Relaxation Induced by GCE on Aortic Rings Pre-Treated with K+ Channel Blockers
4.5.6. Inhibitory Effect of GCE on Vasoconstriction Induced by Angiotensin II
4.6. Measurement of Blood Pressure in SHR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transplant. 2020, 29, 0963689720920830. [Google Scholar] [CrossRef]
- Dunbar, S.B.; Khavjou, O.A.; Bakas, T.; Hunt, G.; Kirch, R.A.; Leib, A.R.; Morrison, R.S.; Poehler, D.C.; Roger, V.L.; Whitsel, L.P.; et al. Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035 A Policy Statement from the American Heart Association. Circulation 2018, 137, E558–E577. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, Y.; Zheng, M.; Pan, A.; Wang, M.; Zhao, M.; Li, Y.; Yao, S.; Chen, S.; Wu, S.; et al. Association of Age of Onset of Hypertension with Cardiovascular Diseases and Mortality. J. Am. Coll. Cardiol. 2020, 75, 2921–2930. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Poulter, N.R.; Borghi, C.; Parati, G.; Pathak, A.; Toli, D.; Williams, B.; Schmieder, R.E. Medication adherence in hypertension. J. Hypertens. 2020, 38, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Singhai, H.; Rathee, S.; Jain, S.K.; Patil, U.K. The Potential of Natural Products in the Management of Cardiovascular Disease. Curr. Pharm. Design 2024, 30, 624–638. [Google Scholar] [CrossRef]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Zhang, X.-R.; Li, T.-N.; Zeng, Y.-J.; Wang, J.; Huang, Q.-W. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J. Ethnopharmacol. 2021, 278, 114247. [Google Scholar] [CrossRef] [PubMed]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis—Folklore to Modern Evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, X.; Yin, W.; Li, M. Gallnuts: A Potential Treasure in Anticancer Drug Discovery. Evid-Based Compl Alt. 2018, 2018, 4930371. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Blood-Pressure-Lowering and Endothelium-Dependent Vasorelaxant Effects of Nutgall Tree in Rats. Foods 2024, 13, 1041. [Google Scholar] [CrossRef] [PubMed]
- Cabou, C.; Martinez, L.O. The Interplay of Endothelial P2Y Receptors in Cardiovascular Health: From Vascular Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 5883. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Nagai, A. The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 2169. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Mallat, R.K.; John, C.M.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Nna, V.U.; Romli, A.C.; Ghazali, W.S.W.; Mohamed, M. Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of eNOS/NO/cGMP-Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4225. [Google Scholar] [CrossRef]
- Bernatova, I.; Conde, M.V.; Kopincova, J.; González, M.C.; Puzserova, A.; Arribas, S.M. Endothelial dysfunction in spontaneously hypertensive rats: Focus on methodological aspects. J. Hypertens. 2009, 27, S27–S31. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion Channels and Vascular Diseases. Arterioscler. Thromb. Vasc. 2019, 39, E146–E156. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Ledoux, J. K+ channels in biological processes: Vascular K+ channels in the regulation of blood pressure. J. Recept. Ligand Channel Res. 2014, 7, 51–60. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef] [PubMed]
- Riet, L.T.; Esch, J.H.M.v.; Roks, A.J.M.; Meiracker, A.H.v.d.; Danser, A.H.J. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. The Renin-Angiotensin System and Cardiovascular-Kidney-Metabolic Syndrome: Focus on Early-Life Programming. Int. J. Mol. Sci. 2024, 25, 3298. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Sakuranetin as a Potential Regulator of Blood Pressure in Spontaneously Hypertensive Rats by Promoting Vasorelaxation through Calcium Channel Blockade. Biomedicines 2024, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, S.; Bu, Y.; Choi, H.-Y.; Lee, K. Vasorelaxant and Blood Pressure-Lowering Effects of Cnidium monnieri Fruit Ethanol Extract in Sprague Dawley and Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2024, 25, 4223. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- McGuire, P.G.; Twietmeyer, T.A. Aortic endothelial junctions in developing hypertension. Hypertension 1985, 7, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Zhang, H.; Srivastava, K. Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension 2000, 35, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Sex differences in hypertension: Lessons from spontaneously hypertensive rats (SHR). Clin. Sci. 2021, 135, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Peng, L.; Yin, Z.; Jia, R.; Hu, Z.; Li, Z.; Ni, X.; Liang, X.; Li, L.; He, C.; et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of Galla chinensis solution. J. Ethnopharmacol. 2015, 162, 181–190. [Google Scholar] [CrossRef]
- Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Vasorelaxant and Hypotensive Effects of Galla chinensis in Rats. Int. J. Mol. Sci. 2024, 25, 7962. https://doi.org/10.3390/ijms25147962
Shin S, Park J, Choi H-Y, Bu Y, Lee K. Vasorelaxant and Hypotensive Effects of Galla chinensis in Rats. International Journal of Molecular Sciences. 2024; 25(14):7962. https://doi.org/10.3390/ijms25147962
Chicago/Turabian StyleShin, Sujin, Junkyu Park, Ho-Young Choi, Youngmin Bu, and Kyungjin Lee. 2024. "Vasorelaxant and Hypotensive Effects of Galla chinensis in Rats" International Journal of Molecular Sciences 25, no. 14: 7962. https://doi.org/10.3390/ijms25147962




